Obesity Is Associated with Changes in Laboratory Biomarkers in Chilean Patients Hospitalized with COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Comorbidities and Clinical Manifestations of Chilean Patients with Obesity Hospitalized with COVID-19
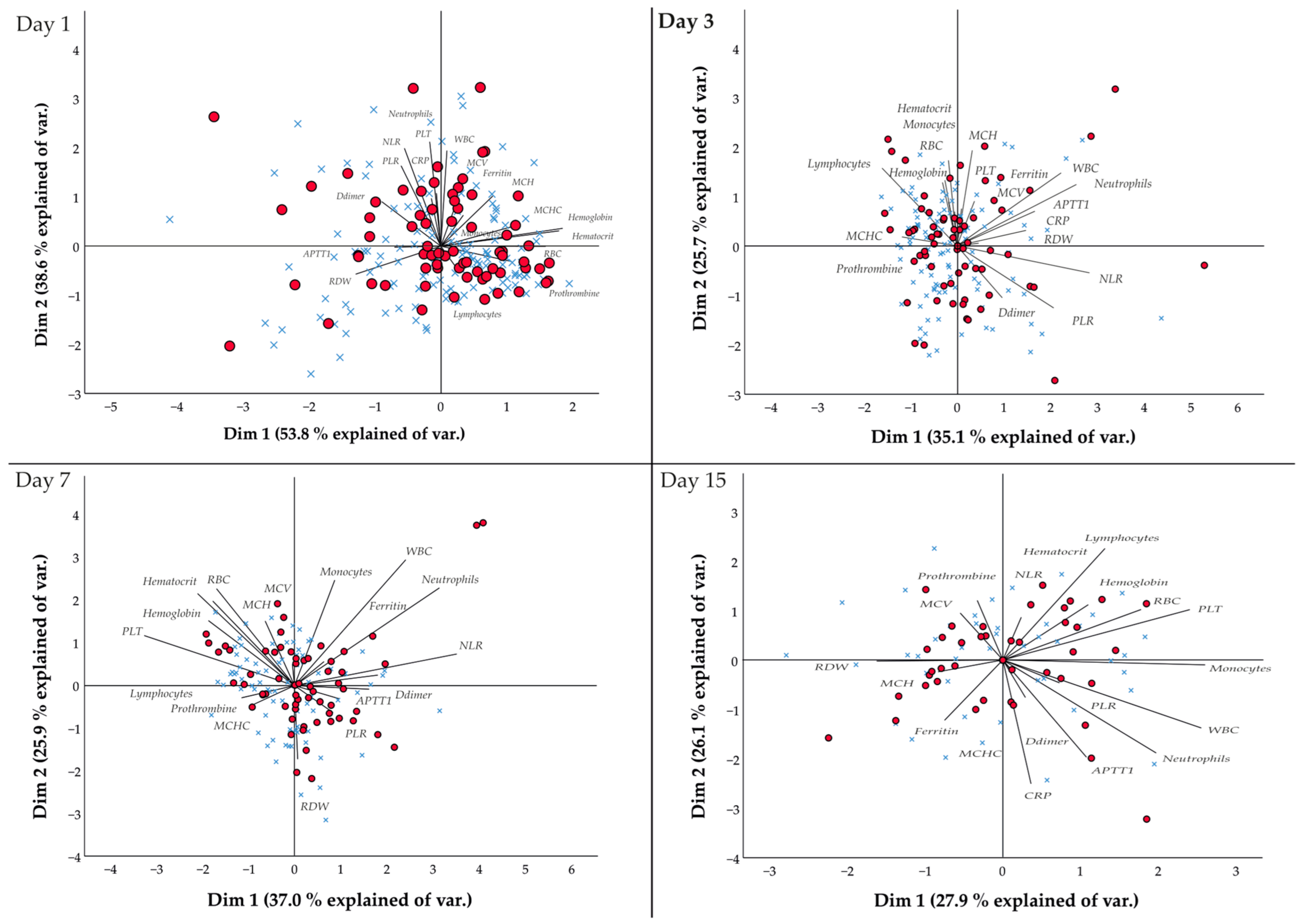
3.2. Dynamic Variations of Laboratory Biomarkers in Chilean Patients with COVID-19
3.3. Association between Laboratory Parameters and Obesity of Chilean Patients Hospitalized with COVID-19
3.4. Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-nCoV and Naming It SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar]
- Liu, K.; Fang, Y.-Y.; Deng, Y.; Liu, W.; Wang, M.-F.; Ma, J.-P.; Xiao, W.; Wang, Y.-N.; Zhong, M.-H.; Li, C.-H.; et al. Clinical Characteristics of Novel Coronavirus Cases in Tertiary Hospitals in Hubei Province. Chin. Med. J. 2020, 133, 1025–1031. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus–Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Perlman, S.; Netland, J. Coronaviruses Post-SARS: Update on Replication and Pathogenesis. Nat. Rev. Microbiol. 2009, 7, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Home. Available online: https://coronavirus.jhu.edu/map.html (accessed on 24 February 2023).
- Datta, P.K.; Liu, F.; Fischer, T.; Rappaport, J.; Qin, X. SARS-CoV-2 Pandemic and Research Gaps: Understanding SARS-CoV-2 Interaction with the ACE2 Receptor and Implications for Therapy. Theranostics 2020, 10, 7448–7464. [Google Scholar] [CrossRef]
- Farkash, E.A.; Wilson, A.M.; Jentzen, J.M. Ultrastructural Evidence for Direct Renal Infection with SARS-CoV-2. J. Am. Soc. Nephrol. 2020, 31, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Aburto, S.; Cisterna, M.; Acuña, J.; Ruíz, C.; Viscardi, S.; Márquez, J.L.; Villano, I.; Letelier, P.; Guzmán, N. Obesity as a Risk Factor for Severe COVID-19 in Hospitalized Patients: Epidemiology and Potential Mechanisms. Healthc. Pap. 2022, 10, 1838. [Google Scholar] [CrossRef]
- Soler, M.J.; Wysocki, J.; Batlle, D. ACE2 Alterations in Kidney Disease. Nephrol. Dial. Transplant 2013, 28, 2687–2697. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, Y.; Zhu, T.; Xia, L. CT Features of Coronavirus Disease 2019 (COVID-19) Pneumonia in 62 Patients in Wuhan, China. Am. J. Roentgenol. 2020, 214, 1287–1294. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.P.; Tian, R.H.; Luo, S.; Zu, Z.Y.; Fan, B.; Wang, X.M.; Xu, K.; Wang, J.T.; Zhu, J.; Shi, J.C.; et al. Risk Factors for Adverse Clinical Outcomes with COVID-19 in China: A Multicenter, Retrospective, Observational Study. Theranostics 2020, 10, 6372–6383. [Google Scholar] [CrossRef] [PubMed]
- Albashir, A.A.D. The Potential Impacts of Obesity on COVID-19. Clin. Med. 2020, 20, e109–e113. [Google Scholar] [CrossRef]
- World Health Organization WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 26 December 2022).
- Jia, X.; Yin, C.; Lu, S.; Chen, Y.; Liu, Q.; Bai, J.; Lu, Y. Two Things About COVID-19 Might Need Attention. Preprints 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Bourgeois, C.; Gorwood, J.; Barrail-Tran, A.; Lagathu, C.; Capeau, J.; Desjardins, D.; Le Grand, R.; Damouche, A.; Béréziat, V.; Lambotte, O. Specific Biological Features of Adipose Tissue, and Their Impact on HIV Persistence. Front. Microbiol. 2019, 10, 1–25. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Nehlsen-Cannarella, S.L.; Ekkens, M.; Utter, A.C.; Butterworth, D.E.; Fagoaga, O.R. Influence of Obesity on Immune Function. J. Am. Diet. Assoc. 1999, 99, 294–299. [Google Scholar] [CrossRef]
- O’Rourke, R.W.; Kay, T.; Scholz, M.H.; Diggs, B.; Jobe, B.A.; Lewinsohn, D.M.; Bakke, A.C. Alterations in T-Cell Subset Frequency in Peripheral Blood in Obesity. Obes. Surg. 2005, 15, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Cuevas, J.; Santos, A.; Armendariz-Borunda, J. Pathophysiological Molecular Mechanisms of Obesity: A Link between MAFLD and NASH with Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 11629. [Google Scholar] [CrossRef]
- Kwok, S.; Adam, S.; Ho, J.H.; Iqbal, Z.; Turkington, P.; Razvi, S.; Le Roux, C.W.; Soran, H.; Syed, A.A. Obesity: A Critical Risk Factor in the COVID-19 Pandemic. Clin. Obes. 2020, 10, e12403. [Google Scholar] [CrossRef]
- Marshall, J.C.; Murthy, S.; Diaz, J.; Adhikari, N.K.; Angus, D.C.; Arabi, Y.M.; Baillie, K.; Bauer, M.; Berry, S.; Blackwood, B.; et al. A Minimal Common Outcome Measure Set for COVID-19 Clinical Research. Lancet Infect. Dis. 2020, 20, e192–e197. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.M.; Gómez-Pérez, A.M.; Fernández-García, J.C.; Barahona San Millan, R.; Aguilera Luque, A.; Hollanda, A.; Jiménez, A.; Jimenez-Murcia, S.; Munguia, L.; Ortega, E.; et al. Coronavirus Disease 2019 (COVID-19) and Obesity. Impact of Obesity and Its Main Comorbidities in the Evolution of the Disease. Eur. Eat. Disord. Rev. 2020, 28, 799–815. [Google Scholar] [CrossRef] [PubMed]
- Celis-Morales, C.A.; Perez-Bravo, F.; Ibañes, L.; Sanzana, R.; Hormazabal, E.; Ulloa, N.; Calvo, C.; Bailey, M.E.S.; Gill, J.M.R. Insulin Resistance in Chileans of European and Indigenous Descent: Evidence for an Ethnicity X Environment Interaction. PLoS ONE 2011, 6, e24690. [Google Scholar] [CrossRef]
- Álvarez Lepin, C.G.; Ramirez, R.; Miranda Fuentes, C.; Ibacache Saavedra, P.; Campos, C.; Cristi-Montero, C.; Molina Sotomayor, E.; Caparrós, C.; Delgado-Floody, P. Lifestyle and Cardiometabolic Risk Factors in the Ethnic and Non-Ethnic Population > 15 Years of Age: Results from the National Chilean Health Survey 2016–2017. Nutr. Hosp. 2023, 2, 1–35. [Google Scholar] [CrossRef]
- da Rosa Mesquita, R.; Francelino Silva Junior, L.C.; Santos Santana, F.M.; Farias de Oliveira, T.; Campos Alcântara, R.; Monteiro Arnozo, G.; Rodrigues da Silva Filho, E.; Galdino dos Santos, A.G.; Oliveira da Cunha, E.J.; Salgueiro de Aquino, S.H.; et al. Clinical Manifestations of COVID-19 in the General Population: Systematic Review. Wien. Klin. Wochenschr. 2021, 133, 377–382. [Google Scholar] [CrossRef]
- Yang, A.-P.; Liu, J.-P.; Tao, W.-Q.; Li, H.-M. The Diagnostic and Predictive Role of NLR, D-NLR and PLR in COVID-19 Patients. Int. Immunopharmacol. 2020, 84, 106504. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Vieyra, R.; Gutiérrez-Castellanos, S.; Álvarez-Aguilar, C.; Baizabal-Aguirre, V.M.; Nuñez-Anita, R.E.; Rocha-López, A.G.; Gómez-García, A. Behavior of Eosinophil Counts in Recovered and Deceased COVID-19 Patients over the Course of the Disease. Viruses 2021, 13, 1675. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F. Clinical Course And Risk Factors For Mortality Of Adult In Patients With COVID-19 In Wuhan, China: A Retrospective Cohort Study. J. Med. Study Res. 2020, 3, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; Zhang, Y.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial Co-Infection and Secondary Infection in Patients with COVID-19: A Living Rapid Review and Meta-Analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Mason, C.Y.; Kanitkar, T.; Richardson, C.J.; Lanzman, M.; Stone, Z.; Mahungu, T.; Mack, D.; Wey, E.Q.; Lamb, L.; Balakrishnan, I.; et al. Exclusion of Bacterial Co-Infection in COVID-19 Using Baseline Inflammatory Markers and Their Response to Antibiotics. J. Antimicrob. Chemother. 2021, 76, 1323–1331. [Google Scholar] [CrossRef]
- Bobcakova, A.; Petriskova, J.; Vysehradsky, R.; Kocan, I.; Kapustova, L.; Barnova, M.; Diamant, Z.; Jesenak, M. Immune Profile in Patients With COVID-19: Lymphocytes Exhaustion Markers in Relationship to Clinical Outcome. Front. Cell. Infect. Microbiol. 2021, 11, 646688. [Google Scholar] [CrossRef] [PubMed]
- Conte, G.; Cei, M.; Evangelista, I.; Colombo, A.; Vitale, J.; Mazzone, A.; Mumoli, N. The Meaning of D-Dimer Value in COVID-19. Clin. Appl. Thromb. Hemost. 2021, 27, 107602962110176. [Google Scholar] [CrossRef]
- Lu, G.; Wang, J. Dynamic Changes in Routine Blood Parameters of a Severe COVID-19 Case. Clin. Chim. Acta 2020, 508, 98–102. [Google Scholar] [CrossRef]
- Mao, J.; Dai, R.; Du, R.-C.; Zhu, Y.; Shui, L.-P.; Luo, X.-H. Hematologic Changes Predict Clinical Outcome in Recovered Patients with COVID-19. Ann. Hematol. 2021, 100, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Yang, J.; Shi, J.; Zhang, P.; Wang, X. Obesity Is Associated with Increased Severity of Disease in COVID-19 Pneumonia: A Systematic Review and Meta-Analysis. Eur. J. Med. Res. 2020, 25, 64. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Piernas, C.; Astbury, N.M.; Hippisley-Cox, J.; O’Rahilly, S.; Aveyard, P.; Jebb, S.A. Associations between Body-Mass Index and COVID-19 Severity in 6·9 Million People in England: A Prospective, Community-Based, Cohort Study. Lancet Diabetes Endocrinol. 2021, 9, 350–359. [Google Scholar] [CrossRef]
| Total | With Obesity | Without Obesity | |||||
|---|---|---|---|---|---|---|---|
| n = 202 (%) | n | (%) | n | (%) | p-Value | ||
| Demographics | |||||||
| Male | 95 | (47.0) | 28 | (13.9) | 67 | (33.2) | 0.111 |
| Female | 107 | (53.0) | 43 | (21.3) | 64 | (31.7) | |
| Age | 60.8 ± 14.8 | 58.9 ± 1.6 | 61.8 ± 1.3 | 0.483 | |||
| Comorbidity | |||||||
| Arterial hypertension | 116 | (57.4) | 45 | (22.3) | 71 | (35.1) | 0.208 |
| Mellitus diabetes 2 (DM2) | 70 | (34.7) | 27 | (13.4) | 43 | (21.3) | 0.458 |
| Cardiovascular disease | 118 | (58.4) | 45 | (22.3) | 73 | (36.1) | 0.292 |
| Chronic respiratory pathology | 26 | (12.9) | 17 | (8.4) | 9 | (4.5) | 0.001 * |
| Chronic kidney disease | 22 | (10.9) | 10 | (5.0) | 12 | (5.9) | 0.283 |
| Chronic liver disease | 8 | 4.0 | 2 | (1.0) | 6 | (3.0) | 0.540 |
| Clinical manifestations | |||||||
| Fever | 115 | (56.9) | 45 | (22.3) | 70 | (34.7) | 0.173 |
| Odynophagia | 33 | (16.3) | 16 | (7.9) | 17 | (8.4) | 0.079 |
| Gastrointestinal symptoms | 43 | (21.3) | 15 | (7.4) | 28 | (13.9) | 0.967 |
| Cough | 126 | (62.4) | 51 | (25.2) | 75 | (37.1) | 0.041 * |
| Headache | 53 | (26.2) | 21 | (10.4) | 32 | (15.8) | 0.427 |
| Dyspnea | 131 | (64.9) | 56 | (27.7) | 75 | (37.1) | 0.002 * |
| Myalgia | 68 | (33.7) | 23 | (11.4) | 45 | (22.3) | 0.779 |
| Fatigue | 36 | (17.8) | 12 | (5.9) | 24 | (11.9) | 0.801 |
| Anosmia | 11 | (5.4) | 7 | (3.5) | 4 | (2.0) | 0.042 * |
| Dysgeusia | 11 | (5.4) | 7 | (3.5) | 4 | (2.0) | 0.042 * |
| Parameter | Reference | Day 1 | Day 3 | Day 7 | Day 15 | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| With Obesity | Without Obesity | With Obesity | Without Obesity | With Obesity | Without Obesity | With Obesity | Without Obesity | ||||||||||||||||||
| Hematocrit, % | 35.0–47.0 | 37.2 | ± | 0.8 | 36.4 | ± | 0.6 | 36.0 | ± | 0.7 | 35.2 | ± | 0.6 | 34.7 | ± | 0.7 | 34.7 | ± | 0.8 | 32.0 | ± | 0.8 | 31.0 | ± | 0.9 |
| Hemoglobin, gr/dL | 14.0–17.5 | 12.6 | ± | 0.3 | 12.3 | ± | 0.2 | 12.0 | ± | 0.3 | 11.8 | ± | 0.2 | 11.4 | ± | 0.3 | 11.6 | ± | 0.3 | 10.5 | ± | 0.3 | 10.2 | ± | 0.3 |
| RBC, × 106 µL | 3.80–5.80 | 4.3 | ± | 0.1 | 4.2 | ± | 0.1 | 4.0 | ± | 0.1 | 4.0 | ± | 0.1 | 4.0 | ± | 0.1 | 3.9 | ± | 0.1 | 3.5 | ± | 0.1 | 3.4 | ± | 0.1 |
| WBC, × 103 µL | 4.00–12.00 | 8.7 | ± | 0.4 | 7.7 | ± | 0.3 | 9.4 | ± | 0.6 | 8.0 | ± | 0.4 | 10.4 | ± | 0.7 | 8.6 | ± | 0.4 | 9.9 | ± | 0.5 | 9.2 | ± | 0.6 |
| PLT, × 103 µL | 150–450 | 232.2 | ± | 10.2 | 219.3 | ± | 10.0 | 262.8 | ± | 13.3 | 261.0 | ± | 12.7 | 301.5 | ± | 14.2 | 299.3 | ± | 15.3 | 267.2 | ± | 20.4 | 287.1 | ± | 22.6 |
| MCV, fL | 82.0–95.0 | 88.1 | ± | 0.7 | 88.7 | ± | 0.5 | 88.9 | ± | 0.7 | 89.3 | ± | 0.5 | 89.9 | ± | 0.8 | 89.8 | ± | 0.5 | 90.9 | ± | 0.9 | 91.2 | ± | 0.8 |
| MCH, pg | 25.0–32.0 | 29.6 | ± | 0.3 | 29.9 | ± | 0.2 | 29.5 | ± | 0.2 | 29.8 | ± | 0.2 | 29.1 | ± | 0.4 | 29.8 | ± | 0.2 | 29.6 | ± | 0.4 | 29.9 | ± | 0.3 |
| MCHC, gr/dL | 32.0–36.0 | 33.5 | ± | 0.2 | 33.7 | ± | 0.1 | 33.2 | ± | 0.1 | 33.4 | ± | 0.1 | 32.7 | ± | 0.2 | 33.2 | ± | 0.1 | 32.6 | ± | 0.2 | 32.8 | ± | 0.2 |
| RDW, % | 11.0–16.0 | 14.0 | ± | 0.2 | 13.8 | ± | 0.1 | 13.9 | ± | 0.2 | 14.1 | ± | 0.2 | 14.2 | ± | 0.2 | 14.8 | ± | 0.5 | 14.8 | ± | 0.3 | 15.6 | ± | 0.8 |
| Lymphocytes, ×103 µL | 0.84–4.2 | 1.1 | ± | 0.1 | 1.2 | ± | 0.1 | 1.2 | ± | 0.2 * | 1.0 | ± | 0.1 * | 1.0 | ± | 0.1 | 1.2 | ± | 0.1 | 1.1 | ± | 0.1 | 1.2 | ± | 0.1 |
| Monocytes, ×103 µL | 0.16–0.96 | 0.6 | ± | 0.1 | 0.5 | ± | 0.0 | 0.6 | ± | 0.0 | 0.5 | ± | 0.0 | 0.7 | ± | 0.1 | 0.6 | ± | 0.0 | 0.6 | ± | 0.0 | 0.7 | ± | 0.0 |
| Neutrophils, ×103 µL | 2–8.2 | 6.9 | ± | 0.4 | 5.9 | ± | 0.3 | 7.6 | ± | 0.6 | 6.4 | ± | 0.4 | 8.5 | ± | 0.7 | 6.6 | ± | 0.4 | 7.9 | ± | 0.5 | 7.2 | ± | 0.6 |
| Eosinophils, × 103 µL | 0.08–0.6 | 0.03 | ± | 0.0 * | 0.01 | ± | 0.0 * | 0.1 | ± | 0.0 | 0.1 | ± | 0.0 | 0.1 | ± | 0.0 | 0.1 | ± | 0.0 | 0.2 | ± | 0.2 | 0.1 | ± | 0.2 |
| Basophils, ×103 µL | 0–0.12 | 0.0 | ± | 0.0 | 0.0 | ± | 0.0 | 0.0 | ± | 0.0 | 0.1 | ± | 0.1 | 0.0 | ± | 0.0 | 0.0 | ± | 0.0 | 0.0 | ± | 0.0 | 0.0 | ± | 0.0 |
| NLR | 0.107–3.19 | 8.6 | ± | 0.9 | 7.4 | ± | 0.7 | 11.7 | ± | 1.6 | 9.6 | ± | 1.3 | 12.3 | ± | 1.6 | 8.4 | ± | 1.2 | 10.9 | ± | 2.0 | 8.0 | ± | 0.9 |
| PLR | 46.79–218.01 | 277.7 | ± | 26.8 | 250.1 | ± | 16.4 | 355.8 | ± | 34.9 | 329.8 | ± | 21.6 | 385.3 | ± | 35.5 | 310.5 | ± | 23.9 | 326.8 | ± | 54.5 | 305.0 | ± | 31.8 |
| CRP (μg/L) | <5 | 125.2 | ± | 16.6 | 102.7 | ± | 8.2 | 86.3 | ± | 11.3 | 95.1 | ± | 9.6 | 51.7 | ± | 7.6 * | 61.1 | ± | 8.9 * | 45.2 | ± | 7.0 | 44.9 | ± | 7.9 |
| Prothrombine Time, % | 70–100 | 88.8 | ± | 2.2 | 84.6 | ± | 1.9 | 86.1 | ± | 2.4 | 81.2 | ± | 2.1 | 81.2 | ± | 2.5 | 83.8 | ± | 1.8 | 77.9 | ± | 3.0 | 79.8 | ± | 2.0 |
| APTT, sec | 26.3–40.3 | 33.2 | ± | 0.9 | 34.2 | ± | 0.7 | 36.4 | ± | 2.6 | 39.0 | ± | 4.0 | 32.9 | ± | 1.0 | 32.2 | ± | 0.8 | 37.0 | ± | 3.4 | 32.8 | ± | 1.2 |
| D-dimer, µg/mL | ≤0.50 | 2.1 | ± | 0.4 | 1.9 | ± | 0.2 | 4.2 | ± | 2.0 | 4.1 | ± | 1.4 | 7.7 | ± | 4.7 * | 2.3 | ± | 0.4 * | 2.0 | ± | 0.2 | 2.5 | ± | 0.4 |
| Ferritin (ng/L) | 30–400 | 1411.9 | ± | 150.1 | 1418.4 | ± | 150.3 | 1519.3 | ± | 196.2 | 1803.1 | ± | 201.0 | 1222.7 | ± | 223.1 | 1377.3 | ± | 175.1 | 1015.6 | ± | 198.8 | 1141.6 | ± | 153.6 |
| Parameter | Day 1 | Day 3 | Day 7 | Day 15 | ||||
|---|---|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | r | p-Value | |
| Hematocrit, % | 0.062 | 0.384 | 0.059 | 0.441 | −0.007 | 0.935 | 0.086 | 0.434 |
| Hemoglobin, gr/dL | 0.057 | 0.423 | 0.039 | 0.607 | −0.040 | 0.634 | 0.065 | 0.557 |
| RBC, ×106 μL | 0.053 | 0.451 | 0.056 | 0.463 | 0.047 | 0.571 | 0.101 | 0.361 |
| WBC, × 103 μL | 0.146 | 0.038 * | 0.155 | 0.042 * | 0.199 | 0.016 * | 0.104 | 0.348 |
| PLT, ×103 μL | 0.059 | 0.405 | 0.007 | 0.922 | 0.012 | 0.890 | −0.068 | 0.541 |
| MCV, fL | −0.046 | 0.518 | −0.036 | 0.634 | 0.007 | 0.937 | −0.036 | 0.747 |
| MCH, pg | −0.063 | 0.374 | −0.068 | 0.374 | −0.137 | 0.100 | −0.082 | 0.456 |
| MCHC, gr/dL | −0.078 | 0.268 | −0.093 | 0.222 | −0.168 | 0.043 * | −0.089 | 0.422 |
| RDW, % | 0.048 | 0.499 | −0.07 | 0.362 | −0.073 | 0.380 | −0.090 | 0.418 |
| Lymphocytes,× 103μL | −0.021 | 0.771 | 0.082 | 0.284 | −0.161 | 0.052 | −0.021 | 0.847 |
| Monocytes, × 103 μL | 0.111 | 0.117 | 0.081 | 0.288 | 0.075 | 0.367 | −0.026 | 0.814 |
| Neutrophils, × 103 μL | 0.151 | 0.032 * | 0.133 | 0.081 | 0.219 | 0.008 * | 0.115 | 0.297 |
| Eosinophils, × 103 μL | 0.081 | 0.251 | −0.050 | 0.514 | 0.014 | 0.867 | 0.118 | 0.286 |
| Basophils, × 103 μL | − | − | −0.057 | 0.459 | 0.073 | 0.379 | 0.076 | 0.492 |
| Prothrombine Time, % | 0.106 | 0.173 | 0.134 | 0.126 | −0.082 | 0.379 | −0.070 | 0.551 |
| NLR | 0.081 | 0.254 | 0.076 | 0.318 | 0.167 | 0.045 * | 0.160 | 0.147 |
| PLR | 0.066 | 0.353 | 0.051 | 0.504 | 0.153 | 0.064 | 0.045 | 0.686 |
| CRP (gmg/L) | 0.099 | 0.180 | −0.047 | 0.554 | −0.063 | 0.459 | 0.006 | 0.958 |
| APTT, sec | −0.072 | 0.358 | −0.045 | 0.615 | 0.052 | 0.581 | 0.147 | 0.213 |
| D-dimer, ug/mL | 0.044 | 0.618 | 0.005 | 0.952 | 0.129 | 0.181 | −0.130 | 0.328 |
| Ferritin (ng/L) | −0.003 | 0.976 | −0.086 | 0.337 | −0.046 | 0.627 | −0.032 | 0.806 |
| With Obesity | Without Obesity | p-Value | |
|---|---|---|---|
| Hospital length of stay, days X + DS | 27.32 + 19.09 | 22.56 + 22.62 | 0.011 |
| ICU admission, n (%) * | 49 (69.0) | 60 (45.8) | 0.001 |
| Invasive mechanical ventilation (n, %) ** | 42 (59.2) | 43 (32.8) | <0.001 |
| Death, n (%) | 11 (15.5) | 21 (16.0) | 0.920 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viscardi, S.; Marileo, L.; Delgado, H.; San Martín, A.; Hernández, L.; Garcés, P.; Guzmán-Oyarzo, D.; Boguen, R.; Medina, G.; Letelier, P.; et al. Obesity Is Associated with Changes in Laboratory Biomarkers in Chilean Patients Hospitalized with COVID-19. J. Clin. Med. 2023, 12, 3392. https://doi.org/10.3390/jcm12103392
Viscardi S, Marileo L, Delgado H, San Martín A, Hernández L, Garcés P, Guzmán-Oyarzo D, Boguen R, Medina G, Letelier P, et al. Obesity Is Associated with Changes in Laboratory Biomarkers in Chilean Patients Hospitalized with COVID-19. Journal of Clinical Medicine. 2023; 12(10):3392. https://doi.org/10.3390/jcm12103392
Chicago/Turabian StyleViscardi, Sharon, Luis Marileo, Hugo Delgado, Andrés San Martín, Loreto Hernández, Paola Garcés, Dina Guzmán-Oyarzo, Rodrigo Boguen, Gustavo Medina, Pablo Letelier, and et al. 2023. "Obesity Is Associated with Changes in Laboratory Biomarkers in Chilean Patients Hospitalized with COVID-19" Journal of Clinical Medicine 12, no. 10: 3392. https://doi.org/10.3390/jcm12103392
APA StyleViscardi, S., Marileo, L., Delgado, H., San Martín, A., Hernández, L., Garcés, P., Guzmán-Oyarzo, D., Boguen, R., Medina, G., Letelier, P., Villano, I., & Guzmán, N. (2023). Obesity Is Associated with Changes in Laboratory Biomarkers in Chilean Patients Hospitalized with COVID-19. Journal of Clinical Medicine, 12(10), 3392. https://doi.org/10.3390/jcm12103392





