Optical-Quality Assessment of a Miniaturized Intraocular Telescope
Abstract
1. Introduction
2. Materials and Methods
2.1. Transmission Spectra Measurement Setup
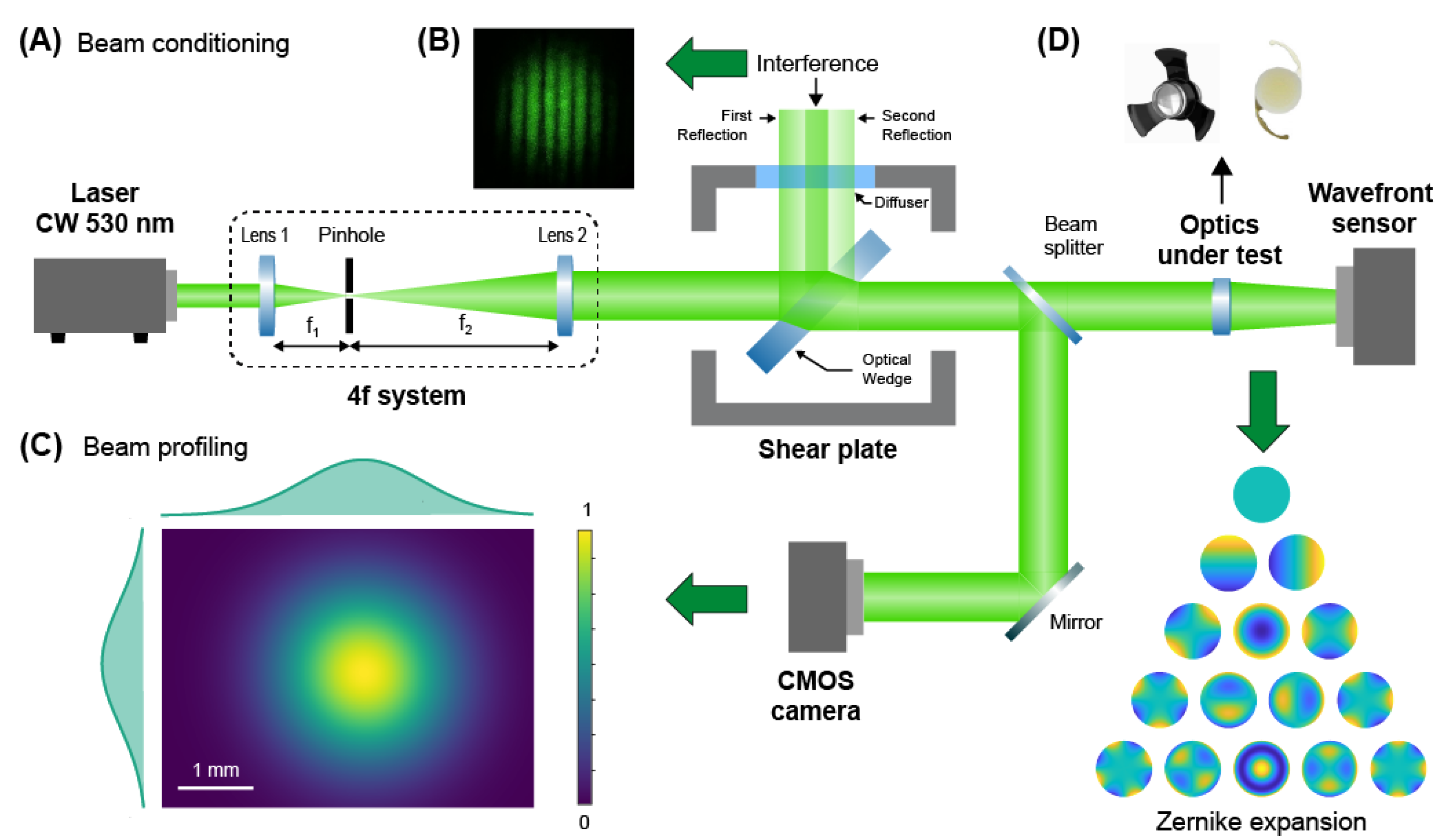
2.2. Geometrical Aberrations Measurement Setup
3. Results
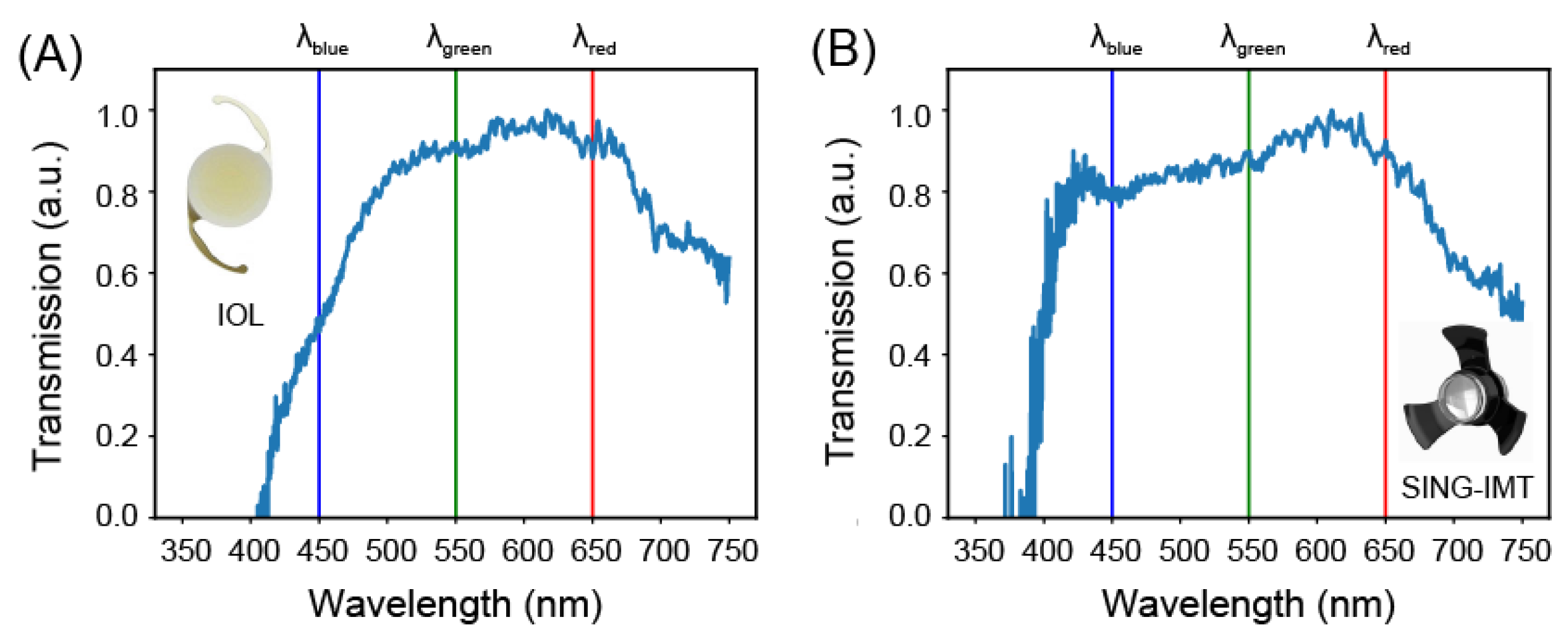
3.1. Transmission Properties
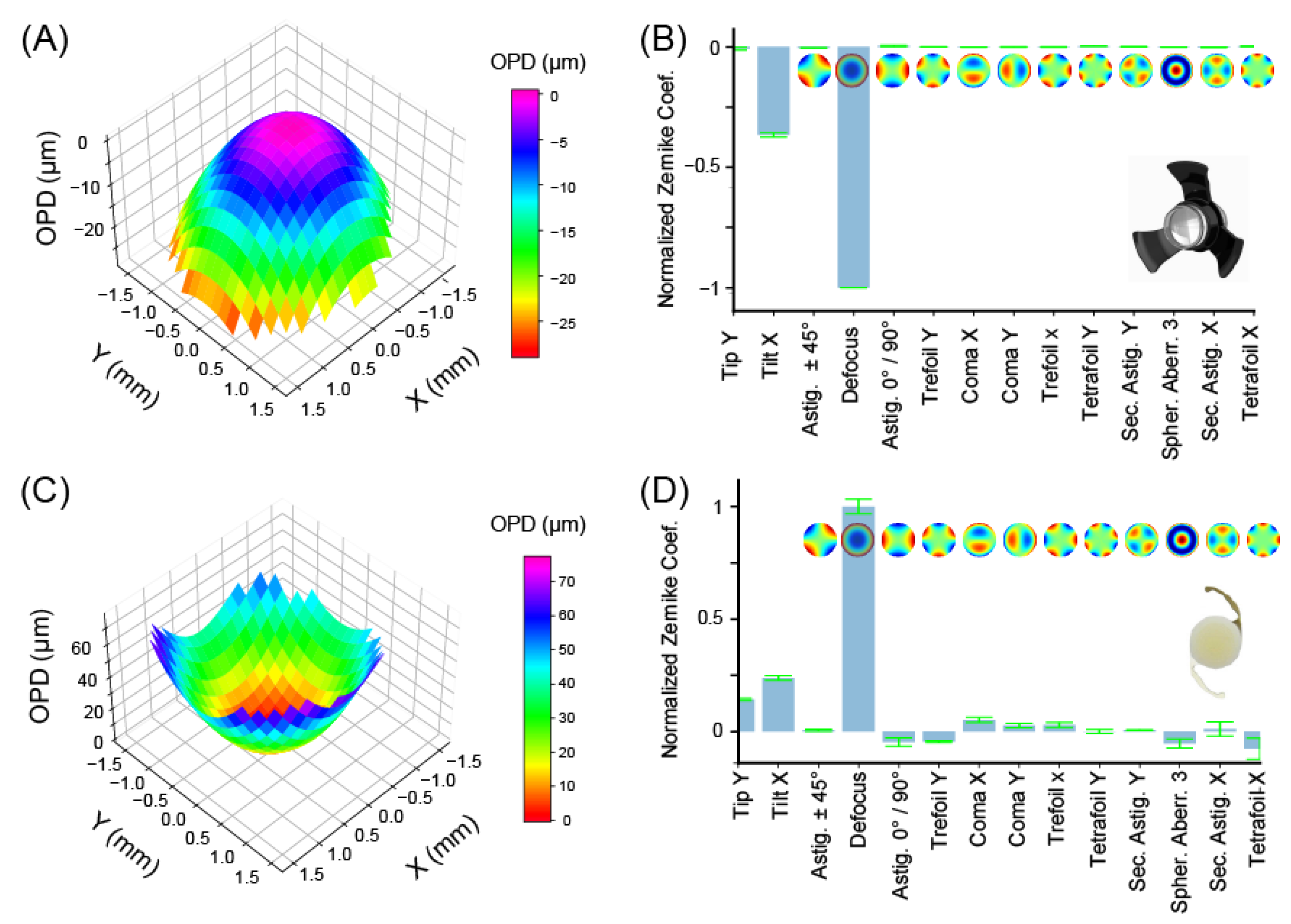
3.2. Geometrical Aberrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michalska-Małecka, K.; Kabiesz, A.; Nowak, M.; Śpiewak, D. Age related macular degeneration—Challenge for future: Pathogenesis and new perspectives for the treatment. Eur. Geriatr. Med. 2015, 6, 69–75. [Google Scholar] [CrossRef]
- Colijn, J.M.; Buitendijk, G.H.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P. Prevalence of Age-Related Macular Degeneration in Europe: The Past and the Future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Camacho, R.M.; Blanco-Llamero, C.; da Ana, R.; Fuertes, M.A.; Señoráns, F.J.; Silva, A.M. Therapeutic Approaches for Age-Related Macular Degeneration. Int. J. Mol. Sci. 2022, 23, 1769. [Google Scholar] [CrossRef] [PubMed]
- Stradiotto, E.; Allegrini, D.; Fossati, G.; Raimondi, R.; Sorrentino, T.; Tripepi, D. Genetic Aspects of Age-Related Macular Degeneration and Their Therapeutic Potential. Int. J. Mol. Sci. 2022, 23, 3280. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, R.; Zollet, P.; De Rosa, F.P.; Tsoutsanis, P.; Stravalaci, M.; Paulis, M. Where Are We with RPE Replacement Therapy? A Translational Review from the Ophthalmologist Perspective. Int. J. Mol. Sci. 2022, 23, 682. [Google Scholar] [CrossRef]
- Borkenstein, A.F.; Borkenstein, E.-M.; Augustin, A.J. Implantable vision-enhancing devices and postoperative rehabilitation in advanced age-related macular degeneration. Eye 2023, 37, 597–606. [Google Scholar] [CrossRef]
- Colby, K.A.; Chang, D.F.; Stulting, R.D.; Lane, S.S. Surgical placement of an optical prosthetic device for end-stage macular degeneration: The implantable miniature telescope. Arch. Ophthalmol. 2007, 125, 1118–1121. [Google Scholar] [CrossRef]
- Hudson, H.L.; Lane, S.S.; Heier, J.S.; Stulting, R.D.; Singerman, L.; Lichter, P.R. Implantable Miniature Telescope for the Treatment of Visual Acuity Loss Resulting from End-Stage Age-Related Macular Degeneration: 1-Year Results. Ophthalmology 2006, 113, 1987–2001. [Google Scholar] [CrossRef]
- Brown, G.C.; Brown, M.M.; Lieske, H.B.; Lieske, P.A.; Brown, K.S.; Lane, S.S. Comparative Effectiveness and Cost-Effectiveness of the Implantable Miniature Telescope. Ophthalmology 2011, 118, 1834–1843. [Google Scholar] [CrossRef]
- Primo, S.A. Implantable miniature telescope: Lessons learned. Optom. J. Am. Optom. Assoc. 2010, 81, 86–93. [Google Scholar] [CrossRef]
- Singer, M.A.; del Cid, M.R.; Stelton, C.R.; Boord, T. Pars Plana Posterior Capsulotomy in a Patient With a Telescope Prosthesis for Age-Related Macular Degeneration. Arch. Ophthalmol. 2010, 128, 1065–1067. [Google Scholar] [CrossRef]
- Carpignano, F.; Surdo, S.; Barillaro, G.; Merlo, S. Silicon Micromachined Device Testing by Infrared Low-Coherence Reflectometry. J. Microelectromech. Syst. 2015, 24, 1960–1964. [Google Scholar] [CrossRef]
- Surdo, S.; Carpignano, F.; Silva, G.; Merlo, S.; Barillaro, G. An all-silicon optical platform based on linear array of vertical high-aspect-ratio silicon/air photonic crystals. Appl. Phys. Lett. 2013, 103, 171103. [Google Scholar] [CrossRef]
- Williams, C.S.; Becklund, O.A. Introduction to the Optical Transfer Function; SPIE Press: Bellingham, WA, USA, 2002. [Google Scholar]
- Goodman, J.W. Introduction to Fourier Optics; McGraw-Hill: New York, NY, USA, 1996. [Google Scholar]
- Porter, J.; Guirao, A.; Cox, I.G.; Williams, D.R. Monochromatic aberrations of the human eye in a large population. J. Opt. Soc. Am. A 2001, 18, 1793–1803. [Google Scholar] [CrossRef]
- Thibos, L.N.; Hong, X.; Bradley, A.; Cheng, X. Statistical variation of aberration structure and image quality in a normal population of healthy eyes. J. Opt. Soc. Am. A 2002, 19, 2329–2348. [Google Scholar] [CrossRef]
- Hernández-Delgado, J.; Malacara-Hernández, Z.; Malacara-Doblado, D.; Vázquez-Dorrío, B.; Hernández, D.M. Local curvatures and its measurements of an optical surface or a wavefront: A review. Opt. Eng. 2022, 61, 50901. [Google Scholar] [CrossRef]
- Surdo, S.; Carzino, R.; Diaspro, A.; Duocastella, M. Single-Shot Laser Additive Manufacturing of High Fill-Factor Microlens Arrays. Adv. Opt. Mater. 2018, 6, 1701190. [Google Scholar] [CrossRef]
- McAlinden, C.; McCartney, M.; Moore, J. Mathematics of Zernike polynomials: A review. Clin. Exp. Ophthalmol. 2011, 39, 820–827. [Google Scholar] [CrossRef]
- Fornasini, P. Uncertainty in Indirect Measurements. In The Uncertainty in Physical Measurements: An Introduction to Data Analysis in the Physics Laboratory; Springer: New York, NY, USA, 2008; pp. 149–162. [Google Scholar] [CrossRef]
- Savastano, A.; Caporossi, T.; Sasso, P.; De Vico, U.; Rizzo, S. A New Intraocular Telescopic Device for Age-Related Macular Degeneration. Ophthalmol. Retin. 2022, 6, 971–972. [Google Scholar] [CrossRef]
- Hudson, H.L.; Stulting, R.D.; Heier, J.S.; Lane, S.S.; Chang, D.F.; Singerman, L.J. Implantable Telescope for End-Stage Age-related Macular Degeneration: Long-term Visual Acuity and Safety Outcomes. Am. J. Ophthalmol. 2008, 146, 664–673.e1. [Google Scholar] [CrossRef]
- Orzalesi, N.; Pierrottet, C.O.; Zenoni, S.; Savaresi, C. The IOL-Vip System: A Double Intraocular Lens Implant for Visual Rehabilitation of Patients with Macular Disease. Ophthalmology 2007, 114, 860–865.e1. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.A.; Robbie, S.J.; Tabernero, J.; Artal, P. Injectable intraocular telescope: Pilot study. J. Cataract. Refract. Surg. 2015, 41, 2125–2135. [Google Scholar] [CrossRef] [PubMed]
- Boyer, D.; Freund, K.B.; Regillo, C.; Levy, M.H.; Garg, S. Long-term (60-month) results for the implantable miniature telescope: Efficacy and safety outcomes stratified by age in patients with end-stage age-related macular degeneration. Clin. Ophthalmol. 2015, 9, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Toro, M.D.; Vidal-Aroca, F.; Montemagni, M.; Xompero, C.; Fioretto, G.; Costagliola, C. Three-Month Safety and Efficacy Outcomes for the Smaller-Incision New-Generation Implantable Miniature Telescope (SING IMT™). J. Clin. Med. 2013, 12, 518. [Google Scholar] [CrossRef]
- Peli, E.; Lipshitz, I.; Dotan, G. Implantable Miniaturized Telescope (IMT) for low vision. In Vision Rehabilitation: Assessment, Intervention and Outcomes. Selected Papers from Vision ’99: International Conference on Low Vision; Stuen, C., Ed.; Swets & Zeitlinger: Lisse, The Netherlands, 2000; pp. 200–203. [Google Scholar]
- Zhang, X.; Qiu, J.; Li, X.; Zhao, J.; Liu, L. Complex refractive indices measurements of polymers in visible and near-infrared bands. Appl. Opt. 2020, 59, 2337–2344. [Google Scholar] [CrossRef]
- Stöhr, M.; Roth, K.; Jähne, B. Measurement of 3D pore-scale flow in index-matched porous media. Exp. Fluids 2003, 35, 159–166. [Google Scholar] [CrossRef]
- Surdo, S.; Diaspro, A.; Duocastella, M. Geometry-controllable micro-optics with laser catapulting. Opt. Mater. Express 2019, 9, 2892–2901. [Google Scholar] [CrossRef]
- Malitson, I.H. Interspecimen Comparison of the Refractive Index of Fused Silica. J. Opt. Soc. Am. 1965, 55, 1205–1209. [Google Scholar] [CrossRef]
- Philipp, H.R. Silicon Dioxide (SiO2) (Glass). In Handbook of Optical Constants of Solids; Palik, E.D., Ed.; Academic Press: Burlington, VT, USA, 1997; pp. 749–763. [Google Scholar] [CrossRef]
- Rodney, W.S.; Spindler, R.J. Index of Refraction of Fused Quartz Glass for Ultraviolet, Visible, and Infrared Wavelengths. J. Opt. Soc. Am. 1954, 44, 677–679. [Google Scholar] [CrossRef]
- Hernandez, V.M.; Cabot, F.; Ruggeri, M.; de Freitas, C.; Ho, A.; Yoo, S. Calculation of crystalline lens power using a modification of the Bennett method. Biomed. Opt. Express 2015, 6, 4501–4515. [Google Scholar] [CrossRef]
- Lane, S.S.; Burgi, P.; Milios, G.S.; Orchowski, M.W.; Vaughan, M.; Schwarte, E. Comparison of the biomechanical behavior of foldable intraocular lenses. J. Cataract. Refract. Surg. 2004, 30, 2397–2402. [Google Scholar] [CrossRef]
- Lane, S.; Collins, S.; Das, K.K.; Maass, S.; Thatthamla, I.; Schatz, H. Evaluation of intraocular lens mechanical stability. J. Cataract. Refract. Surg. 2019, 45, 501–506. [Google Scholar] [CrossRef]
- Guthoff, R.; Abramo, F.; Draeger, J.; Chumbley, L. Measurement of elastic resisting forces of intraocular haptic loops of varying geometrical designs and material composition. J. Cataract. Refract. Surg. 1990, 16, 551–558. [Google Scholar] [CrossRef]
- ISO standard No. 11979-2:2014; Ophthalmic Implants—Intraocular Lenses—Part 2: Optical Properties and Test Methods. International Organization for Standardization, 2014. Available online: https://www.iso.org/obp/ui/#iso:std:55682:en (accessed on 20 April 2023).
- Lee, J.-S.; Li, P.-R.; Hou, C.-H.; Lin, K.-K.; Kuo, C.-F.; See, L.-C. Effect of Blue Light-Filtering Intraocular Lenses on Age-Related Macular Degeneration: A Nationwide Cohort Study With 10-Year Follow-up. Am. J. Ophthalmol. 2022, 234, 138–146. [Google Scholar] [CrossRef]
- Luo, C.; Wang, H.; Chen, X.; Xu, J.; Yin, H.; Yao, K. Recent Advances of Intraocular Lens Materials and Surface Modification in Cataract Surgery. Front. Bioeng. Biotechnol. 2022, 10, 913383. [Google Scholar] [CrossRef]
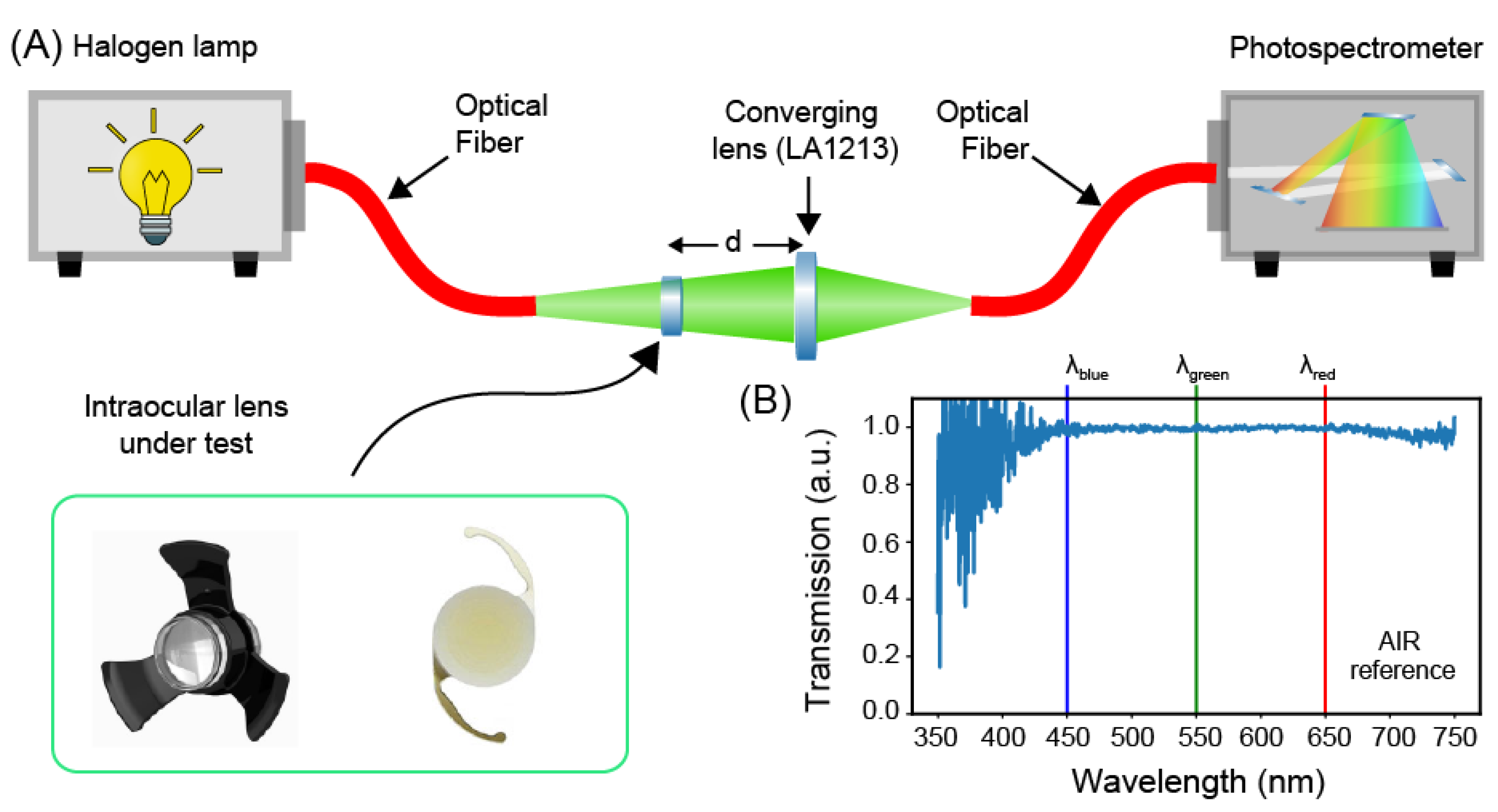
| Bandwidth (Inf-Sup) (nm) | Flatness (rms) | Extinction Ratio (Blue to Green) | Extinction Ratio (Blue to Red) | |
|---|---|---|---|---|
| AIR | 350–750 | 0.08 | 0.98 ± 0.11 | 0.98 ± 0.11 |
| SY60WF IOL | 453.41–749.75 | 0.13 | 0.45 ± 0.17 | 0.45 ± 0.17 |
| SING IMT™ | 397.08–749.75 | 0.12 | 0.94 ± 0.25 | 0.91 ± 0.25 |
| Aberrations | SING-IMT (µm) | SY60WF-IOL (µm) | ||
|---|---|---|---|---|
| Mean | Std | Mean | Std | |
| Tip Y | −0.057 | 0.014 | 2.727 | 0.031 |
| Tip X | −2.652 | 0.020 | 4.529 | 0.071 |
| Astigmatism ± 45° | −0.034 | 0.002 | 0.115 | 0.026 |
| Defocus | −7.247 | 0.002 | 18.988 | 0.206 |
| Astigmatism 0°/90° | −0.028 | 0.007 | −0.878 | 0.119 |
| Trefoil Y | 0.005 | 0.002 | −0.849 | 0.028 |
| Coma X | −0.007 | 0.002 | 0.992 | 0.073 |
| Coma Y | −0.004 | 0.004 | 0.514 | 0.065 |
| Trefoil X | −0.009 | 0.004 | 0.559 | 0.069 |
| Tetrafoil Y | 0.025 | 0.001 | −0.005 | 0.056 |
| Sec. Astig. Y | 0.003 | 0.004 | 0.115 | 0.014 |
| Spher. Aberr. 3 | −0.011 | 0.001 | −1.005 | 0.130 |
| Sec. Astig. X | −0.016 | 0.002 | 0.213 | 0.200 |
| Tetrafoil X | 0.017 | 0.004 | −1.435 | 0.295 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nepita, I.; Raimondi, R.; Piazza, S.; Diaspro, A.; Vidal-Aroca, F.; Surdo, S.; Romano, M.R. Optical-Quality Assessment of a Miniaturized Intraocular Telescope. J. Clin. Med. 2023, 12, 3375. https://doi.org/10.3390/jcm12103375
Nepita I, Raimondi R, Piazza S, Diaspro A, Vidal-Aroca F, Surdo S, Romano MR. Optical-Quality Assessment of a Miniaturized Intraocular Telescope. Journal of Clinical Medicine. 2023; 12(10):3375. https://doi.org/10.3390/jcm12103375
Chicago/Turabian StyleNepita, Irene, Raffaele Raimondi, Simonluca Piazza, Alberto Diaspro, Faustino Vidal-Aroca, Salvatore Surdo, and Mario R. Romano. 2023. "Optical-Quality Assessment of a Miniaturized Intraocular Telescope" Journal of Clinical Medicine 12, no. 10: 3375. https://doi.org/10.3390/jcm12103375
APA StyleNepita, I., Raimondi, R., Piazza, S., Diaspro, A., Vidal-Aroca, F., Surdo, S., & Romano, M. R. (2023). Optical-Quality Assessment of a Miniaturized Intraocular Telescope. Journal of Clinical Medicine, 12(10), 3375. https://doi.org/10.3390/jcm12103375







