IgG and IgE Autoantibodies to IgE Receptors in Chronic Spontaneous Urticaria and Their Role in the Response to Omalizumab
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zuberbier, T.; Abdul Latiff, A.H.; Abuzakouk, M.; Aquilina, S.; Asero, R.; Baker, D.; Ballmer-Weber, B.; Bangert, C.; Ben-Shoshan, M.; Bangert, C.; et al. The international EAACI/GA2LEN/EuroGuiDerm/APAAACI guideline for the definition, classification, diagnosis, and management of urticaria. Allergy 2022, 77, 734–766. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Boccon-Gibod, I.; Gonçalo, M.; Serhat İnalöz, H.; Knulst, A.; Lapeere, H.; Parthasaradhi, A.; Stingl, G.; Tagka, A.; Valenzuela, F.; et al. Expert opinion: Defining response to omalizumab in patients with chronic spontaneous urticaria. Eur. J. Dermatol. 2017, 27, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Fok, J.S.; Kolkhir, P.; Church, M.K.; Maurer, M. Predictors of treatment response in chronic spontaneous urticaria. Allergy 2021, 76, 2965–2981. [Google Scholar] [CrossRef] [PubMed]
- Schmetzer, O.; Lakin, E.; Topal, F.A.; Preusse, P.; Freier, D.; Church, M.K.; Maurer, M. IL-24 is a common and specific autoantigen of IgE in patients with chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2018, 142, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Cugno, M.; Asero, R.; Ferrucci, S.; Lorini, M.; Carbonelli, V.; Tedeschi, A.; Marzano, A.V. Elevated IgE to tissue factor and thyroglobulin are abated by omalizumab in chronic spontaneous urticaria. Allergy 2018, 73, 2408–2411. [Google Scholar] [CrossRef] [PubMed]
- Asero, R.; Marzano, A.V.; Ferrucci, S.; Lorini, M.; Carbonelli, V.; Cugno, M. Co-occurrence of IgE and IgG autoantibodies in patients with chronic spontaneous urticaria. Clin. Exp. Immunol. 2020, 200, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Kolkhir, P.; Muñoz, M.; Asero, R.; Ferrer, M.; Kocatürk, E.; Metz, M.; Xiang, Y.K.; Maurer, M. Autoimmune chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2022, 149, 1819–1831. [Google Scholar] [CrossRef] [PubMed]
- Cugno, M.; Mancuso, M.E.; Tedeschi, A.; Santagostino, E.; Lorini, M.; Carbonelli, V.; Peyvandi, F.; Mannucci, P.M. Involvement of the IgE-basophil system and mild complement activation in haemophilia B with anti-factor IX neutralizing antibodies and anaphylaxis. Haemophilia 2017, 23, e348–e353. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Arnau, A.M.; DeMontojoye, L.; Asero, R.; Cugno, M.; Kulthanan, K.; Yanase, Y.; Hide, M.; Kaplan, A.P. The Pathogenesis of Chronic Spontaneous Urticaria: The Role of Infiltrating Cells. J. Allergy Clin. Immunol. Pract. 2021, 9, 2195–2208. [Google Scholar] [CrossRef] [PubMed]
- MacGlashan, D., Jr.; Saini, S.; Schroeder, J.T. Response of peripheral blood basophils in subjects with chronic spontaneous urticaria during treatment with omalizumab. J. Allergy Clin. Immunol. 2021, 147, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
| CSU Patients (n = 18) | Healthy Non-Atopic Controls (n = 18) | p-Value | |
|---|---|---|---|
| Gender | |||
| Male, n (%) | 6 (33.3%) | 8 (44.4%) | - |
| Female, n (%) | 12 (66.7%) | 10 (55.6%) | - |
| Age (years), median (IQR) | 52 (32.75–60.25) | 49 (34–58) | - |
| Time from onset to omalizumab (months), median (IQR) | 14.5 (8.25–63) | - | |
| Angioedema, n (%) | 8 (44.4%) | - | - |
| Response to omalizumab | |||
| Early response, n (%) | 8 (44.4%) | - | - |
| Late-response, n (%) | 8 (44.4%) | - | - |
| Non-response, n (%) | 2 (11.2%) | - | - |
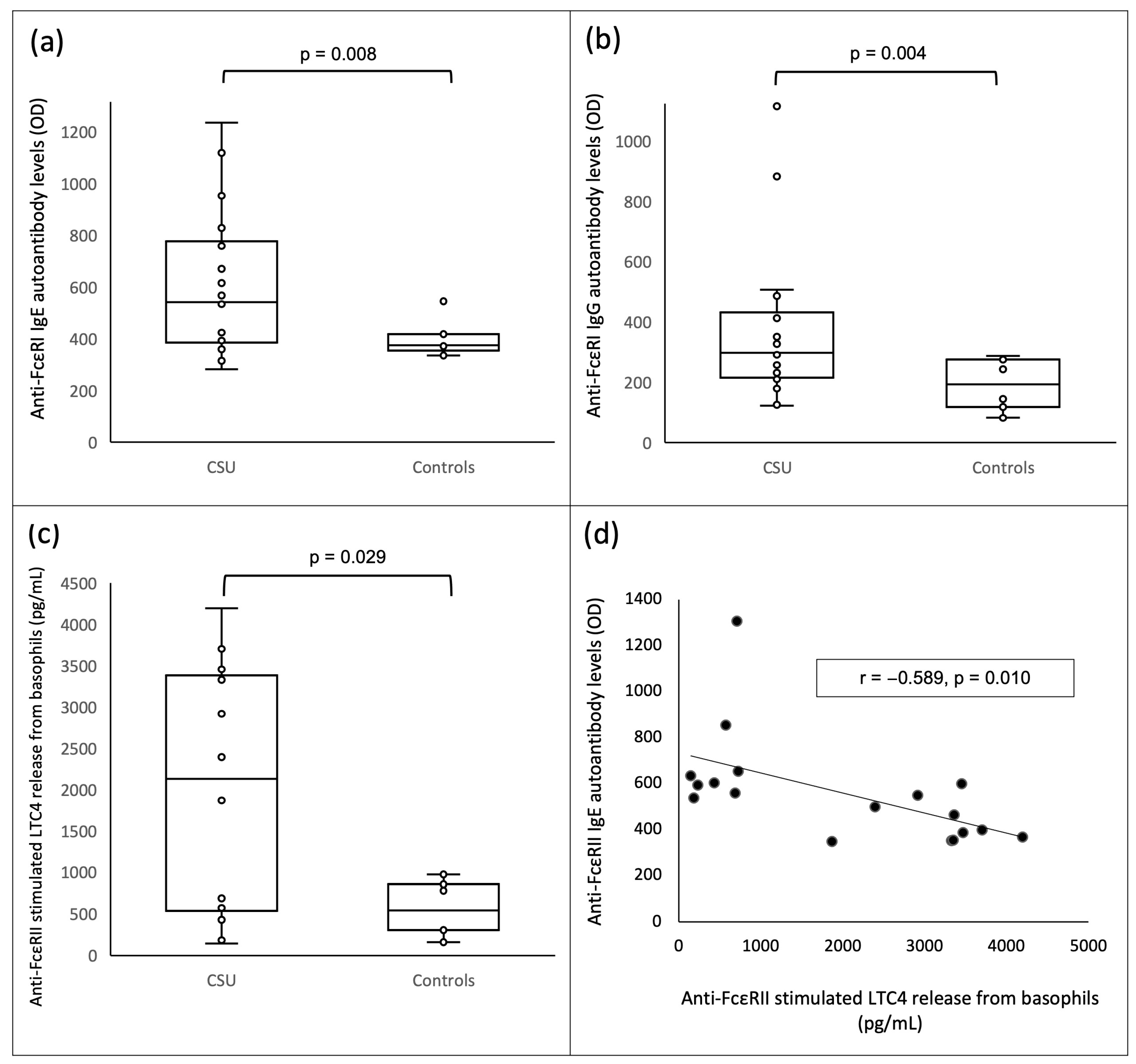
| anti-FcεRI IgE autoantibody levels (OD), median (IQR) | 542 (386.25–776.5) | 375 (355–418) | 0.008 |
| anti-FcεRII IgE autoantibody levels (OD), median (IQR) | 544 (383–610.75) | 505.5 (431–676) | 0.775 |
| anti-FcεRI IgG autoantibody levels (OD), median (IQR) | 297 (214.5–431.25) | 193.5 (118–275) | 0.004 |
| anti-FcεRII IgG autoantibody levels (OD), median (IQR) | 314.5 (191–506.5) | 346 (257–463) | 0.775 |
| Spontaneous LTC4 release (pg/mL), median (IQR) | 70.5 (38.2–129.4) | 89.1 (71.2–110.3) | 0.296 |
| anti-IgE stimulated LTC4 release (pg/mL), median (IQR) | 724.1 (418.9–1104.5) | 436.1 (295.6–1287.2) | 0.587 |
| anti-FcεRI stimulated LTC4 release (pg/mL), median (IQR) | 746.35 (245.025–1072.075) | 357.85 (223.4–793.7) | 0.183 |
| anti-FcεRII stimulated LTC4 release (pg/mL), median (IQR) | 2130.15 (535.325–3383.425) | 543.6 (304.5–854.5) | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maronese, C.A.; Ferrucci, S.M.; Moltrasio, C.; Lorini, M.; Carbonelli, V.; Asero, R.; Marzano, A.V.; Cugno, M. IgG and IgE Autoantibodies to IgE Receptors in Chronic Spontaneous Urticaria and Their Role in the Response to Omalizumab. J. Clin. Med. 2023, 12, 378. https://doi.org/10.3390/jcm12010378
Maronese CA, Ferrucci SM, Moltrasio C, Lorini M, Carbonelli V, Asero R, Marzano AV, Cugno M. IgG and IgE Autoantibodies to IgE Receptors in Chronic Spontaneous Urticaria and Their Role in the Response to Omalizumab. Journal of Clinical Medicine. 2023; 12(1):378. https://doi.org/10.3390/jcm12010378
Chicago/Turabian StyleMaronese, Carlo Alberto, Silvia Mariel Ferrucci, Chiara Moltrasio, Maurizio Lorini, Vincenzo Carbonelli, Riccardo Asero, Angelo Valerio Marzano, and Massimo Cugno. 2023. "IgG and IgE Autoantibodies to IgE Receptors in Chronic Spontaneous Urticaria and Their Role in the Response to Omalizumab" Journal of Clinical Medicine 12, no. 1: 378. https://doi.org/10.3390/jcm12010378
APA StyleMaronese, C. A., Ferrucci, S. M., Moltrasio, C., Lorini, M., Carbonelli, V., Asero, R., Marzano, A. V., & Cugno, M. (2023). IgG and IgE Autoantibodies to IgE Receptors in Chronic Spontaneous Urticaria and Their Role in the Response to Omalizumab. Journal of Clinical Medicine, 12(1), 378. https://doi.org/10.3390/jcm12010378









