Incidence and Associations of Acute Kidney Injury after General Thoracic Surgery: A System Review and Meta-Analysis
Abstract
1. Introduction
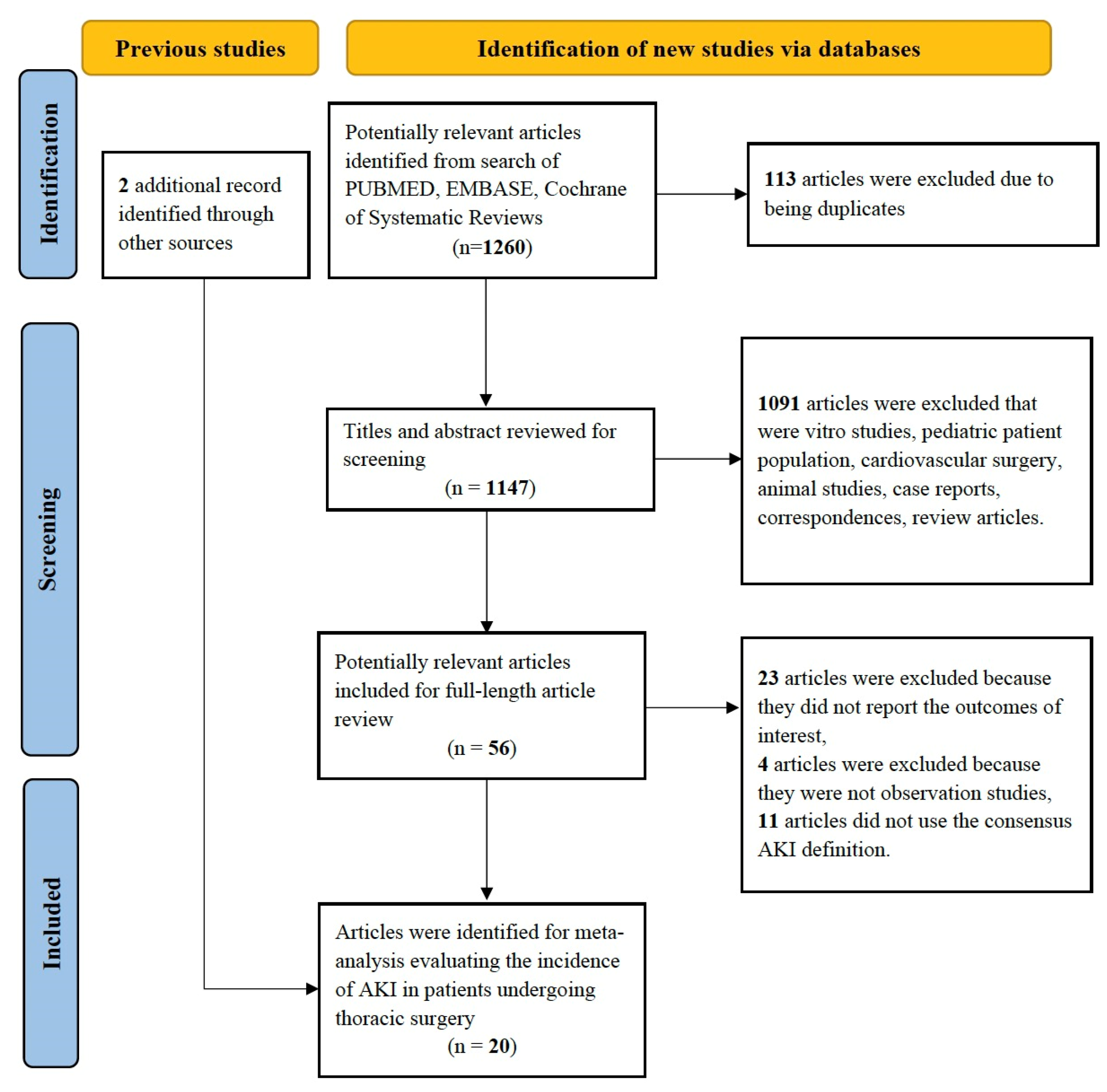
2. Materials and Methods
2.1. Study Selection
2.2. Search Strategy
2.3. Data Abstraction
2.4. Study Quality
2.5. Statistical Analysis
3. Results
3.1. Incidence of AKI among Patients after Thoracic Surgery
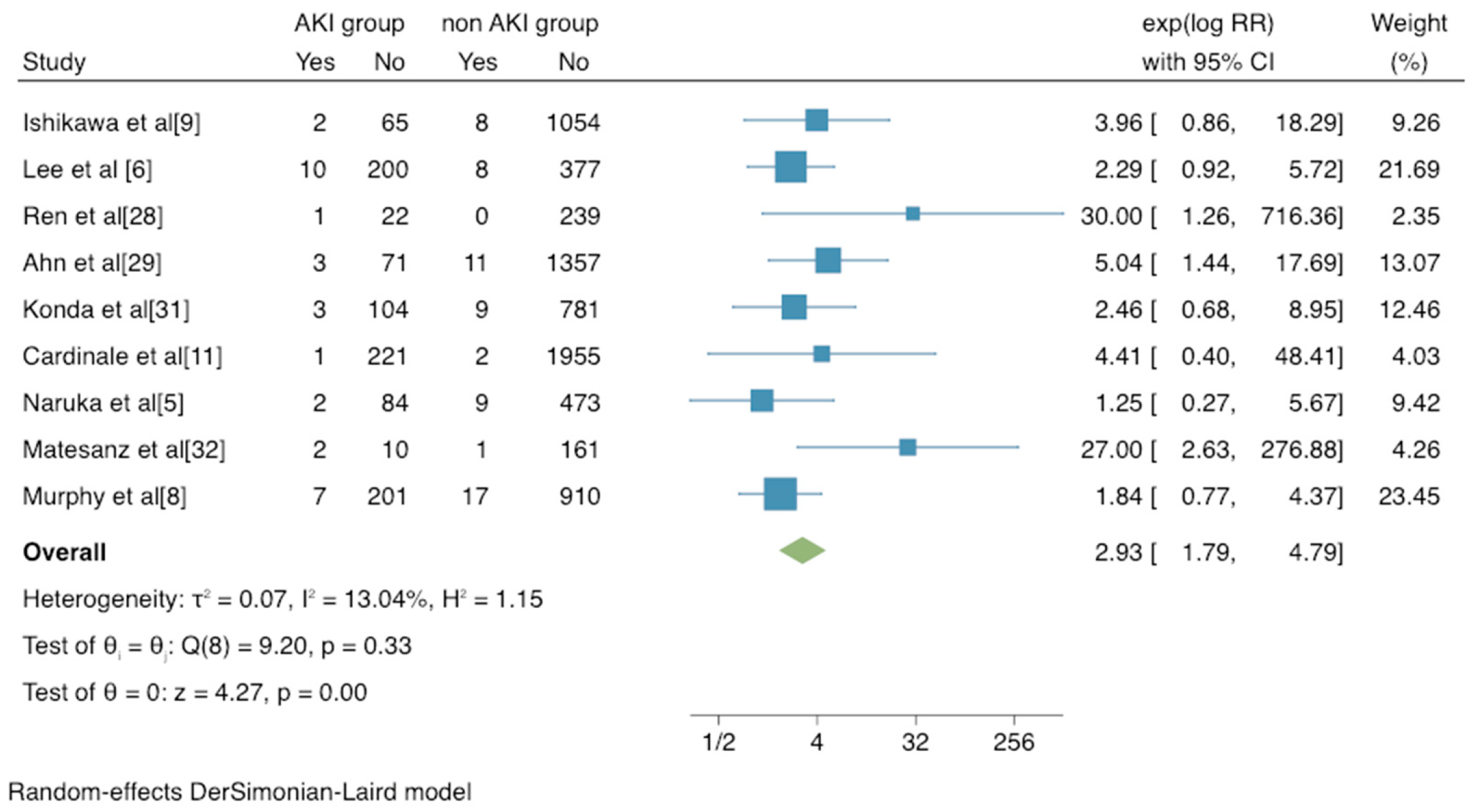
3.2. Mortality and Complications Risk of AKI in Patients after Thoracic Surgery
3.3. Evaluation for Publication Bias and Sensitivity Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nooreldeen, R.; Bach, H. Current and Future Development in Lung Cancer Diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, K.B.; Stein, L.; Bogatyreva, L.; Ulbrich, F.; Kaifi, J.T.; Hauschke, D.; Loop, T.; Goebel, U. Oesophageal Doppler guided goal-directed haemodynamic therapy in thoracic surgery—A single centre randomized parallel-arm trial. Br. J. Anaesth. 2017, 118, 852–861. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saftic, I.; Bille, A.; Asemota, N.; Berjon de la Vega, L.; Routledge, T.; King, J.; Phipps, K.H.; Pilling, J. Risks and rewards of the surgical treatment of lung cancer in octogenarians. Interact. Cardiovasc. Thorac. Surg. 2021, 33, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Naruka, V.; McKie, M.A.; Khushiwal, R.; Clayton, J.; Aresu, G.; Peryt, A.; Villar, S.S.; MacKay, J.; Coonar, A.S. Acute kidney injury after thoracic surgery: A proposal for a multicentre evaluation (MERITS). Interact. Cardiovasc. Thorac. Surg. 2019, 29, 861–866. [Google Scholar] [CrossRef]
- Lee, E.H.; Kim, H.R.; Baek, S.H.; Kim, K.M.; Chin, J.H.; Choi, D.K.; Kim, W.J.; Choi, I.C. Risk factors of postoperative acute kidney injury in patients undergoing esophageal cancer surgery. J. Cardiothorac. Vasc. Anesth. 2014, 28, 936–942. [Google Scholar] [CrossRef]
- Wu, Y.; Zhou, Y.; Gao, S.; Du, C.; Yao, L.; Yang, R. Effects of preoperative pulmonary function on short-term outcomes and overall survival after video-assisted thoracic surgery lobectomy. Ann. Transl. Med. 2021, 9, 1651. [Google Scholar] [CrossRef]
- Murphy, C.F.; Dunne, T.; Elliott, J.A.; Kamarajah, S.K.; Leighton, J.; Evans, R.P.T.; Bundred, J.; King, S.; Ravi, N.; Donohoe, C.L.; et al. Acute Kidney Injury After Esophageal Cancer Surgery: Incidence, Risk Factors, and Impact on Oncologic Outcomes. Ann. Surg. 2022, 275, e683–e689. [Google Scholar] [CrossRef]
- Ishikawa, S.; Griesdale, D.E.; Lohser, J. Acute kidney injury after lung resection surgery: Incidence and perioperative risk factors. Anesth. Analg. 2012, 114, 1256–1262. [Google Scholar] [CrossRef]
- Wang, W.; Wang, T.; Feng, X.; Sun, L. Incidence and risk factors of acute kidney injury after esophageal cancer surgery: A nested case-control study. Int. J. Surg. 2017, 39, 11–15. [Google Scholar] [CrossRef]
- Cardinale, D.; Cosentino, N.; Moltrasio, M.; Sandri, M.T.; Petrella, F.; Colombo, A.; Bacchiani, G.; Tessitore, A.; Bonomi, A.; Veglia, F.; et al. Acute kidney injury after lung cancer surgery: Incidence and clinical relevance, predictors, and role of N-terminal pro B-type natriuretic peptide. Lung Cancer. 2018, 123, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Assaad, S.; Kyriakides, T.; Tellides, G.; Kim, A.W.; Perkal, M.; Perrino, A. Extravascular Lung Water and Tissue Perfusion Biomarkers After Lung Resection Surgery Under a Normovolemic Fluid Protocol. J. Cardiothorac. Vasc. Anesth. 2015, 29, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, W.; Gao, M.; Gu, C.; Yu, Y. Preoperative Prophylactic Intraaortic Balloon Pump Reduces the Incidence of Postoperative Acute Kidney Injury and Short-Term Death of High-Risk Patients Undergoing Coronary Artery Bypass Grafting: A Meta-Analysis of 17 Studies. Ann. Thorac. Surg. 2016, 101, 2007–2019. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Lertjitbanjong, P.; Cheungpasitporn, W.; Hansrivijit, P.; Fulop, T.; Kovvuru, K.; Kanduri, S.R.; Davis, P.W.; Vallabhajosyula, S.; Bathini, T.; et al. Incidence and impact of acute kidney injury on patients with implantable left ventricular assist devices: A Meta-analysis. Ren. Fail. 2020, 42, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Tinica, G.; Brinza, C.; Covic, A.; Popa, I.V.; Tarus, A.; Bacusca, A.E.; Burlacu, A. Determinants of acute kidney injury after cardiac surgery: A systematic review. Rev. Cardiovasc. Med. 2020, 21, 601–610. [Google Scholar] [PubMed]
- Fernandes, M.; Majoni, M.; Garg, A.X.; Dubois, L. Systematic Review and Meta-Analysis of Preventative Strategies for Acute Kidney Injury in Patients Undergoing Abdominal Aortic Aneurysm Repair. Ann. Vasc. Surg. 2021, 74, 419–430. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.E.; Kirwan, C.J.; Pearse, R.M.; Prowle, J.R. Incidence and associations of acute kidney injury after major abdominal surgery. Intensive Care Med. 2016, 42, 521–530. [Google Scholar] [CrossRef]
- Hu, J.; Chen, R.; Liu, S.; Yu, X.; Zou, J.; Ding, X. Global Incidence and Outcomes of Adult Patients With Acute Kidney Injury After Cardiac Surgery: A Systematic Review and Meta-Analysis. J. Cardiothorac. Vasc. Anesth. 2016, 30, 82–89. [Google Scholar] [CrossRef]
- Grams, M.E.; Sang, Y.; Coresh, J.; Ballew, S.; Matsushita, K.; Molnar, M.Z.; Szabo, Z.; Kalantar-Zadeh, K.; Kovesdy, C.P. Acute Kidney Injury After Major Surgery: A Retrospective Analysis of Veterans Health Administration Data. Am. J. Kidney Dis. 2016, 67, 872–880. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care 2004, 8, R204–R212. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A.; Acute Kidney Injury, N. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, R31. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Licker, M.; Cartier, V.; Robert, J.; Diaper, J.; Villiger, Y.; Tschopp, J.M.; Inan, C. Risk factors of acute kidney injury according to RIFLE criteria after lung cancer surgery. Ann. Thorac. Surg. 2011, 91, 844–850. [Google Scholar] [CrossRef]
- Ren, H.; Meng, L. Acute kidney injury treatment for elderly patients after esophageal cancer operation. Zhonghua Yi Xue Za Zhi 2015, 95, 2000–2002. [Google Scholar]
- Ahn, H.J.; Kim, J.A.; Lee, A.R.; Yang, M.; Jung, H.J.; Heo, B. The Risk of Acute Kidney Injury from Fluid Restriction and Hydroxyethyl Starch in Thoracic Surgery. Anesth. Analg. 2016, 122, 186–193. [Google Scholar] [CrossRef]
- Moon, T.; Tsai, J.Y.; Vachhani, S.; Peng, S.P.; Feng, L.; Vaporciyan, A.A.; Cata, J.P. The Use of Intraoperative Dexmedetomidine Is Not Associated With a Reduction in Acute Kidney Injury After Lung Cancer Surgery. J. Cardiothorac. Vasc. Anesth. 2016, 30, 51–55. [Google Scholar] [CrossRef]
- Konda, P.; Ai, D.; Guerra, C.E.; Rodriguez-Restrepo, A.; Mehran, R.J.; Rice, D.; Hofstetter, W.; Heir, J.; Kwater, P.; Gottumukkala, V.; et al. Identification of Risk Factors Associated With Postoperative Acute Kidney Injury After Esophagectomy for Esophageal Cancer. J. Cardiothorac. Vasc. Anesth. 2017, 31, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Monteserín Matesanz, C.; de la Gala, F.; Rancan, L.; Piñeiro, P.; Simón, C.; Tejedor, A.; Vara, E.; Gonzalez-Cantero, J.L.; Garutti, I. Predictive value of plasma cytokines for acute kidney injury following lung resection surgery: Prospective observational study. Braz. J. Anesthesiol. 2019, 69, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.K.; Kim, J.; Han, S.; Kim, K.; Jheon, S.; Ji, E. Effect of sevoflurane-based or propofol-based anaesthesia on the incidence of postoperative acute kidney injury: A retrospective propensity score-matched analysis. Eur. J. Anaesthesiol. 2019, 36, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Ahn, H.J.; Oh, A.R.; Choi, J. Restrictive intraoperative fluid management was associated with higher incidence of composite complications compared to less restrictive strategies in open thoracotomy: A retrospective cohort study. Sci. Rep. 2020, 10, 8449. [Google Scholar] [CrossRef]
- Meng, Z.T.; Mu, D.L. Impact of oliguria during lung surgery on postoperative acute kidney injury. Beijing Da Xue Xue Bao Yi Xue Ban 2020, 53, 188–194. [Google Scholar]
- Zhao, B.C.; Lei, S.H.; Yang, X.; Zhang, Y.; Qiu, S.D.; Liu, W.F.; Li, C.; Liu, K.X. Assessment of prognostic value of intraoperative oliguria for postoperative acute kidney injury: A retrospective cohort study. Br. J. Anaesth. 2021, 126, 799–807. [Google Scholar] [CrossRef]
- Garutti, I.; De la Gala, F.; Pineiro, P.; Rancan, L.; Vara, E.; Reyes, A.; Puente-Maestu, L.; Bellon, J.M.; Simon, C. Usefulness of combining clinical and biochemical parameters for prediction of postoperative pulmonary complications after lung resection surgery. J. Clin. Monit. Comput. 2019, 33, 1043–1054. [Google Scholar] [CrossRef]
- Palmer, T.M.; Sterne, J. Meta-Analysis in Stata; BMJ Publishing Group: London, UK, 2008. [Google Scholar]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Hobson, C.; Lysak, N.; Huber, M.; Scali, S.; Bihorac, A. Epidemiology, outcomes, and management of acute kidney injury in the vascular surgery patient. J. Vasc. Surg. 2018, 68, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Lassnigg, A.; Schmidlin, D.; Mouhieddine, M.; Bachmann, L.M.; Druml, W.; Bauer, P.; Hiesmayr, M. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: A prospective cohort study. J. Am. Soc. Nephrol. 2004, 15, 1597–1605. [Google Scholar] [CrossRef]
- Lopes, J.A.; Jorge, S. The RIFLE and AKIN classifications for acute kidney injury: A critical and comprehensive review. Clin. Kidney J. 2013, 6, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Silver, S.A.; Harel, Z.; McArthur, E.; Nash, D.M.; Acedillo, R.; Kitchlu, A.; Garg, A.X.; Chertow, G.M.; Bell, C.M.; Wald, R. Causes of Death after a Hospitalization with AKI. J. Am. Soc. Nephrol. 2018, 29, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Gelman, S. Acute Kidney Injury after Surgery: Where Does the Journey Lead? Anesthesiology 2020, 132, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Uchino, S. Creatinine. Curr. Opin. Crit. Care 2010, 16, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Matot, I.; Paskaleva, R.; Eid, L.; Cohen, K.; Khalaileh, A.; Elazary, R.; Keidar, A. Effect of the volume of fluids administered on intraoperative oliguria in laparoscopic bariatric surgery: A randomized controlled trial. Arch. Surg. 2012, 147, 228–234. [Google Scholar] [CrossRef]
- Myles, P.S.; McIlroy, D.R.; Bellomo, R.; Wallace, S. Importance of intraoperative oliguria during major abdominal surgery: Findings of the Restrictive versus Liberal Fluid Therapy in Major Abdominal Surgery trial. Br. J. Anaesth. 2019, 122, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Inácio, R.; Gameiro, J.; Amaro, S.; Duarte, M. Intraoperative oliguria does not predict postoperative acute kidney injury in major abdominal surgery: A cohort analysis. J. Bras. Nefrol. 2021, 43, 9–19. [Google Scholar] [CrossRef]
- Huo, W.; Zhang, K.; Nie, Z.; Li, Q.; Jin, F. Kidney injury molecule-1 (KIM-1): A novel kidney-specific injury molecule playing potential double-edged functions in kidney injury. Transplant. Rev. 2010, 24, 143–146. [Google Scholar] [CrossRef]
- Sinna, M.M.; Altaf, F.M.N.; Mosa, O.F. Serum and Urinary NGAL and Cystatin C Levels as Diagnostic Tools for Acute Kidney Injury and Chronic Kidney Disease: A Histobiochemical Comparative Study. Curr. Pharm. Des. 2019, 25, 1122–1133. [Google Scholar] [CrossRef]
- Yi, A.; Lee, C.H.; Yun, Y.M.; Kim, H.; Moon, H.W.; Hur, M. Effectiveness of Plasma and Urine Neutrophil Gelatinase-Associated Lipocalin for Predicting Acute Kidney Injury in High-Risk Patients. Ann. Lab. Med. 2021, 41, 60–67. [Google Scholar] [CrossRef]
- Meersch, M.; Schmidt, C.; Hoffmeier, A.; Van Aken, H.; Wempe, C.; Gerss, J.; Zarbock, A. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high risk patients identified by biomarkers: The PrevAKI randomized controlled trial. Intensive Care Med. 2017, 43, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Göcze, I.; Jauch, D.; Götz, M.; Kennedy, P.; Jung, B.; Zeman, F.; Gnewuch, C.; Graf, B.M.; Gnann, W.; Banas, B.; et al. Biomarker-guided Intervention to Prevent Acute Kidney Injury After Major Surgery: The Prospective Randomized BigpAK Study. Ann. Surg. 2018, 267, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Küllmar, M.; Ostermann, M.; Lucchese, G.; Baig, K.; Cennamo, A.; Rajani, R.; McCorkell, S.; Arndt, C.; Wulf, H.; et al. Prevention of Cardiac Surgery-Associated Acute Kidney Injury by Implementing the KDIGO Guidelines in High-Risk Patients Identified by Biomarkers: The PrevAKI-Multicenter Randomized Controlled Trial. Anesth. Analg. 2021, 133, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Esmeijer, K.; Schoe, A.; Ruhaak, L.R.; Hoogeveen, E.K.; Soonawala, D.; Romijn, F.; Shirzada, M.R.; van Dissel, J.T.; Cobbaert, C.M.; de Fijter, J.W. The predictive value of TIMP-2 and IGFBP7 for kidney failure and 30-day mortality after elective cardiac surgery. Sci. Rep. 2021, 11, 1071. [Google Scholar] [CrossRef]
- Gameiro, J.; Fonseca, J.A.; Neves, M.; Jorge, S.; Lopes, J.A. Acute kidney injury in major abdominal surgery: Incidence, risk factors, pathogenesis and outcomes. Ann. Intensive Care 2018, 8, 22. [Google Scholar] [CrossRef]
- Meersch, M.; Schmidt, C.; Zarbock, A. Perioperative Acute Kidney Injury: An Under-Recognized Problem. Anesth. Analg. 2017, 125, 1223–1232. [Google Scholar] [CrossRef]
- Yuan, X.; Lee, J.W.; Bowser, J.L.; Neudecker, V.; Sridhar, S.; Eltzschig, H.K. Targeting Hypoxia Signaling for Perioperative Organ Injury. Anesth. Analg. 2018, 126, 308–321. [Google Scholar] [CrossRef]
- Singh, P.; Ricksten, S.E.; Bragadottir, G.; Redfors, B.; Nordquist, L. Renal oxygenation and haemodynamics in acute kidney injury and chronic kidney disease. Clin. Exp. Pharmacol. Physiol. 2013, 40, 138–147. [Google Scholar] [CrossRef]
- Hollmann, C.; Fernandes, N.L.; Biccard, B.M. A Systematic Review of Outcomes Associated With Withholding or Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Before Noncardiac Surgery. Anesth. Analg. 2018, 127, 678–687. [Google Scholar] [CrossRef]
- Takeuchi, K.; Hayashida, M.; Kudoh, O.; Niimi, N.; Kataoka, K.; Kakemizu-Watanabe, M.; Yamamoto, M.; Hara, A.; Kawagoe, I.; Yamaguchi, K. Continuing versus withholding angiotensin receptor blocker (ARB)/calcium channel blocker (CCB) combination tablets during perioperative periods in patients undergoing minor surgery: A single-blinded randomized controlled trial. J. Anesth. 2022, 36, 374–382. [Google Scholar] [CrossRef]
- Norman, J.T.; Stidwill, R.; Singer, M.; Fine, L.G. Angiotensin II Blockade Augments Renal Cortical Microvascular pO2 Indicating a Novel, Potentially Renoprotective Action. Nephron Physiol. 2003, 94, p39–p46. [Google Scholar] [CrossRef] [PubMed]
- Gayat, E.; Hollinger, A.; Cariou, A.; Deye, N.; Vieillard-Baron, A.; Jaber, S.; Chousterman, B.G.; Lu, Q.; Laterre, P.F.; Monnet, X.; et al. Impact of angiotensin-converting enzyme inhibitors or receptor blockers on post-ICU discharge outcome in patients with acute kidney injury. Intensive Care Med. 2018, 44, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Qiao, H.; Guo, J.F.; Yang, H.Y.; Li, X.Y.; Li, S.L.; Wang, D.X.; Yang, L. Preoperative hypoalbuminemia was associated with acute kidney injury in high-risk patients following non-cardiac surgery: A retrospective cohort study. BMC Anesthesiol. 2019, 19, 171. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, C.J.; Wiedermann, W.; Joannidis, M. Causal relationship between hypoalbuminemia and acute kidney injury. World J. Nephrol. 2017, 6, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Degoul, S.; Chazard, E.; Lamer, A.; Lebuffe, G.; Duhamel, A.; Tavernier, B. lntraoperative administration of 6% hydroxyethyl starch 130/0.4 is not associated with acute kidney injury in elective non-cardiac surgery: A sequential and propensity-matched analysis. Anaesth. Crit. Care Pain. Med. 2020, 39, 199–206. [Google Scholar] [CrossRef] [PubMed]
- He, S.J.; Liu, Q.; Li, H.Q.; Tian, F.; Chen, S.Y.; Weng, J.X. Role of statins in preventing cardiac surgery-associated acute kidney injury: An updated meta-analysis of randomized controlled trials. Ther. Clin. Risk Manag. 2018, 14, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Tie, H.T. Dexmedetomidine as a promising prevention strategy for cardiac surgery-associated acute kidney injury: A meta-analysis. Crit. Care. 2017, 21, 198. [Google Scholar] [CrossRef]
- Alsabbagh, M.M.; Asmar, A.; Ejaz, N.I.; Aiyer, R.K.; Kambhampati, G.; Ejaz, A.A. Update on clinical trials for the prevention of acute kidney injury in patients undergoing cardiac surgery. Am. J. Surg. 2013, 206, 86–95. [Google Scholar] [CrossRef]
- Zhao, B.C.; Shen, P.; Liu, K.X. Perioperative Statins Do Not Prevent Acute Kidney Injury After Cardiac Surgery: A Meta-analysis of Randomized Controlled Trials. J. Cardiothorac. Vasc. Anesth. 2017, 31, 2086–2092. [Google Scholar] [CrossRef]
- Song, J.W.; Shim, J.K.; Soh, S.; Jang, J.; Kwak, Y.L. Double-blinded, randomized controlled trial of N-acetylcysteine for prevention of acute kidney injury in high risk patients undergoing off-pump coronary artery bypass. Nephrology 2015, 20, 96–102. [Google Scholar] [CrossRef]
- Piggott, K.D.; Liu, A.; Monczka, J.; Fakioglu, H.; Narasimhulu, S.S.; Pourmoghadam, K.; DeCampli, W. Inadequate preoperative nutrition might be associated with acute kidney injury and greater illness severity postoperatively. J. Thorac. Cardiovasc. Surg. 2018, 155, 2104–2109. [Google Scholar] [CrossRef] [PubMed]
- Al Adas, Z.; Lodewyk, K.; Robinson, D.; Qureshi, S.; Kabbani, L.S.; Sullivan, B.; Shepard, A.D.; Weaver, M.R.; Nypaver, T.J. Contrast-induced nephropathy after peripheral vascular intervention: Long-term renal outcome and risk factors for progressive renal dysfunction. J. Vasc. Surg. 2019, 69, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.S.; Bellomo, R.; Corcoran, T.; Forbes, A.; Peyton, P.; Story, D.; Christophi, C.; Leslie, K.; McGuinness, S.; Parke, R.; et al. Restrictive versus Liberal Fluid Therapy for Major Abdominal Surgery. N. Engl. J. Med. 2018, 378, 2263–2274. [Google Scholar] [CrossRef] [PubMed]
- Prowle, J.R.; Kirwan, C.J.; Bellomo, R. Fluid management for the prevention and attenuation of acute kidney injury. Nat. Rev. Nephrol. 2014, 10, 37–47. [Google Scholar] [CrossRef]
| Study (Year) | Country | Setting | Patients Type (Non-AKI vs. AKI) | Number of Patients | Number with AKI | AKI Incidence | RRT Incidence | AKI Definition |
|---|---|---|---|---|---|---|---|---|
| Licker et al. (2011) [27] | Switzerland | Lung | Excluding hemodialysis Age: 63 (11) vs. 66 (9) Preoperative eGFR: 83 (23) vs. 75 (21) DM: 9.3% vs. 23.3% CAD: 10.5% vs. 12.5% | 1345 | 91 | 0.068 | -- | RIFLE |
| Ishikawa et al. (2012) [9] | Canada | Lung | Excluding hemodialysis Age: 61 (15) vs. 67 (10) Preoperative eGFR: 74 (22) vs. 62 (23) Preoperative SCr: 79.56 (17.68) vs. 97.24 (35.36) DM: 9% vs. 19% CAD: 11% vs. 19% | 1129 | 67 | 0.059 | 1/1129 (0.09%) | AKIN |
| Lee et al. (2014) [6] | Korea | Esophageal | Excluding hemodialysis Age: 61.7 (8.2) vs. 62.6 (8.2) Preoperative eGFR < 60 mL/min/1.73 m2: 3.1% vs. 4.8% Preoperative SCr: 70.72 (61.88–79.56) vs. 70.72 (61.88–79.56) DM: 15.1% vs. 21.4% CAD: 1.3% vs. 0.5% | 595 | 210 | 0.353 | 11/595 (1.85%) | AKIN |
| Ren et al. (2015) [28] | China | Esophageal | Normal Age: 63 (15) vs. 74 (12) Preoperative SCr: 78 (11) vs. 75 (13) DM: 18% vs. 39.1% CAD: 23% vs. 26.1% | 362 | 23 | 0.064 | 2/362 (0.55%) | KDIGO |
| Assaad et al. (2015) [12] | USA | Lung | Excluding SCr > 2 mg/dL Mean age of all: 67 (from 54 to 83) | 40 | 3 | 0.075 | 0 | AKIN |
| Grams et al. (2016) [19] | USA | Thoracic | Excluding hemodialysis Mean age of all: 64 (10) Preoperative eGFR < 60 mL/min/1.73 m2: 1.2% DM: 27% CAD: 27% | 11,779 | 1413 | 0.120 | 23/11779 (0.2%) | KDIGO |
| Ahn et al. (2016) [29] | Korea | Thoracic | Excluding hemodialysis Age: 59.4 (12.5) vs. 64.5 (10.3) Preoperative eGFR: 92 (21) vs. 93 (41) DM: 13% vs. 39% | 1442 | 74 | 0.051 | 2/1142 (0.18%) | AKIN |
| Moon et al. (2016) [30] | USA | Lung | Unselected Mean age of all: 66 (from 59 to 73) | 1207 | 98 | 0.081 | 0 | AKIN |
| Konda et al. (2017) [31] | USA | Esophageal | Excluding hemodialysis Age: 60 (10) vs. 63 (9) Preoperative SCr: 79.6 (17.7) vs. 91.1 (26.5) DM: 13.3% vs. 26.2% CAD: 15.4% vs. 23.4% | 897 | 107 | 0.119 | 0 | AKIN |
| Wang et al. (2017) [10] | China | Esophageal | Excluding hemodialysis Age: 63 (8) vs. 63 (9) Preoperative SCr: 76 (13) vs. 85 (23) DM: 8.3% vs. 11.8% CAD: 4.9% vs. 7.8% | 2094 | 51 | 0.024 | -- | KDIGO |
| Cardinale et al. (2018) [11] | Italy | Lung | Excluding hemodialysis Age: 62 (10) vs. 68 (9) Preoperative eGFR: 102 (84–117) vs. 83 (67–103) Preoperative SCr: 67.18 (56.58–78.67) vs. 81.33 (68.07–98.12) DM: 8% vs. 8% CAD: 4% vs. 20% | 2179 | 222 | 0.102 | 5/2179 (0.23%) | AKIN |
| Naruka et al. (2019) [5] | UK | Lung | Excluding hemodialysis Old (>60 years):58.7% vs. 75.6 | 568 | 86 | 0.151 | -- | KDIGO |
| Matesanz et al. (2019) [32] | Spain | Lung | Unselected Age: 65 (56–70) vs. 73 (64–77) | 174 | 12 | 0.069 | 2/174 (1.15%) | AKIN |
| Oh et al. (2019) [33] | Korea | Lung | Excluding hemodialysis Aged 19 yr or older | 2872 | 140 | 0.049 | -- | KDIGO |
| Garutti et al. (2019) [37] | Spain | Lung | Unselected Aged 19 yr or older | 174 | 12 | 0.069 | -- | AKIN |
| Murphy et al. (2020) [8] | UK | Esophageal | Unselected Mean age of all: 64.2 ± 9.2 | 1135 | 208 | 0.183 | 10/1135 (0.88%) | AKIN |
| Meng et al. (2020) [35] | China | Lung | Excluding hemodialysis Age: 59.8 (10.6) vs. 58.8 (10.7) Preoperative eGFR < 30 mL/min/1.73 m2: 0.3% vs. 9.7% DM: 17.4% vs. 32.3% CAD: 11.4% vs. 12.9% | 1393 | 31 | 0.022 | 1/1393 (0.07%) | KDIGO |
| Kim et al. (2020) [34] | Korea | Lung | Unselected | 1031 | 63 | 0.061 | -- | AKIN |
| Zhao et al. (2021) [36] | China | Lung | Excluding hemodialysis Age: 58 (51–65) vs. 63 (56–69) Preoperative SCr: 70 (60–82) vs. 75 (62–90) DM: 11% vs. 23% CAD: 5% vs. 13% | 3862 | 205 | 0.053 | 0 | KDIGO |
| Wu et al. (2021) [7] | China | Lung | Normal | 548 | 12 | 0.022 | -- | AKIN |
| Number of Studies | Number of Patients | AKI Incidence | 95% CI | I2 | p | |
|---|---|---|---|---|---|---|
| All patients | 20 | 34,826 | 0.088 | 0.067–0.108 | 98.3% | |
| AKI definition | 0.136 | |||||
| RIFLE | 1 | 1345 | 0.068 | 0.054–0.081 | -- | |
| AKIN | 12 | 10,551 | 0.103 | 0.071–0.135 | 97.4% | |
| KDIGO | 7 | 22,930 | 0.068 | 0.035–0.101 | 99.1% | |
| Surgical type | 0.203 | |||||
| Lung | 13 | 16,522 | 0.066 | 0.050–0.081 | 94.2% | |
| Esophageal | 5 | 5083 | 0.148 | 0.055–0.240 | 99.2% | |
| Thoracic | 2 | 13,221 | 0.086 | 0.019–0.153 | 99.1% | |
| Preoperative renal function | 0.092 | |||||
| Unselected | 5 | 3721 | 0.093 | 0.048–0.138 | 95.3% | |
| Excluding hemodialysis | 12 | 30,155 | 0.094 | 0.068–0.120 | 98.8% | |
| Normal | 3 | 950 | 0.046 | 0.009–0.082 | 79.3% |
| Study (Year) | Setting | Patients Type | Risk Factors for AKI |
|---|---|---|---|
| Licker et al. (2011) [27] | Lung | Excluding hemodialysis | ASA 3 or 4, low FEV1, use of vasopressors, prolonged anesthesia time |
| Ishikawa et al. (2012) [9] | Lung | Excluding hemodialysis | hypertension, peripheral vascular disease, low eGFR, use of ARB, intraoperative hydroxyethyl starch administration, thoracotomy procedure |
| Lee et al. (2014) [6] | Esophageal | Excluding hemodialysis | BMI, low serum albumin level, use of ACEI or ARB, intraoperative hydroxyethyl starch administration, postoperative 2-day CRP |
| Ren et al. (2015) [28] | Esophageal | Normal | elderly, DM, intraoperative hypotension |
| Assaad et al. (2015) [12] | Lung | Normal | elderly, ASA 3 or 4, prolonged surgery time |
| Grams et al. (2016) [19] | Thoracic | Excluding hemodialysis | elderly, male, African American, higher BMI, hypertension, DM, lung disease, malignancy, low eGFR, use of ACEI/ARB, diuretic use, later timing of surgery during the hospital stay |
| Ahn et al. (2016) [29] | Thoracic | Excluding hemodialysis | use of ACRI/ARB, open thoracotomy, pneumonectomy/esophagectomy, DM, cerebrovascular disease, low serum albumin level, decreased renal function(eGFR < 60 mL/min/1.73 m2) |
| Moon et al. (2016) [30] | Lung | Unselected | BMI, male, ASA 3 or 4, hypertension, smoking status, thoracotomy procedure |
| Konda et al. (2017) [31] | Esophageal | Excluding hemodialysis | higher BMI, a number of comorbidities, high preoperative creatinine level |
| Wang et al. (2017) [10] | Esophageal | Excluding hemodialysis | preoperative serum creatinine level, duration of surgery, smoking status, hypertension |
| Cardinale et al. (2018) [11] | Lung | Excluding hemodialysis | hypertension, preoperative serum creatinine level, forced vital capacity, preoperative NT-proBNP, pneumonectomy, intraoperative blood loss |
| Naruka et al. (2019) [5] | Lung | Excluding hemodialysis | 60 years or older |
| Matesanz et al. (2019) [32] | Lung | Unselected | hypertension, ASA 3 or 4, prolonged surgery time, plasma IL-6 level at 6 h after surgery |
| Murphy et al. (2020) [8] | Esophageal | Unselected | elderly, male, increased BMI, dyslipidemia |
| Meng et al. (2020) [35] | Lung | Excluding hemodialysis | intraoperative urine output < 0.8 mL/(kg·h), preoperative Hb ≤ 120.0 g/L, preoperative eGFR < 30 mL/min/1.73 m2) |
| Zhao et al. (2021) [36] | Lung | Excluding hemodialysis | elderly, hypertension, DM, use of ACEI/ARB, preoperative serum albumin and creatinine level, blood loss, intraoperative lowest MAP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Xu, S.; Yan, B.; Tang, X.; Zhang, H.; Pan, C.; Zhu, S. Incidence and Associations of Acute Kidney Injury after General Thoracic Surgery: A System Review and Meta-Analysis. J. Clin. Med. 2023, 12, 37. https://doi.org/10.3390/jcm12010037
Yu Y, Xu S, Yan B, Tang X, Zhang H, Pan C, Zhu S. Incidence and Associations of Acute Kidney Injury after General Thoracic Surgery: A System Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(1):37. https://doi.org/10.3390/jcm12010037
Chicago/Turabian StyleYu, Yang, Shanshan Xu, Bing Yan, Xiaodong Tang, Honggang Zhang, Caifei Pan, and Shengmei Zhu. 2023. "Incidence and Associations of Acute Kidney Injury after General Thoracic Surgery: A System Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 1: 37. https://doi.org/10.3390/jcm12010037
APA StyleYu, Y., Xu, S., Yan, B., Tang, X., Zhang, H., Pan, C., & Zhu, S. (2023). Incidence and Associations of Acute Kidney Injury after General Thoracic Surgery: A System Review and Meta-Analysis. Journal of Clinical Medicine, 12(1), 37. https://doi.org/10.3390/jcm12010037






