Heparin-Induced Thrombocytopenia in Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
- Take-home message:
- Prevalence of HIT is 3.5% in patients on VA-ECMO treatment.
- HIT was not associated with a significantly higher mortality rate.
- Argatroban seems to be an appropriate and safe therapeutic option for confirmed HIT-positive patients undergoing VA-ECMO support.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baran, D.A.; Grines, C.L.; Bailey, S.; Burkhoff, D.; Hall, S.A.; Henry, T.D.; Hollenberg, S.M.; Kapur, N.K.; O’Neill, W.; Ornato, J.P.; et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter. Cardiovasc. Interv. 2019, 94, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Khalpey, Z.; Smith, R.; Burkhoff, D.; Kociol, R.D. Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock and Cardiac Arrest. Circ. Heart Fail. 2018, 11, e004905. [Google Scholar] [CrossRef] [PubMed]
- Guglin, M.; Zucker, M.J.; Bazan, V.M.; Bozkurt, B.; El Banayosy, A.; Estep, J.D.; Gurley, J.; Nelson, K.; Malyala, R.; Panjrath, G.S.; et al. Venoarterial ECMO for Adults: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 698–716. [Google Scholar] [CrossRef]
- Abrams, D.; Combes, A.; Brodie, D. Extracorporeal membrane oxygenation in cardiopulmonary disease in adults. J. Am. Coll. Cardiol. 2014, 63, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Abrams, D.; Baldwin, M.R.; Champion, M.; Agerstrand, C.; Eisenberger, A.; Bacchetta, M.; Brodie, D. Thrombocytopenia and extracorporeal membrane oxygenation in adults with acute respiratory failure: A cohort study. Intensive Care Med. 2016, 42, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Lüsebrink, E.; Orban, M.; Kupka, D.; Scherer, C.; Hagl, C.; Zimmer, S.; Luedike, P.; Thiele, H.; Westermann, D.; Massberg, S.; et al. Prevention and treatment of pulmonary congestion in patients undergoing venoarterial extracorporeal membrane oxygenation for cardiogenic shock. Eur. Heart J. 2020, 41, 3753–3761. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Combes, A.; Rozé, H.; Chevret, S.; Mercat, A.; Roch, A.; Mourvillier, B.; Ara-Somohano, C.; Bastien, O.; Zogheib, E.; et al. REVA Research Network (Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: A cohort study and propensity-matched analysis. Am. J. Respir. Crit. Care Med. 2013, 187, 276–285. [Google Scholar] [CrossRef]
- Lüsebrink, E.; Zimmer, S.; Schrage, B.; Dabboura, S.; Majunke, N.; Scherer, C.; Aksoy, A.; Krogmann, A.; Hoffmann, S.; Szczanowicz, L.; et al. ICH-VA-ECMO Investigator Group (Intracranial haemorrhage in adult patients on venoarterial extracorporeal membrane oxygenation. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 303–311. [Google Scholar] [CrossRef]
- Opfermann, P.; Bevilacqua, M.; Felli, A.; Mouhieddine, M.; Bachleda, T.; Pichler, T.; Hiesmayr, M.; Zuckermann, A.; Dworschak, M.; Steinlechner, B. Prognostic Impact of Persistent Thrombocytopenia During Extracorporeal Membrane Oxygenation: A Retrospective Analysis of Prospectively Collected Data from a Cohort of Patients With Left Ventricular Dysfunction After Cardiac Surgery. Crit. Care Med. 2016, 44, e1208–e1218. [Google Scholar] [CrossRef]
- Millar, J.E.; Fanning, J.P.; McDonald, C.I.; McAuley, D.F.; Fraser, J.F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit. Care 2016, 20, 387. [Google Scholar] [CrossRef]
- Haneya, A.; Philipp, A.; Diez, C.; Ried, M.; Puehler, T.; Camboni, D.; Zausig, Y.; Lehle, K.; Schmid, C. Comparison of two different minimized extracorporeal circulation systems: Hematological effects after coronary surgery. ASAIO J. 2009, 55, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.C. Anticoagulation and coagulation management for ECMO. Semin. Cardiothorac Vasc Anesth. 2009, 13, 154–175. [Google Scholar] [CrossRef] [PubMed]
- Kimmoun, A.; Oulehri, W.; Sonneville, R.; Grisot, P.H.; Zogheib, E.; Amour, J.; Aissaoui, N.; Megarbane, B.; Mongardon, N.; Renou, A.; et al. Prevalence and outcome of heparin-induced thrombocytopenia diagnosed under veno-arterial extracorporeal membrane oxygenation: A retrospective nationwide study. Intensive Care Med. 2018, 44, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A. CLINICAL PRACTICE. Heparin-Induced Thrombocytopenia. N. Engl. J. Med. 2015, 373, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Sokolovic, M.; Pratt, A.K.; Vukicevic, V.; Sarumi, M.; Johnson, L.S.; Shah, N.S. Platelet Count Trends and Prevalence of Heparin-Induced Thrombocytopenia in a Cohort of Extracorporeal Membrane Oxygenator Patients. Crit. Care Med. 2016, 44, e1031–e1037. [Google Scholar] [CrossRef]
- Salter, B.S.; Weiner, M.M.; Trinh, M.A.; Heller, J.; Evans, A.S.; Adams, D.H.; Fischer, G.W. Heparin-Induced Thrombocytopenia: A Comprehensive Clinical Review. J. Am. Coll. Cardiol. 2016, 67, 2519–2532. [Google Scholar] [CrossRef]
- Lo, G.K.; Juhl, D.; Warkentin, T.E.; Sigouin, C.S.; Eichler, P.; Greinacher, A. Evaluation of pretest clinical score (4 T’s) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J. Thromb. Haemost. 2006, 4, 759–765. [Google Scholar] [CrossRef]
- Glick, D.; Dzierba, A.L.; Abrams, D.; Muir, J.; Eisenberger, A.; Diuguid, D.; Abel, E.; Agerstrand, C.; Bacchetta, M.; Brodie, D. Clinically suspected heparin-induced thrombocytopenia during extracorporeal membrane oxygenation. J. Crit. Care 2015, 30, 1190–1194. [Google Scholar] [CrossRef]
- Jiritano, F.; Serraino, G.F.; Ten Cate, H.; Fina, D.; Matteucci, M.; Mastroroberto, P.; Lorusso, R. Platelets and extra-corporeal membrane oxygenation in adult patients: A systematic review and meta-analysis. Intensive Care Med. 2020, 46, 1154–1169. [Google Scholar] [CrossRef]
- Sullivan, J.; Bak, E.; Sullivan, M.J.; Gurnani, P.K. Predictive value of scoring tools in determining heparin-induced thrombocytopenia in patients on extracorporeal membrane oxygenation. Perfusion 2020, 35, 378–383. [Google Scholar] [CrossRef]
- Althaus, K.; Straub, A.; Häberle, H.; Rosenberger, P.; Hidiatov, O.; Hammer, S.; Nowak-Harnau, S.; Enkel, S.; Riessen, R.; Bakchoul, T. Heparin-induced thrombocytopenia: Diagnostic challenges in intensive care patients especially with extracorporeal circulation. Thromb. Res. 2020, 188, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Zaaqoq, A.M.; Brammer, R.C.; Chan, C.M.; Shorr, A.F. Heparin-induced thrombocytopenia in extra-corporeal membrane oxygenation: Epidemiology, outcomes, and diagnostic challenges. J. Thromb. Thrombolysis. 2022, 53, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Lubnow, M.; Berger, J.; Schneckenpointner, R.; Zeman, F.; Lunz, D.; Philipp, A.; Foltan, M.; Lehle, K.; Heimerl, S.; Hart, C.; et al. Prevalence and outcomes of patients developing heparin-induced thrombocytopenia during extracorporeal membrane oxygenation. PLoS ONE 2022, 17, e0272577. [Google Scholar] [CrossRef] [PubMed]
- To, L.; Attar, D.; Lines, B.; McCarty, M.; Nemeh, H.; Lopez-Plaza, I.; Smith, Z.; Coba, V.; Lekura, J. Incidence of Heparin-Induced Thrombocytopenia in Patients with Newly Implanted Mechanical Circulatory Support Devices. Ann. Pharmacother. 2021, 56, 565–571. [Google Scholar] [CrossRef]
- Renou, A.; Neuschwander, A.; Kimmoun, A.; Brodie, D.; Pirracchio, R.; HIT-ECMO Study Group. Modified 4T score for heparin-induced thrombocytopenia diagnosis in VA-ECMO patients. Intensive Care Med. 2020, 46, 1481–1483. [Google Scholar] [CrossRef]
- Bloom, M.B.; Johnson, J.; Volod, O.; Lee, E.Y.; White, T.; Margulies, D.R. Improved prediction of HIT in the SICU using an improved model of the Warkentin 4-T system: 3-T. Am. J. Surg. 2020, 219, 54–57. [Google Scholar] [CrossRef]
- Vayne, C.; May, M.A.; Bourguignon, T.; Lemoine, E.; Guery, E.A.; Rollin, J.; Gruel, Y.; Pouplard, C. Frequency and Clinical Impact of Platelet Factor 4-Specific Antibodies in Patients Undergoing Extracorporeal Membrane Oxygenation. Thromb. Haemost. 2019, 119, 1138–1146. [Google Scholar] [CrossRef]
- Thomas, J.; Kostousov, V.; Teruya, J. Bleeding and Thrombotic Complications in the Use of Extracorporeal Membrane Oxygenation. Semin Thromb. Hemost. 2018, 44, 20–29. [Google Scholar] [CrossRef]
- Lukito, P.; Wong, A.; Jing, J.; Arthur, J.F.; Marasco, S.F.; Murphy, D.A.; Bergin, P.J.; Shaw, J.A.; Collecutt, M.; Andrews, R.K.; et al. Mechanical circulatory support is associated with loss of platelet receptors glycoprotein Ibα and glycoprotein VI. J. Thromb. Haemost. 2016, 14, 2253–2260. [Google Scholar] [CrossRef]
- Sibbing, D.; Aradi, D.; Alexopoulos, D.; Ten Berg, J.; Bhatt, D.L.; Bonello, L.; Collet, J.P.; Cuisset, T.; Franchi, F.; Gross, L.; et al. Updated Expert Consensus Statement on Platelet Function and Genetic Testing for Guiding P2Y12 Receptor Inhibitor Treatment in Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2019, 12, 1521–1537. [Google Scholar] [CrossRef]
- Choi, J.H.; Luc, J.G.Y.; Weber, M.P.; Reddy, H.G.; Maynes, E.J.; Deb, A.K.; Samuels, L.E.; Morris, R.J.; Massey, H.T.; Loforte, A.; et al. Heparin-induced thrombocytopenia during extracorporeal life support: Incidence, management and outcomes. Ann. Cardiothorac. Surg. 2019, 8, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Arachchillage, D.R.J.; Laffan, M.; Khanna, S.; Vandenbriele, C.; Kamani, F.; Passariello, M.; Rosenberg, A.; Aw, T.C.; Banya, W.; Ledot, S.; et al. Frequency of Thrombocytopenia and Heparin-Induced Thrombocytopenia in Patients Receiving Extracorporeal Membrane Oxygenation Compared With Cardiopulmonary Bypass and the Limited Sensitivity of Pretest Probability Score. Crit. Care Med. 2020, 48, e371–e379. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, H.; Yang, J.H.; Sung, K.; Suh, G.Y.; Chung, C.R.; Yang, J.H.; Cho, Y.H. Use of argatroban for extracorporeal life support in patients with nonheparin-induced thrombocytopenia: Analysis of 10 consecutive patients. Medicine 2018, 97, e13235. [Google Scholar] [CrossRef] [PubMed]
- Rougé, A.; Pelen, F.; Durand, M.; Schwebel, C. Argatroban for an alternative anticoagulant in HIT during ECMO. J. Intensive Care 2017, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, F.; Asmussen, S.; Maybauer, D.M.; Santonocito, C.; Fraser, J.F.; Erdoes, G.; Maybauer, M.O. Bivalirudin for Alternative Anticoagulation in Extracorporeal Membrane Oxygenation: A Systematic Review. J. Intensive Care Med. 2017, 32, 312–319. [Google Scholar] [CrossRef]
- Hanna, D.J.; Torbic, H.; Militello, M.; Strnad, K.; Krishnan, S.; Hohlfelder, B. Evaluation of anticoagulation with bivalirudin for heparin-induced thrombocytopenia during extracorporeal membrane oxygenation. Int. J. Artif. Organs. 2022, 45, 688–694. [Google Scholar] [CrossRef]
- Seelhammer, T.G.; Bohman, J.K.; Schulte, P.J.; Hanson, A.C.; Aganga, D.O. Comparison of Bivalirudin Versus Heparin for Maintenance Systemic Anticoagulation During Adult and Pediatric Extracorporeal Membrane Oxygenation. Crit. Care Med. 2021, 49, 1481–1492. [Google Scholar] [CrossRef]
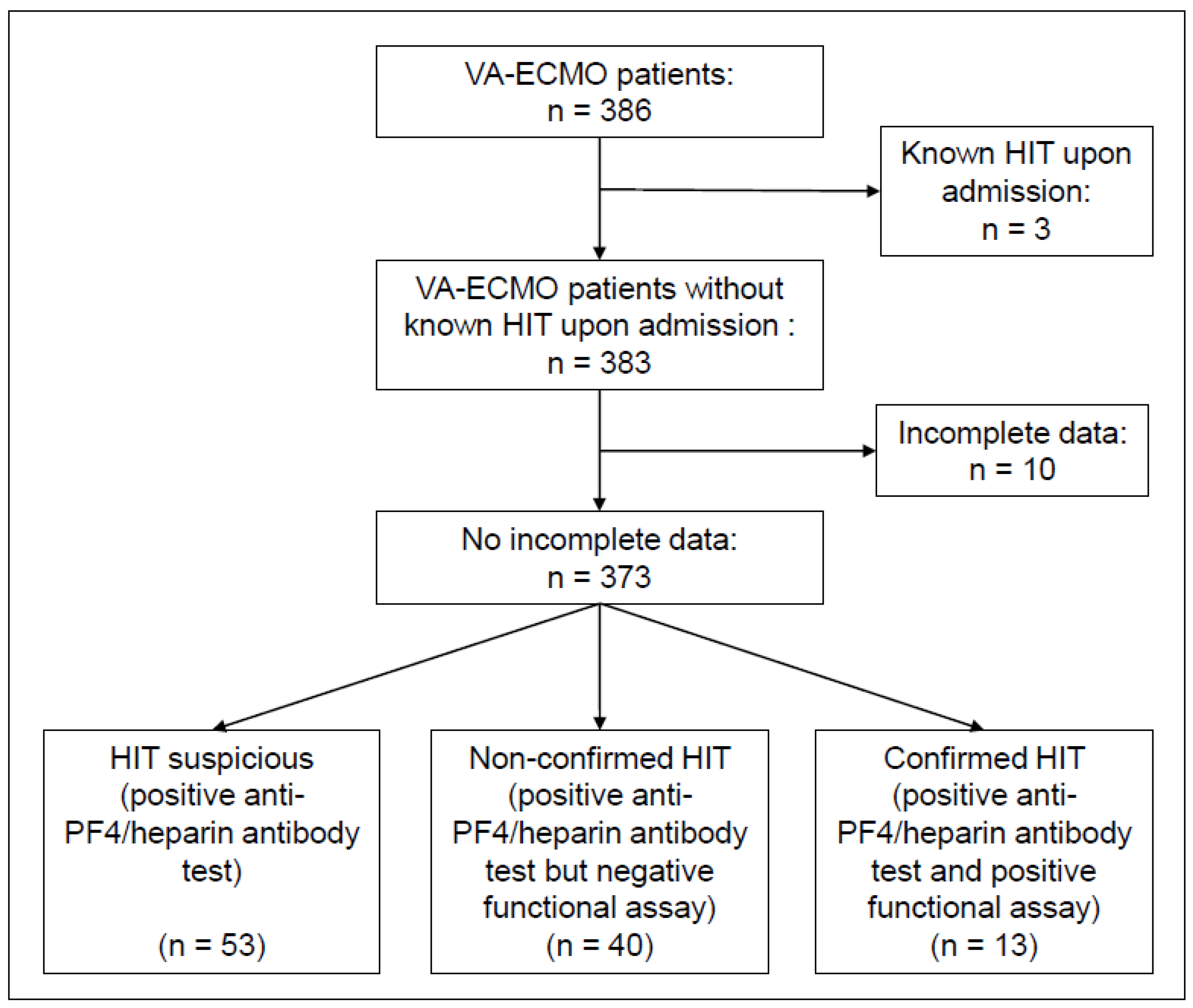

| Characteristics | Overall | Patients with HIT Suspicion | Patients with Excluded HIT | Patients with Confirmed HIT | p-Value (III vs. IV) | |
|---|---|---|---|---|---|---|
| (n = 373) (I) | (n = 53) (II) | (n = 40) (III) | (n = 13) (IV) | |||
| Demographics | ||||||
| Age [years], median [IQR] | 59.0 [51.0, 67.0] | 59.0 [44.0, 63.0] | 59.0 [45.5, 64.2] | 56.0 [35.0, 63.0] | 0.45 | |
| Sex [male], n (%) | 306 (82) | 44 (83) | 32 (80) | 12 (92) | 0.424 | |
| Body mass index [kg/m²], median [IQR] | 26.8 [24.5, 29.4] | 26.8 [24.2, 29.6] | 26.5 [23.8, 29.7] | 27.8 [25.9, 29.4] | 0.357 | |
| Morbidity at admission | ||||||
| History of stroke, n (%) | 36 (10) | 4 (8) | 4 (10) | 0 (0) | 0.561 | |
| History of cancer, n (%) | 17 (5) | 2 (4) | 2 (5) | 0 (0) | >0.999 | |
| History of atrial fibrillation, n (%) | 50 (13) | 6 (11) | 5 (13) | 1 (8) | >0.999 | |
| Coronary artery disease, n (%) | 111 (30) | 13 (25) | 11 (28) | 2 (15) | 0.48 | |
| Previous myocardial infarction, n (%) | 71 (19) | 7 (13) | 6 (15) | 1 (8) | 0.667 | |
| Previous percutaneous coronary intervention, n (%) | 71 (19) | 7 (13) | 6 (15) | 1 (8) | 0.424 | |
| Previous coronary artery bypass graft, n (%) | 16 (4) | 2 (4) | 2 (5) | 0 (0) | >0.999 | |
| Peripheral artery disease, n (%) | 27 (7) | 3 (6) | 3 (8) | 0 (0) | 0.567 | |
| Hypertension, n (%) | 199 (53) | 28 (53) | 24 (60) | 4 (31) | 0.109 | |
| Diabetes mellitus, n (%) | 82 (22) | 10 (19) | 9 (23) | 1 (8) | 0.419 | |
| Chronic renal disease, n (%) | 28 (8) | 7 (13) | 7 (18) | 0 (0) | 0.174 | |
| Pre-cannulation SAPS II score, median [IQR] | 75.0 [67.0, 81.0] | 71.0 [63.0, 77.0] | 71.5 [63.8, 78.0] | 66.0 [62.0, 76.0] | 0.469 | |
| Pre-cannulation SOFA score, median [IQR] | 13.0 [11.0, 15.0] | 13.0 [11.0, 15.0] | 13.0 [10.0, 15.0] | 13.0 [11.0, 15.0] | 0.37 | |
| Cardiac arrest, n (%) | 258 (69) | 29 (55) | 19 (48) | 10 (77) | 0.108 | |
| Extracorporeal cardiopulmonary resuscitation, n (%) | 65 (17) | 6 (11) | 5 (13) | 1 (8) | >0.999 | |
| Medication at admission | ||||||
| Aspirin, n (%) | 99 (27) | 14 (26) | 13 (33) | 1 (8) | 0.145 | |
| P2Y12-inhibitor, n (%) | 86 (23) | 9 (17) | 8 (20) | 1 (8) | 0.424 | |
| Aspirin + P2Y12-inhibitor, n (%) | 56 (15) | 7 (13) | 6 (15) | 1 (8) | 0.667 | |
| Gas exchange at ICU admission | ||||||
| pH, median [IQR] * | 7.3 [7.3, 7.4] | 7.4 [7.3, 7.5] | 7.4 [7.3, 7.5] | 7.4 [7.3, 7.4] | 0.82 | |
| Arterial PaO2 [mmHg], median [IQR] * | 132.0 [90.0, 253.0] | 119.0 [93.3, 192.0] | 112.0 [92.6, 198.2] | 153.0 [109.0, 175.0] | 0.788 | |
| Arterial PaCO2 [mmHg], median [IQR] * | 33.9 [29.1, 40.0] | 32.1 [27.9, 39.3] | 31.1 [27.7, 37.8] | 34.8 [29.7, 40.3] | 0.584 | |
| Laboratory values at ICU admission | ||||||
| Lactate [mmol/L], median [IQR] * | 6.8 [2.7, 11.1] | 3.3 [1.7, 7.9] | 3.3 [1.7, 8.0] | 3.9 [1.5, 6.3] | >0.999 | |
| Creatinine [mg/dl], median [IQR] * | 1.5 [1.2, 2] | 1.5 [1.2, 2.2] | 1.5 [1.2, 2.2] | 1.6 [1.2, 2.2] | 0.959 | |
| Bilirubin [mg/dl], median [IQR] * | 0.9 [0.5, 1.6] | 1.1 [0.8, 2.4] | 1.3 [0.7, 2.5] | 0.9 [0.8, 1.4] | 0.326 | |
| Aspartate aminotransferase [U/I], median [IQR] * | 338.0 [125.0, 936.0] | 322.0 [118.0, 672.0] | 325.5 [124.0, 645.8] | 288.0 [65.0, 1002.0] | 0.894 | |
| Alanine aminotransferase [U/I], median [IQR] * | 147.0 [67.0, 398.0] | 121.0 [73.0, 508.0] | 122.5 [75.2, 491.2] | 93.0 [53.0, 508.0] | 0.877 | |
| Hemoglobin [g/dl], median [IQR] * | 11.7 [9.6, 13.6] | 12.2 [9.8, 14.4] | 12.9 [10.4, 14.6] | 9.7 [9.2, 13.1] | 0.038 | |
| Platelet count [G/L], median [IQR] * | 189.0 [127.0, 240.0] | 197.0 [118.0, 250.0] | 200.0 [132.5, 243.0] | 176.0 [112.0, 250.0] | 0.358 | |
| aPTT [sec], median [IQR] * | 63.0 [33.0, 160.0] | 46.0 [33.0, 115.0] | 46.0 [32.5, 108.5] | 40.0 [35.0, 160.0] | 0.561 | |
| INR, median [IQR] * | 1.8 [1.3, 2.6] | 1.7 [1.4, 2.3] | 1.8 [1.4, 2.2] | 1.5 [1.3, 3.4] | 0.686 | |
| VA-ECMO set-up | ||||||
| VA-ECMO indication | ST-elevation myocardial infarction, n (%) | 157 (42) | 19 (36) | 15 (38) | 4 (31) | 0.749 |
| Non-ST segment elevation myocardial infarction, n (%) | 69 (18) | 4 (8) | 2 (5) | 2 (15) | 0.249 | |
| Cardiomyopathy, n (%) | 71 (19) | 17 (32) | 13 (33) | 4 (31) | >0.999 | |
| Myocarditis, n (%) | 22 (6) | 6 (11) | 4 (10) | 2 (15) | 0.627 | |
| Cardiac arrhythmia, n (%) | 22 (6) | 4 (8) | 4 (10) | 0 (0) | 0.561 | |
| Pulmonary embolism, n (%) | 7 (2) | 1 (2) | 0 (0) | 1 (8) | 0.245 | |
| Septic shock, n (%) | 3 (0.8) | 1 (2) | 1 (3) | 0 (0) | >0.999 | |
| Others, n (%) | 22 (6) | 1 (2) | 1 (3) | 0 (0) | >0.999 | |
| Peripheral cannulation, n (%) | 373 (100) | 53 (100) | 40 (100) | 13 (100) | 1 | |
| Intra-aortic balloon pump therapy, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 | |
| Impella therapy, n (%) | 58 (16) | 14 (26) | 11 (28) | 3 (23) | >0.999 | |
| Mechanical ventilation, n (%) | 335 (90) | 45 (85) | 33 (83) | 12 (92) | 0.662 | |
| Renal replacement therapy, n (%) | 108 (29) | 13 (25) | 9 (23) | 4 (31) | 0.712 | |
| Vasopressors | Epinephrine, n (%) | 209 (56) | 19 (36) | 16 (40) | 3 (23) | 0.334 |
| Norepinephrine, n (%) | 285 (76) | 42 (79) | 29 (73) | 13 (100) | 0.047 | |
| Dobutamine, n (%) | 56 (15) | 20 (38) | 16 (40) | 4 (31) | 0.744 | |
| Vasopressin, n (%) | 47 (13) | 6 (11) | 4 (10) | 2 (15) | 0.627 | |
| Red blood cell transfusion [units], median [IQR] | 6 [2, 10] | 6 [4, 11] | 6 [2, 10] | 8 [5, 13] | 0.228 | |
| Platelet transfusions [units], median [IQR] | 0 [0, 0] | 0 [0, 0] | 0 [0, 0] | 0 [0, 0] | 0.937 | |
| Plasma transfusions [units], median [IQR] | 6 [2, 12] | 8 [4, 13] | 8 [4, 12.2] | 11 [4, 17] | 0.52 | |
| Total duration of mechanical ventilation [h], median [IQR] | 132 [23, 313] | 220 [84, 398] | 189 [53, 364] | 398 [304, 617] | 0.015 | |
| Total duration of VA-ECMO treatment [h], median [IQR] | 89 [39, 144] | 157 [90, 176] | 149 [88, 169] | 176 [99, 215] | 0.13 | |
| Characteristics | Patients with HIT Suspicion | Patients with Excluded HIT | Patients with Confirmed HIT | p-Value | |
|---|---|---|---|---|---|
| (n = 53) (II) | (n = 40) (III) | (n = 13) (IV) | (III vs. IV) | ||
| HIT Diagnosis | |||||
| Continuous unfractionated heparin therapy before HIT suspicion, n (%) | 53 (100) | 40 (100) | 13 (100) | 1 | |
| Heparin-bonded VA-ECMO circuit, n (%) | 53 (100) | 40 (100) | 13 (100) | 1 | |
| Duration of heparin therapy before anti-PF4/heparin antibody testing [d], median [IQR] | 5 [3, 10] | 5 [3, 10] | 8 [2, 11] | 0.959 | |
| Positive anti-PF4/heparin antibody testing, n (%) | 53 (100) | 40 (100) | 13 (100) | ||
| Duration of heparin therapy before | 7 [3, 10] | 6 [3, 10] | 8 [2, 11] | 0.959 | |
| Confirmed HIT-functional assay [d], median [IQR] | |||||
| Positive HIT-functional assay, n (%) | 13 (25) | 0 (0) | 13 (100) | ||
| HIT-4T-Score, median [IQR] | 4 [4, 5] | 4 [3, 5] | 5 [4, 6] | 0.054 | |
| HIT Management | |||||
| Anticoagulant therapy after HIT confirmation | Argatroban, n (%) | 13 (100) | |||
| Danaparoid, n (%) | 0 (0) | ||||
| Bivalirudin, n (%) | 0 (0) | ||||
| Duration of heparin therapy before anticoagulation change [d], median [IQR] | 8 [2, 11] | ||||
| Platelet Counts | |||||
| Platelet count at admission [G/L], median [IQR] | 197 [118, 250] | 200 [133, 243] | 176 [112, 250] | 0.358 | |
| Platelet count at the beginning of VA-ECMO therapy [G/L], median [IQR] | 164 [114, 208] | 170 [113, 210] | 137 [117, 188] | 0.605 | |
| Platelet count at day 3 of VA-ECMO therapy [G/L], median [IQR] | 75 [50, 100] | 73 [53, 101] | 82 [43, 99] | 0.8 | |
| Platelet count at day 7 of VA-ECMO therapy [G/L], median [IQR] | 81 [58, 123] | 81 [56, 117.2] | 82 [65, 156] | 0.513 | |
| Platelet count at day 14 of VA-ECMO therapy [G/L], median [IQR] | 230 [145, 299] | 242 [151, 309] | 200 [142, 260] | 0.693 | |
| Minimum platelet count during heparin therapy [G/L], median [IQR] | 45 [33, 71] | 44 [33, 71] | 50 [26, 65] | 0.885 | |
| Maximum platelet count during heparin therapy [G/L], median [IQR] | 252 [176, 303] | 286 [192, 360] | 176 [124, 206] | 0.012 | |
| Date 1 | Date 2 | Patients with Excluded HIT (n = 40) | Patients with Confirmed HIT (n = 13) | ||
|---|---|---|---|---|---|
| Median [IQR] of | p-Value for Pairwise | Median [IQR] of | p-Value for Pairwise | ||
| Change in Platelet Count | Comparison | Change in Platelet Count | Comparison | ||
| Date 1 vs. Date 2 | Date 1 vs. Date 2 | ||||
| Admission | Beginning of VA-ECMO therapy | 0.0 [−65.8, 0.0] | 0.13 | 0.0 [−8.0, 0.0] | 0.681 |
| Admission | Day 3 of VA-ECMO | −120.0 [−174.5, −51.0] | <0.001 | −76.0 [−135.0, −30.0] | 0.008 |
| Admission | Day 7 of VA-ECMO | −103.5 [−164.2, −50.0] | <0.001 | −40.0 [−108.0, −26.0] | 0.04 |
| Admission | Day 14 of VA-ECMO | 55.0 [−50.0, 148.0] | 0.246 | 37.5 [−63.2, 132.0] | 0.264 |
| Characteristics | Overall | Patients with HIT Suspicion | Patients with Excluded HIT | Patients with Confirmed HIT | p-Value (III vs. IV) | |
|---|---|---|---|---|---|---|
| (n = 373) (I) | (n = 53) (II) | (n = 40) (III) | (n = 13) (IV) | |||
| Total ICU length of stay [d], median [IQR] | 8.9 [3.0, 16.0] | 14.6 [8.2, 22.3] | 10.5 [7.1, 19.4] | 20.6 [16.7, 30.8] | 0.018 | |
| Total hospital length of stay [d], median [IQR] | 13.8 [4.7, 25.3] | 26.3 [13.3, 49.3] | 26.1 [9.5, 48.3] | 28 [20.8, 51.8] | 0.468 | |
| Hospital mortality, n (%) | 213 (57) | 22 (42) | 17 (43) | 5 (38) | >0.999 | |
| 1-month mortality, n (%) | 203 (54) | 19 (36) | 14 (35) | 5 (38) | >0.999 | |
| 3-month mortality, n (%) | 222 (60) | 23 (43) | 17 (43) | 6 (46) | >0.999 | |
| 1-year mortality, n (%) | 239 (64) | 27 (51) | 21 (53) | 6 (46) | 0.938 | |
| Cerebral performance category of survivors at hospital discharge | CPC1, n (%) | 19 (12) | 5 (16) | 4 (17) | 1 (13) | >0.999 |
| CPC2, n (%) | 37 (23) | 4 (13) | 3 (13) | 1 (13) | >0.999 | |
| CPC3, n (%) | 75 (47) | 13 (25) | 10 (43) | 3 (38) | >0.999 | |
| CPC4, n (%) | 29 (18) | 9 (29) | 6 (26) | 3 (38) | 0.672 | |
| Characteristics | Overall | Patients with HIT Suspicion | Patients With Excluded HIT | Patients with Confirmed HIT | p-Value (III vs. IV) | |
|---|---|---|---|---|---|---|
| (n = 373) (I) | (n = 53) (II) | (n = 40) (III) | (n = 13) (IV) | |||
| Adverse Events during VA-ECMO Therapy | ||||||
| Hemorrhage | BARC 3, n (%) | 126 (34) | 15 (28) | 11 (28) | 4 (31) | >0.999 |
| BARC 4, n (%) | 1 (0.3) | 0 (0) | 0 (0) | 0 (0) | 1 | |
| BARC 5, n (%) | 13 (3) | 1 (2) | 1 (3) | 0 (0) | >0.999 | |
| Stroke, n (%) | 16 (4) | 2 (4) | 1 (3) | 1 (8) | 0.434 | |
| Hemolysis, n (%) | 48 (13) | 11 (21) | 8 (20) | 3 (23) | >0.999 | |
| Myocardial infarction, n (%) | 9 (2) | 0 (0) | 0 (0) | 0 (0) | 1 | |
| Arterial thrombosis, n (%) | 21 (6) | 6 (11) | 4 (10) | 2 (15) | 0.627 | |
| Venous thrombosis, n (%) | 14 (4) | 5 (9) | 3 (8) | 2 (15) | 0.586 | |
| Device-related peripheral ischemic complications, n (%) | 18 (5) | 0 (0) | 0 (0) | 0 (0) | 1 | |
| Device malfunction, n (%) | 5 (1) | 0 (0) | 0 (0) | 0 (0) | 1 | |
| VA-ECMO oxygenator exchange, n (%) | 7 (2) | 2 (4) | 2 (5) | 0 (0) | >0.999 | |
| VA-ECMO circuit exchange, n (%) | 13 (3) | 6 (11) | 5 (13) | 1 (8) | >0.999 | |
| VA-ECMO oxygenator and circuit exchange, n (%) | 4 (1) | 1 (2) | 1 (3) | 0 (0) | >0.999 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lüsebrink, E.; Scherer, C.; Binzenhöfer, L.; Hoffmann, S.; Höpler, J.; Kellnar, A.; Thienel, M.; Joskowiak, D.; Peterß, S.; Petzold, T.; et al. Heparin-Induced Thrombocytopenia in Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation. J. Clin. Med. 2023, 12, 362. https://doi.org/10.3390/jcm12010362
Lüsebrink E, Scherer C, Binzenhöfer L, Hoffmann S, Höpler J, Kellnar A, Thienel M, Joskowiak D, Peterß S, Petzold T, et al. Heparin-Induced Thrombocytopenia in Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation. Journal of Clinical Medicine. 2023; 12(1):362. https://doi.org/10.3390/jcm12010362
Chicago/Turabian StyleLüsebrink, Enzo, Clemens Scherer, Leonhard Binzenhöfer, Sabine Hoffmann, Julia Höpler, Antonia Kellnar, Manuela Thienel, Dominik Joskowiak, Sven Peterß, Tobias Petzold, and et al. 2023. "Heparin-Induced Thrombocytopenia in Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation" Journal of Clinical Medicine 12, no. 1: 362. https://doi.org/10.3390/jcm12010362
APA StyleLüsebrink, E., Scherer, C., Binzenhöfer, L., Hoffmann, S., Höpler, J., Kellnar, A., Thienel, M., Joskowiak, D., Peterß, S., Petzold, T., Deseive, S., Hein, R., Brunner, S., Kääb, S., Braun, D., Theiss, H., Hausleiter, J., Hagl, C., Massberg, S., & Orban, M. (2023). Heparin-Induced Thrombocytopenia in Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation. Journal of Clinical Medicine, 12(1), 362. https://doi.org/10.3390/jcm12010362







