Nomograms Combining PHI and PI-RADS in Detecting Prostate Cancer: A Multicenter Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection and Clinical Variables
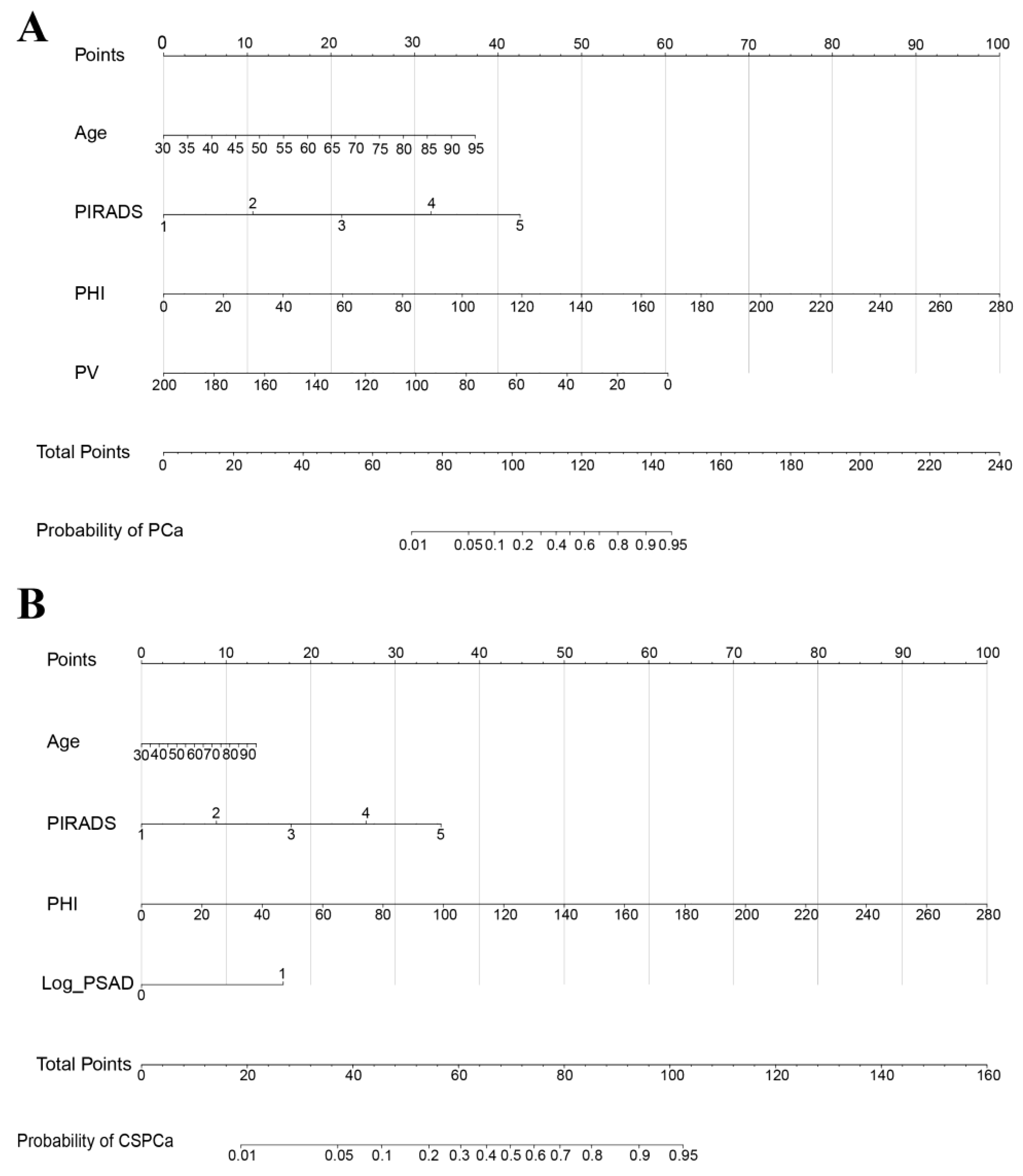
2.3. Construction of the PCa and CSPCa Nomograms
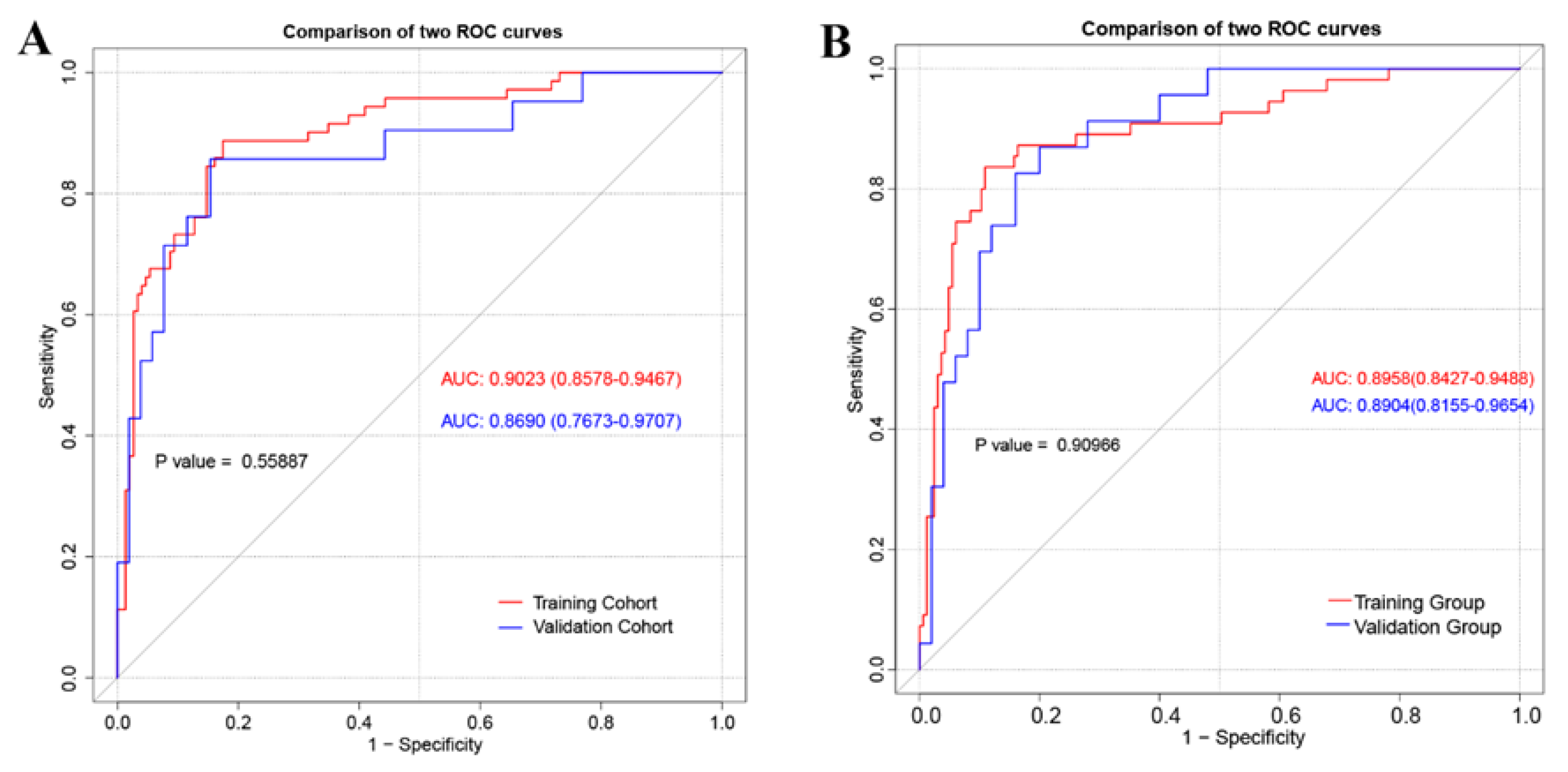
2.4. Nomogram Performance
2.5. Statistical Analysis
3. Results
3.1. Univariable and Multivariable Regression Analyses in Predicting PCa and CSPCa
3.2. The Construction and Performance of Nomogram
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, P.C. Prostate cancer screening with prostate-specific antigen: Where are we going? Cancer 2018, 124, 453–455. [Google Scholar] [CrossRef]
- Catalona, W.J.; Partin, A.W.; Sanda, M.G.; Wei, J.T.; Klee, G.G.; Bangma, C.H.; Slawin, K.M.; Marks, L.S.; Loeb, S.; Broyles, D.L.; et al. A Multicenter Study of [-2]Pro-Prostate Specific Antigen Combined With Prostate Specific Antigen and Free Prostate Specific Antigen for Prostate Cancer Detection in the 2.0 to 10.0 ng/mL Prostate Specific Antigen Range. J. Urol. 2011, 185, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Vertosick, E.A.; Sjoberg, D.D. Value of a Statistical Model Based on Four Kallikrein Markers in Blood, Commercially Available as 4Kscore, in All Reasonable Prostate Biopsy Subgroups. Eur. Urol. 2018, 74, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.H.; Nason, G.; Ajib, K.; Woon, D.T.S.; Herrera-Caceres, J.; Alhunaidi, O.; Perlis, N. Smarter screening for prostate cancer. World J. Urol. 2019, 37, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Mendhiratta, N.; Rosenkrantz, A.B.; Meng, X.; Wysock, J.S.; Fenstermaker, M.; Huang, R.; Deng, F.M.; Melamed, J.; Zhou, M.; Huang, W.C.; et al. Magnetic Resonance Imag-ing-Ultrasound Fusion Targeted Prostate Biopsy in a Consecutive Cohort of Men with No Previous Biopsy: Reduction of Over Detection through Improved Risk Stratification. J. Urol. 2015, 194, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Qi, W.; Cui, J.; Zhong, M.; Lv, G.; Qu, S.; Chen, S.; Li, R.; Shi, B.; Zhu, Y. Construction and Comparison of Different Models in Detecting Prostate Cancer and Clinically Significant Prostate Cancer. Front. Oncol. 2022, 12, 911725. [Google Scholar] [CrossRef]
- Chiu, P.K.-F.; Ng, C.-F.; Semjonow, A.; Zhu, Y.; Vincendeau, S.; Houlgatte, A.; Lazzeri, M.; Guazzoni, G.; Stephan, C.; Haese, A.; et al. A Multicentre Evaluation of the Role of the Prostate Health Index (PHI) in Regions with Differing Prevalence of Prostate Cancer: Adjustment of PHI Reference Ranges is Needed for European and Asian Settings. Eur. Urol. 2019, 75, 558–561. [Google Scholar] [CrossRef]
- Mikolajczyk, S.D.; Grauer, L.S.; Millar, L.S.; Hill, T.M.; Kumar, A.; Rittenhouse, H.G.; Wolfert, R.L.; Saedi, M.S. A precursor form of PSA (pPSA) is a com-ponent of the free PSA in prostate cancer serum. Urology 1997, 50, 710–714. [Google Scholar] [CrossRef]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef]
- Nilsson, O.; Peter, A.T.; Andersson, I.; Nilsson, K.; Grundström, B.; Karlsson, B. Antigenic determinants of prostate-specific antigen (PSA) and development of assays specific for different forms of PSA. Br. J. Cancer 1997, 75, 789–797. [Google Scholar] [CrossRef][Green Version]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; Margolis, D.; Schnall, M.D.; Shtern, F.; Tempany, C.M.; et al. PI-RADS Prostate Imaging—Reporting and Data System: 2015, Version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Weinreb, J.; Rosenkrantz, A.B.; Villeirs, G.; Turkbey, B.; Barentsz, J. Prostate Imaging-Reporting and Data System Steering Committee: PI-RADS v2 Status Update and Future Directions. Eur. Urol. 2019, 75, 385–396. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- Matoso, A.; Epstein, J. Defining clinically significant prostate cancer on the basis of pathological findings. Histopathology 2019, 74, 135–145. [Google Scholar] [CrossRef]
- Obuchowski, N.A.; Bullen, J.A. Receiver operating characteristic (ROC) curves: Review of methods with applications in diagnostic medicine. Phys. Med. Biol. 2018, 63, 07TR01. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, J.P.; Donchin, E. Resampling (bootstrapping) the mean: A definite do. Psychophysiology 2015, 52, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Noureldin, M.; Eldred-Evans, D.; Khoo, C.C.; Winkler, M.; Sokhi, H.; Tam, H.; Ahmed, H.U. Review article: MRI-targeted biopsies for prostate cancer diagnosis and management. World J. Urol. 2021, 39, 57–63. [Google Scholar] [CrossRef]
- Hsieh, P.-F.; Li, W.-J.; Lin, W.-C.; Chang, H.; Chang, C.-H.; Huang, C.-P.; Yang, C.-R.; Chen, W.-C.; Chang, Y.-H.; Wu, H.-C. Combining prostate health index and multiparametric magnetic resonance imaging in the diagnosis of clinically significant prostate cancer in an Asian population. World J. Urol. 2020, 38, 1207–1214. [Google Scholar] [CrossRef]
- Foj, L.; Filella, X. Development and internal validation of a novel PHI-nomogram to identify aggressive prostate cancer. Clin. Chim. Acta 2020, 501, 174–178. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; OuYang, J. A novel nomogram combined PIRADS v2 and neutrophil-to-lymphocyte ratio to predict the risk of clinically significant prostate cancer in men with PSA < 10 ng/mL at first biopsy. Urol. Oncol. Semin. Orig. Investig. 2020, 38, 401–409. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Liu, F.; Wang, W.J.; Liu, X.; Sun, L.J.; Zhu, Y.; Ye, D.W.; Zhang, G.M. Development and validation of a nomogram including lympho-cyte-to-monocyte ratio for initial prostate biopsy: A double-center retrospective study. Asian J. Androl. 2021, 23, 41–46. [Google Scholar]
- van der Leest, M.; Cornel, E.; Israël, B.; Hendriks, R.; Padhani, A.R.; Hoogenboom, M.; Zamecnik, P.; Bakker, D.; Setiasti, A.Y.; Veltman, J.; et al. Head-to-head Comparison of Transrectal Ultrasound-guided Prostate Biopsy Versus Multiparametric Prostate Resonance Imaging with Subsequent Magnetic Reso-nance-guided Biopsy in Biopsy-naïve Men with Elevated Prostate-specific Antigen: A Large Prospective Multicenter Clinical Study. Eur. Urol. 2019, 75, 570–578. [Google Scholar] [PubMed]
- Grey, A.D.; Chana, M.S.; Popert, R.; Wolfe, K.; Liyanage, S.H.; Acher, P.L. Diagnostic accuracy of magnetic resonance imaging (MRI) prostate imaging reporting and data system (PI-RADS) scoring in a transperineal prostate biopsy setting. Br. J. Urol. 2015, 115, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Drost, F.-J.H.; Osses, D.F.; Nieboer, D.; Steyerberg, E.W.; Bangma, C.H.; Roobol, M.J.; Schoots, I.G. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst. Rev. 2019, 4, CD012663. [Google Scholar] [CrossRef] [PubMed]
- Barentsz, J.O.; Richenberg, J.; Clements, R.; Choyke, P.; Verma, S.; Villeirs, G.; Rouviere, O.; Logager, V.; Fütterer, J.J. ESUR prostate MR guidelines 2012. Eur. Radiol. 2012, 22, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Berry, B.; Parry, M.G.; Sujenthiran, A.; Nossiter, J.; Cowling, T.E.; Aggarwal, A.; Cathcart, P.; Payne, H.; van der Meulen, J.; Clarke, N.; et al. Comparison of complications after transrectal and transperineal prostate biopsy: A national population-based study. BJU Int. 2020, 126, 97–103. [Google Scholar] [CrossRef]
- Schwen, Z.R.; Mamawala, M.; Tosoian, J.J.; Druskin, S.C.; Ross, A.E.; Sokoll, L.J.; Epstein, J.I.; Carter, H.B.; Gorin, M.A.; Pavlovich, C.P. Prostate Health Index and multiparametric magnetic resonance imaging to predict prostate cancer grade reclassification in active surveillance. BJU Int. 2020, 126, 373–378. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Abdollah, F.; Sun, M.; Thuret, R.; Jeldres, C.; Tian, Z.; Briganti, A.; Shariat, S.F.; Perrotte, P.; Rigatti, P.; Montorsi, F.; et al. A Competing-Risks Analysis of Survival After Alternative Treatment Modalities for Prostate Cancer Patients: 1988–2006. Eur. Urol. 2011, 59, 88–95. [Google Scholar] [CrossRef]
- Bechis, S.K.; Carroll, P.R.; Cooperberg, M.R. Impact of age at diagnosis on prostate cancer treatment and survival. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Barisiene, M.; Bakavicius, A.; Stanciute, D.; Jurkeviciene, J.; Zelvys, A.; Ulys, A.; Vitkus, D.; Jankevicius, F. Prostate Health Index and Prostate Health Index Density as Diagnostic Tools for Improved Prostate Cancer Detection. BioMed Res. Int. 2020, 2020, 9872146. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.; Sanda, M.G.; Broyles, D.L.; Shin, S.S.; Bangma, C.H.; Wei, J.T.; Partin, A.W.; Klee, G.G.; Slawin, K.M.; Marks, L.S.; et al. The Prostate Health Index Selectively Identifies Clinically Significant Prostate Cancer. J. Urol. 2015, 193, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Fütterer, J.J.; Briganti, A.; De Visschere, P.; Emberton, M.; Giannarini, G.; Kirkham, A.; Taneja, S.S.; Thoeny, H.; Villeirs, G.; Villers, A. Can Clinically Significant Prostate Cancer Be Detected with Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. Eur. Urol. 2015, 68, 1045–1053. [Google Scholar] [CrossRef]

| Characteristics | All Cohort | PCa | CSPCa | ||||
|---|---|---|---|---|---|---|---|
| Training Cohort | Validation Cohort | p Value | Training Cohort | Validation Cohort | p Value | ||
| N (%) | 293 (100) | 220 (75.09) | 73 (24.91) | - | 220 (75.09) | 73 (24.91) | - |
| Age (years), median (IQR) | 66.00 (60.00–72.00) | 66.00 (59.25–72.75) | 66.00 (61.00–72.00) | 0.787 | 66.00 (60.00–72.00) | 66.00 (60.00–74.00) | 0.355 |
| TPSA (ng/mL), median (IQR) | 8.51 (5.97–12.11) | 8.59 (5.96–12.13) | 8.31 (5.95–11.93) | 0.956 | 8.51 (5.88–11.96) | 8.84 (6.11–12.99) | 0.478 |
| fPSA (ng/mL), median (IQR) | 1.13 (0.79–1.61) | 1.12 (0.79–1.60) | 1.14 (0.76–1.73) | 0.697 | 1.13 (0.75–1.60) | 1.21 (0.91–1.67) | 0.396 |
| P2PSA (ng/mL), median (IQR) | 17.89 (12.01–28.90) | 17.97 (12.95–22.35) | 17.89 (10.98–29.76) | 0.783 | 17.83 (11.80–28.62) | 20.54 (14.25–29.87) | 0.226 |
| PHI, median (IQR) | 47.15 (35.36–67.90) | 47.65 (35.18–67.91) | 46.28 (35.16–68.51) | 0.842 | 46.51 (25.09–69.60) | 48.80 (37.42–63.95) | 0.574 |
| f/T, median (IQR) | 0.14 (0.10–0.19) | 0.14 (0.09–0.19) | 0.13 (0.11–0.19) | 0.690 | 0.14 (0.09–0.20) | 0.14 (0.10–0.19) | 0.679 |
| %P2PSA, median (IQR) | 1.70 (1.27–2.27) | 1.71 (1.30–2.23) | 1.70 (1.15–2.28) | 0.955 | 1.69 (1.26–2.27) | 1.73 (1.33–2.28) | 0.582 |
| PV (mL), median (IQR) | 44.13 (28.84–65.54) | 44.45 (28.84–66.23) | 43.68 (28.53–63.86) | 0.820 | 42.46 (28.22–63.10) | 45.45 (31.43–67.27) | 0.381 |
| PI-RADS, n (%) | 0.963 | 0.359 | |||||
| ≤2 | 117 (39.9) | 85 (38.6) | 32 (43.8) | 91 (41.4) | 26 (35.6) | ||
| 3 | 92 (31.4) | 76 (34.5) | 16 (21.9) | 69 (31.4) | 23 (31.5) | ||
| ≥4 | 84 (28.7) | 59 (26.8) | 25 (34.2) | 60 (27.3) | 24 (32.9) | ||
| PSAD (ng/mL2), median (IQR) | 0.19 (0.13–0.31) | 0.18 (0.12–0.31) | 0.21 (0.13–0.33) | 0.589 | 0.18 (0.12–0.31) | 0.21 | 0.871 |
| Characteristics | Training Cohort | Validation Cohort | ||||
|---|---|---|---|---|---|---|
| Non-PCa | PCa | p Value | Non-PCa | PCa | p Value | |
| Age (years), median (IQR) | 66.00 (59.00–71.50) | 67.00 (64.00–74.00) | 0.094 | 63.50 (58.00–69.75) | 71.00 (66.00–77.00) | 0.001 |
| TPSA (ng/mL), median (IQR) | 8.38 (5.57–11.59) | 8.97 (6.38–13.58) | 0.106 | 7.98 (5.65–10.48) | 11.03 (7.14–13.69) | 0.023 |
| fPSA (ng/mL), median (IQR) | 1.23 (0.81–1.70) | 1.06 (0.79–1.39) | 0.196 | 1.11 (0.85–1.69) | 1.40 (0.74–1.78) | 0.622 |
| P2PSA (ng/mL), median (IQR) | 16.57 (10.93–23.62) | 25.57 (31.58–52.10) | 0.000 | 15.20 (9.65–24.64) | 30.44 (15.45–49.74) | 0.001 |
| PHI, median (IQR) | 42.07 (31.58–52.10) | 72.57 (51.67–110.15) | 0.000 | 40.74 (28.62–53.92) | 73.11 (59.45–98.91) | 0.000 |
| f/T, median (IQR) | 0.15 (0.10–0.21) | 0.12 (0.09–0.15) | 0.001 | 0.14 (0.11–0.20) | 0.13 (0.08–0.17) | 0.338 |
| %P2PSA, median (IQR) | 1.47 (1.03–1.85) | 2.44 (1.87–3.20) | 0.000 | 1.58 (1.05–2.03) | 2.30 (1.76–3.23) | 0.000 |
| PV (mL), median (IQR) | 50.39 (35.03–73.81) | 32.85 (23.06–47.67) | 0.000 | 50.16 (32.90–66.55) | 30.40 (20.14–48.64) | 0.010 |
| PI-RADS, n (%) | 0.000 | 0.002 | ||||
| ≤2 | 77 (51.7) | 8 (11.3) | 29 (55.8) | 3 (14.3) | ||
| 3 | 51 (34.2) | 25 (35.2) | 11 (21.2) | 5 (23.8) | ||
| ≥4 | 21 (14.1) | 38 (53.5) | 12 (23.1) | 13 (61.9) | ||
| PSAD (ng/mL2), median (IQR) | 0.16 (0.11–0.24) | 0.25 (0.18–0.47) | 0.000 | 0.17 (0.11–0.25) | 0.36 (0.23–0.43) | 0.000 |
| Characteristics | Training Cohort | Validation Cohort | ||||
|---|---|---|---|---|---|---|
| Non-PCa | PCa | p Value | Non-PCa | PCa | p Value | |
| Age (years), median (IQR) | 66.00 (59.00–71.50) | 67.00 (64.00–74.00) | 0.094 | 63.50 (58.00–69.75) | 71.00 (66.00–77.00) | 0.001 |
| TPSA (ng/mL), median (IQR) | 8.38 (5.57–11.59) | 8.97 (6.38–13.58) | 0.106 | 7.98 (5.65–10.48) | 11.03 (7.14–13.69) | 0.023 |
| fPSA (ng/mL), median (IQR) | 1.23 (0.81–1.70) | 1.06 (0.79–1.39) | 0.196 | 1.11 (0.85–1.69) | 1.40 (0.74–1.78) | 0.622 |
| P2PSA (ng/mL), median (IQR) | 16.57 (10.93–23.62) | 25.57 (31.58–52.10) | 0.000 | 15.20 (9.65–24.64) | 30.44 (15.45–49.74) | 0.001 |
| PHI, median (IQR) | 42.07 (31.58–52.10) | 72.57 (51.67–110.15) | 0.000 | 40.74 (28.62–53.92) | 73.11 (59.45–98.91) | 0.000 |
| f/T, median (IQR) | 0.15 (0.10–0.21) | 0.12 (0.09–0.15) | 0.001 | 0.14 (0.11–0.20) | 0.13 (0.08–0.17) | 0.338 |
| %P2PSA, median (IQR) | 1.47 (1.03–1.85) | 2.44 (1.87–3.20) | 0.000 | 1.58 (1.05–2.03) | 2.30 (1.76–3.23) | 0.000 |
| PV (mL), median (IQR) | 50.39 (35.03–73.81) | 32.85 (23.06–47.67) | 0.000 | 50.16 (32.90–66.55) | 30.40 (20.14–48.64) | 0.010 |
| PI-RADS, n (%) | 0.000 | 0.002 | ||||
| ≤2 | 77 (51.7) | 8 (11.3) | 29 (55.8) | 3 (14.3) | ||
| 3 | 51 (34.2) | 25 (35.2) | 11 (21.2) | 5 (23.8) | ||
| ≥4 | 21 (14.1) | 38 (53.5) | 12 (23.1) | 13 (61.9) | ||
| PSAD (ng/mL2), median (IQR) | 0.16 (0.11–0.24) | 0.25 (0.18–0.47) | 0.000 | 0.17 (0.11–0.25) | 0.36 (0.23–0.43) | 0.000 |
| Variable | PCa | CSPCa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis | Univariable Analysis | Multivariable Analysis | |||||||||
| OR | 95% CI | p Value | OR | 95% CI | p Value | OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age | 1.028 | 0.996–1.061 | 0.084 | 0.970 | 0.952–0.988 | 0.014 | 1.038 | 1.002–1.076 | 0.040 | |||
| TPSA | 1.057 | 0.993–1.124 | 0.081 | 1.097 | 1.024–1.176 | 0.008 | ||||||
| fPSA | 0.736 | 0.499–1.085 | 0.122 | 0.794 | 0.527–1.196 | 0.269 | ||||||
| P2PSA | 1.045 | 1.025–1.066 | 0.000 | 1.047 | 1.026–1.068 | 0.000 | ||||||
| PHI | 1.044 | 1.030–1.059 | 0.000 | 1.037 | 1.022–1.052 | 0.000 | 1.046 | 1.032–1.061 | 0.000 | 1.033 | 1.020–1.045 | 0.000 |
| f/T | 0.002 | 0.000–0.196 | 0.007 | 0.001 | 0.000–0.078 | 0.003 | ||||||
| %P2PSA | 3.652 | 2.389–5.583 | 0.000 | 3.004 | 2.058–4.383 | 0.000 | ||||||
| PV | 0.970 | 0.956–0.984 | 0.000 | 0.970 | 0.952–0.988 | 0.002 | 0.964 | 0.947–0.981 | 0.000 | |||
| PI-RADS | 3.385 | 2.319–4.941 | 0.000 | 2.936 | 1.873–4.601 | 0.000 | 2.805 | 1.970–3.994 | 0.000 | 2.458 | 1.709–3.535 | 0.000 |
| Log (PSAD) | 22.300 | 6.809–73.042 | 0.000 | 72.227 | 16.817–310.206 | 0.000 | 9.758 | 2.458–39.220 | 0.001 | |||
| Risk Factors | Coefficient | SE | OR (95% CI) | p |
|---|---|---|---|---|
| PCa | ||||
| Intercept | −8.508 | 1.754 | 0.000 | 0.000 |
| Age | 0.058 | 0.024 | 0.970 (0.952–0.988) | 0.014 |
| PHI | 0.036 | 0.008 | 1.037 (1.022–1.052) | 0.000 |
| PV | −0.030 | 0.010 | 0.970 (0.952–0.988) | 0.002 |
| PI-RADS | 1.077 | 0.229 | 2.936 (1.873–4.601) | 0.000 |
| CSPCa | ||||
| Intercept | −5.341 | 1.717 | 0.005 | 0.002 |
| Age | 0.020 | 0.023 | 1.020 (0.975–1.067) | 0.383 |
| PHI | 0.032 | 0.007 | 1.032 (1.018–1.047) | 0.000 |
| PI-RADS | 0.850 | 0.217 | 2.340 (1.529–3.580) | 0.000 |
| Log (PASD) | 2.515 | 0.835 | 12.370 (2.406–63.583) | 0.003 |
| Sensitivity | Specificity | PPV | NPV | % Biopsy Avoided | % PCa Missed | %CSPCa Missed | |
|---|---|---|---|---|---|---|---|
| PHI ≥ 35 | 95.77 | 34.90 | 41.21 | 94.55 | 23.64 | 1.36 | 1.36 |
| PHI ≥ 40 | 90.14 | 45.64 | 44.14 | 90.67 | 30.91 | 3.18 | 1.82 |
| PHI ≥ 45 | 81.69 | 59.73 | 49.15 | 87.25 | 40.45 | 5.91 | 3.18 |
| PHI ≥ 50 | 76.06 | 71.81 | 56.25 | 86.29 | 48.64 | 7.73 | 4.09 |
| PHI ≥ 55 | 74.65 | 79.87 | 63.86 | 86.86 | 54.09 | 8.18 | 4.55 |
| a NP ≥ 27% | 88.73 | 82.55 | 70.79 | 93.89 | 55.91 | 3.67 | 1.82 |
| b NP ≥ 31% | 83.64 | 89.09 | 71.88 | 94.23 | 63.64 | 7.27 | 4.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Fu, Q.; Shao, Z.; Zhang, K.; Qi, W.; Geng, S.; Wang, W.; Cui, J.; Jiang, X.; Li, R.; et al. Nomograms Combining PHI and PI-RADS in Detecting Prostate Cancer: A Multicenter Prospective Study. J. Clin. Med. 2023, 12, 339. https://doi.org/10.3390/jcm12010339
Zhou Y, Fu Q, Shao Z, Zhang K, Qi W, Geng S, Wang W, Cui J, Jiang X, Li R, et al. Nomograms Combining PHI and PI-RADS in Detecting Prostate Cancer: A Multicenter Prospective Study. Journal of Clinical Medicine. 2023; 12(1):339. https://doi.org/10.3390/jcm12010339
Chicago/Turabian StyleZhou, Yongheng, Qiang Fu, Zhiqiang Shao, Keqin Zhang, Wenqiang Qi, Shangzhen Geng, Wenfu Wang, Jianfeng Cui, Xin Jiang, Rongyang Li, and et al. 2023. "Nomograms Combining PHI and PI-RADS in Detecting Prostate Cancer: A Multicenter Prospective Study" Journal of Clinical Medicine 12, no. 1: 339. https://doi.org/10.3390/jcm12010339
APA StyleZhou, Y., Fu, Q., Shao, Z., Zhang, K., Qi, W., Geng, S., Wang, W., Cui, J., Jiang, X., Li, R., Zhu, Y., Chen, S., & Shi, B. (2023). Nomograms Combining PHI and PI-RADS in Detecting Prostate Cancer: A Multicenter Prospective Study. Journal of Clinical Medicine, 12(1), 339. https://doi.org/10.3390/jcm12010339





