Improvement in Glucocorticoid-Induced Osteoporosis on Switching from Bisphosphonates to Once-Weekly Teriparatide: A Randomized Open-Label Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Intervention
2.3. Efficacy Outcomes
2.4. Analytical Populations
2.5. Statistical Analysis
3. Results
3.1. Study Participants
3.2. Primary Endpoints
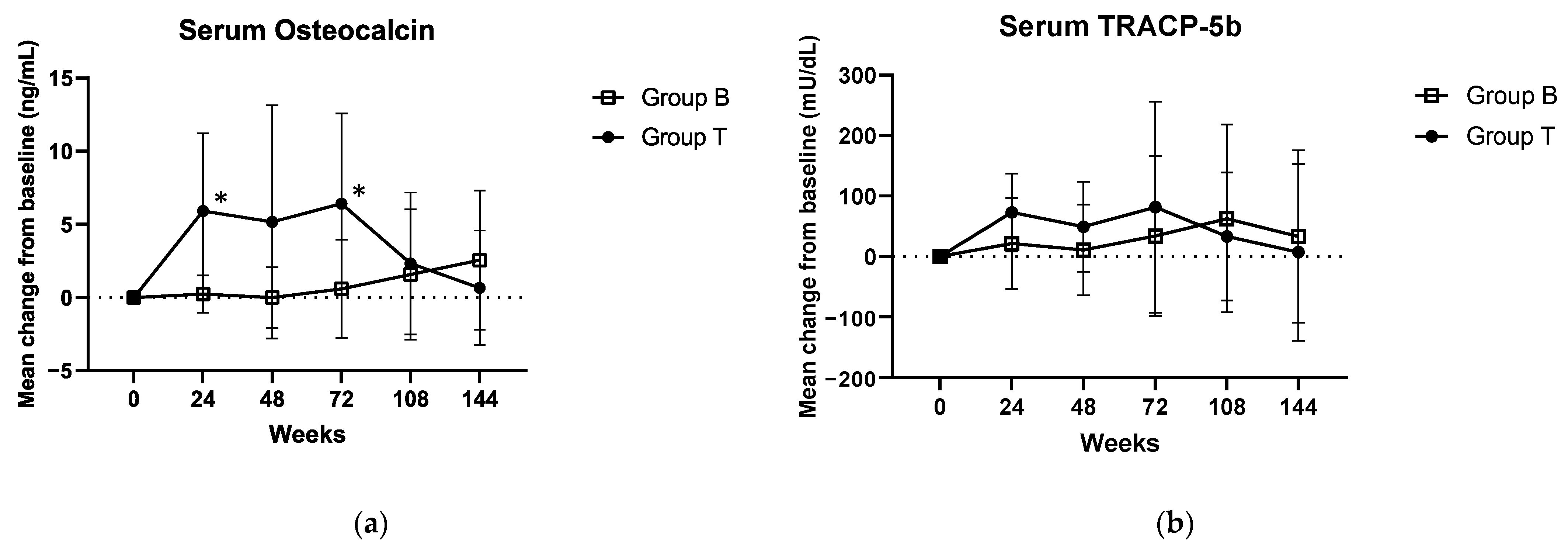
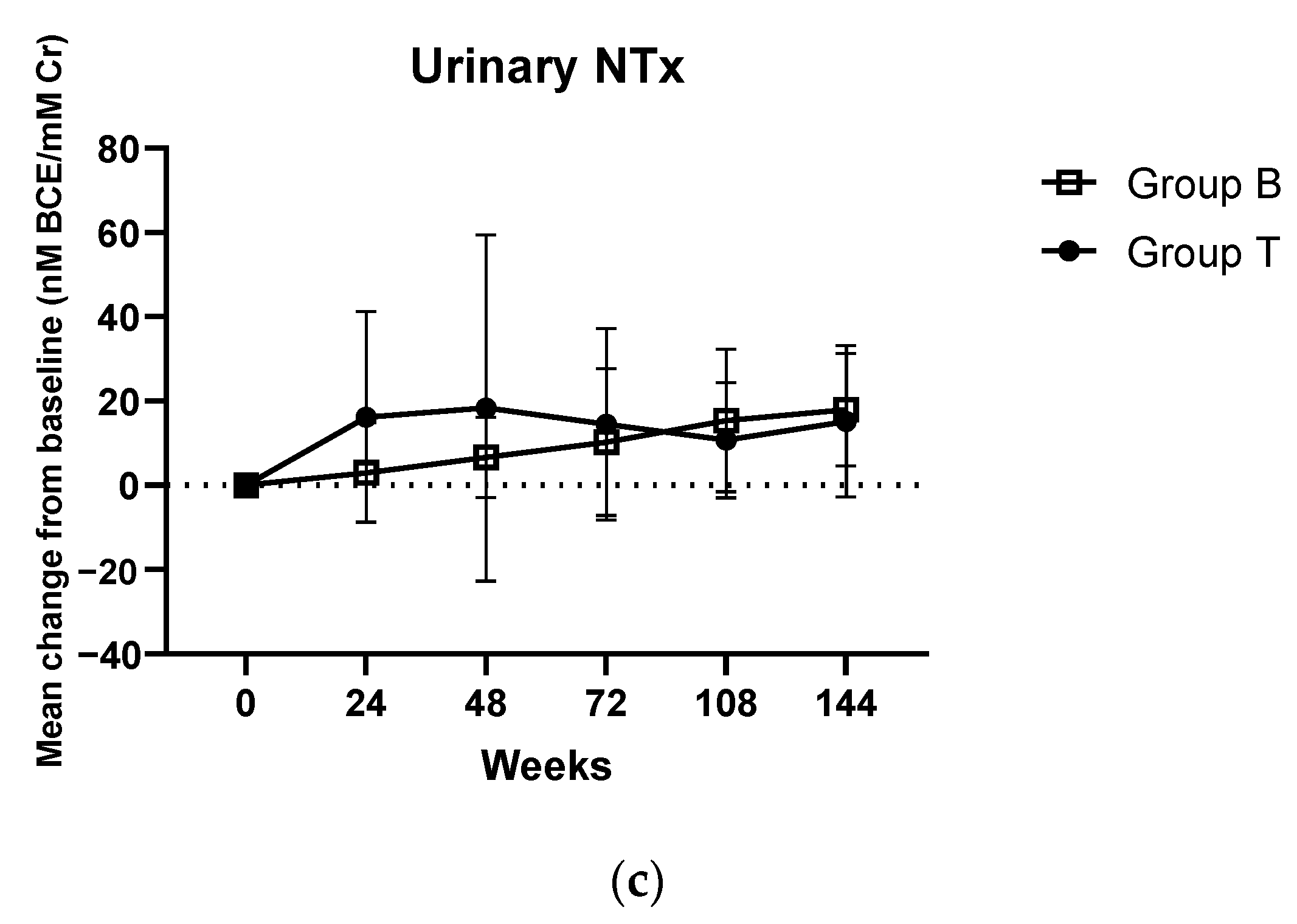
3.3. Secondary Endpoints
3.4. Adverse Events
3.5. New Fractures
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chotiyarnwong, P.; McCloskey, E.V. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat. Rev. Endocrinol. 2020, 15, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Bénard-Laribière, A.; Pariente, A.; Pambrun, E.; Bégaud, B.; Fardet, L.; Noize, P. Prevalence and prescription patterns of oral glucocorticoids in adults: A retrospective cross-sectional and cohort analysis in France. BMJ Open 2017, 7, e015905. [Google Scholar] [CrossRef] [PubMed]
- Laugesen, K.; Jørgensen, J.O.; Petersen, I.; Sørensen, H.T. Fifteen-year nationwide trends in systemic glucocorticoid drug use in Denmark. Eur. J. Endocrinol. 2019, 181, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Silverman, S.; Curtis, J.; Saag, K.; Flahive, J.; Adachi, J.; Anderson, F.; Chapurlat, R.; Cooper, C.; Diez-Perez, A.; Greenspan, S.; et al. International management of bone health in glucocorticoid-exposed individuals in the observational GLOW study. Osteopor. Int. 2015, 26, 419–420. [Google Scholar] [CrossRef][Green Version]
- Overman, R.A.; Yeh, J.Y.; Deal, C.L. Prevalence of oral glucocorticoid usage in the United States: A general population perspective. Arthritis Care Res. 2013, 65, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.S.; Zhou, H.; Seibel, M.J.; Cooper, M.S. Glucocorticoids and bone: Consequences of endogenous and exogenous excess and replacement therapy. Endocr. Rev. 2018, 39, 519–548. [Google Scholar] [CrossRef]
- Briot, K.; Roux, C. Glucocorticoid-induced osteoporosis. RMD Open 2015, 1, e000014. [Google Scholar] [CrossRef]
- Nam, B.; Sung, Y.K.; Choi, C.B.; Kim, T.H.; Jun, J.B.; Bae, S.C.; Yoo, D.H.; Cho, S.K. Fracture Risk and its Prevention Patterns in Korean Patients with Polymyalgia Rheumatica: A Retrospective Cohort Study. J. Korean Med. Sci. 2021, 36, e263. [Google Scholar] [CrossRef]
- Oh, T.K.; Song, I. Trends in long-term glucocorticoid use and risk of 5-year mortality: A historical cohort study in South Korea. Endocrine 2020, 69, 634–641. [Google Scholar] [CrossRef]
- Kim, D.; Cho, S.K.; Park, B.; Jang, E.J.; Bae, S.C.; Sung, Y.K. Glucocorticoids are associated with an increased risk for vertebral fracture in patients with rheumatoid arthritis. J. Rheumatol. 2018, 45, 612–620. [Google Scholar] [CrossRef]
- Compston, J. Glucocorticoid-induced osteoporosis: An update. Endocrine 2018, 61, 7–16. [Google Scholar] [CrossRef]
- Hodsman, A.B.; Bauer, D.C.; Dempster, D.W.; Dian, L.; Hanley, D.A.; Harris, S.T.; Kendler, D.L.; McClung, M.R.; Miller, P.D.; Olszynski, W.P.; et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: A review of the evidence and suggested guidelines for its use. Endocr. Rev. 2005, 26, 688–703. [Google Scholar] [CrossRef]
- Lindsay, R.; Krege, J.H.; Marin, F.; Jin, L.; Stepan, J.J. Teriparatide for osteoporosis: Importance of the full course. Osteoporos. Int. 2016, 27, 2395–2410. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Sung, Y.K. Update on Glucocorticoid Induced Osteoporosis. Endocrinol. Metabol. 2021, 36, 536–543. [Google Scholar] [CrossRef]
- Saag, K.G.; Shane, E.; Boonen, S.; Marín, F.; Donley, D.W.; Taylor, K.A.; Dalsky, G.P.; Marcus, R. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N. Engl. J. Med. 2007, 357, 2028–2039. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Nawata, H.; Soen, S.; Fujiwara, S.; Nakayama, H.; Tanaka, I.; Ozono, K.; Sagawa, A.; Takayanagi, R.; Tanaka, H.; et al. Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research: 2014 update. J. Bone Miner. Metabol. 2014, 32, 337–350. [Google Scholar] [CrossRef]
- Buckley, L.; Guyatt, G.; Fink, H.A.; Cannon, M.; Grossman, J.; Hansen, K.E.; Humphrey, M.B.; Lane, N.E.; Magrey, M.; Miller, M.; et al. 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-Induced osteoporosis. Arthritis Rheumatol. 2017, 69, 1521–1537. [Google Scholar] [CrossRef]
- Kobza, A.O.; Herman, D.; Papaioannou, A.; Lau, A.N.; Adachi, J.D. Understanding and Managing Corticosteroid-Induced Osteoporosis. Open Access Rheumatol. 2021, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.; Nieves, J.; Formica, C.; Henneman, E.; Woelfert, L.; Shen, V.; Dempster, D.; Cosman, F. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 1997, 350, 550–555. [Google Scholar] [CrossRef]
- Nakamura, T.; Sugimoto, T.; Nakano, T.; Kishimoto, H.; Ito, M.; Fukunaga, M.; Hagino, H.; Sone, T.; Yoshikawa, H.; Nishizawa, Y.; et al. Randomized Teriparatide [human parathyroid hormone (PTH) 1–34] Once-Weekly Efficacy Research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J. Clin. Endocrinol. Metab. 2012, 97, 3097–3106. [Google Scholar] [CrossRef]
- Tanaka, I.; Tanaka, Y.; Soen, S.; Oshima, H. Efficacy of once-weekly teriparatide in patients with glucocorticoid-induced osteoporosis: The TOWER-GO study. J. Bone Miner. Metab. 2021, 39, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kuroda, T.; Sugimoto, T.; Nakamura, T.; Shiraki, M. Changes in bone mineral density, bone turnover markers, and vertebral fracture risk reduction with once weekly teriparatide. Curr. Med. Res. Opinion 2014, 30, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Shiraki, M.; Fukunaga, M.; Hagino, H.; Sone, T.; Nakano, T.; Kishimoto, H.; Ito, M.; Yoshikawa, H.; Kishida, M.; et al. 24-month open-label teriparatide once-weekly efficacy research trial examining bone mineral density in subjects with primary osteoporosis and high fracture risk. Adv. Ther. 2017, 34, 1727–1740. [Google Scholar] [CrossRef] [PubMed]
- Zebaze, R.; Takao-Kawabata, R.; Peng, Y.; Zadeh, A.G.; Hirano, K.; Yamane, H.; Takakura, A.; Isogai, Y.; Ishizuya, T.; Seeman, E. Increased cortical porosity is associated with daily, not weekly, administration of equivalent doses of teriparatide. Bone 2017, 99, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, T.; Nakamura, T.; Nakamura, Y.; Isogai, Y.; Shiraki, M. Profile of changes in bone turnover markers during once-weekly teriparatide administration for 24 weeks in postmenopausal women with osteoporosis. Osteoporos. Int. 2017, 25, 1173–1180. [Google Scholar] [CrossRef]
- Takeuchi, Y. How different is the once-weekly teriparatide from the daily one or the same? Osteoporos. Sarcopenia 2019, 5, 27–28. [Google Scholar] [CrossRef]
- Miyauchi, A.; Matsumoto, T.; Sugimoto, T.; Tsujimoto, M.; Warner, M.R.; Nakamura, T. Effects of teriparatide on bone mineral density and bone turnover markers in Japanese subjects with osteoporosis at high risk of fracture in a 24-month clinical study: 12-month, randomized, placebo-controlled, double-blind and 12-month open-label phases. Bone 2010, 47, 493–502. [Google Scholar] [CrossRef]
- Fujita, T.; Inoue, T.; Morii, H.; Morita, R.; Norimatsu, H.; Orimo, H.; Takahashi, H.E.; Yamamoto, K.; Fukunaga, M. Effect of an intermittent weekly dose of human parathyroid hormone (1-34) on osteoporosis: A randomized double-masked prospective study using three dose levels. Osteoporos. Int. 1999, 9, 296–306. [Google Scholar] [CrossRef]
- Napoli, N.; Langdahl, B.; Ljunggren, Ö.; Lespessailles, E.; Kapetanos, G.; Kocjan, T.; Nikolic, T.; Eiken, P.; Petto, H.; Moll, T.; et al. Effects of teriparatide in patients with osteoporosis in clinical practice: 42-month results during and after discontinuation of treatment from the European Extended Forsteo® Observational Study (ExFOS). Calcif. Tissue Int. 2018, 103, 359–371. [Google Scholar] [CrossRef]
- Obermayer-Pietsch, B.M.; Marin, F.; McCloskey, E.V.; Hadji, P.; Farrerons, J.; Boonen, S.; Audran, M.; Barker, C.; Anastasilakis, A.D.; Fraser, W.D.; et al. Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J. Bone Miner. Res. 2008, 23, 1591–1600. [Google Scholar] [CrossRef]
- Ettinger, B.; Martin, S.J.; Crans, G.; Pavo, I. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J. Bone Miner. Res. 2004, 19, 745–751. [Google Scholar] [CrossRef]
- Hagino, H.; Sugimoto, T.; Tanaka, S.; Sasaki, K.; Sone, T.; Nakamura, T.; Soen, S.; Mori, S. A randomized, controlled trial of once-weekly teriparatide injection versus alendronate in patients at high risk of osteoporotic fracture: Primary results of the Japanese Osteoporosis Intervention Trial-05. Osteoporos. Int. 2021, 32, 2301–2311, Erratum in Osteoporos. Int. 2021, 32, 2143. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Miller, P.D.; Delmas, P.D.; Misurski, D.A.; Krege, J.H. Change in lumbar spine BMD and vertebral fracture risk reduction in teriparatide-treated postmenopausal women with osteoporosis. J. Bone Miner. Res. 2006, 21, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Zanchetta, J.R.; Bogado, C.E.; Ferretti, J.L.; Wang, O.; Wilson, M.G.; Sato, M.; Gaich, G.A.; Dalsky, G.P.; Myers, S.L. Effects of teriparatide [recombinant human parathyroid hormone (1-34)] on cortical bone in postmenopausal women with osteoporosis. J. Bone Miner. Res. 2003, 18, 539–543. [Google Scholar] [CrossRef]
- Seno, T.; Yamamoto, A.; Kukida, Y.; Hirano, A.; Kida, T.; Nakabayashi, A.; Fujioka, K.; Nagahara, H.; Fujii, W.; Murakami, K.; et al. Once-weekly teriparatide improves glucocorticoid-induced osteoporosis in patients with inadequate response to bisphosphonates. SpringerPlus 2016, 5, 1056. [Google Scholar] [CrossRef][Green Version]
- Cosman, F.; Keaveny, T.M.; Kopperdahl, D.; Wermers, R.A.; Wan, X.; Krohn, K.D.; Krege, H. Hip and spine strength effects of adding versus switching to teriparatide in postmenopausal women with osteoporosis treated with prior alendronate or raloxifene. J. Bone Miner. Res. 2013, 28, 1328–1336. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, Y.; Nozaki, Y.; Okuda, S.; Sugiyama, M.; Kinoshita, K.; Funauchi, M.; Matsumura, I. Our-Year Teriparatide Followed by Denosumab vs. Continuous Denosumab in Glucocorticoid-Induced Osteoporosis Patients with Prior Bisphosphonate Treatment. Front. Endocrinol. 2017, 12, 753185. [Google Scholar] [CrossRef]
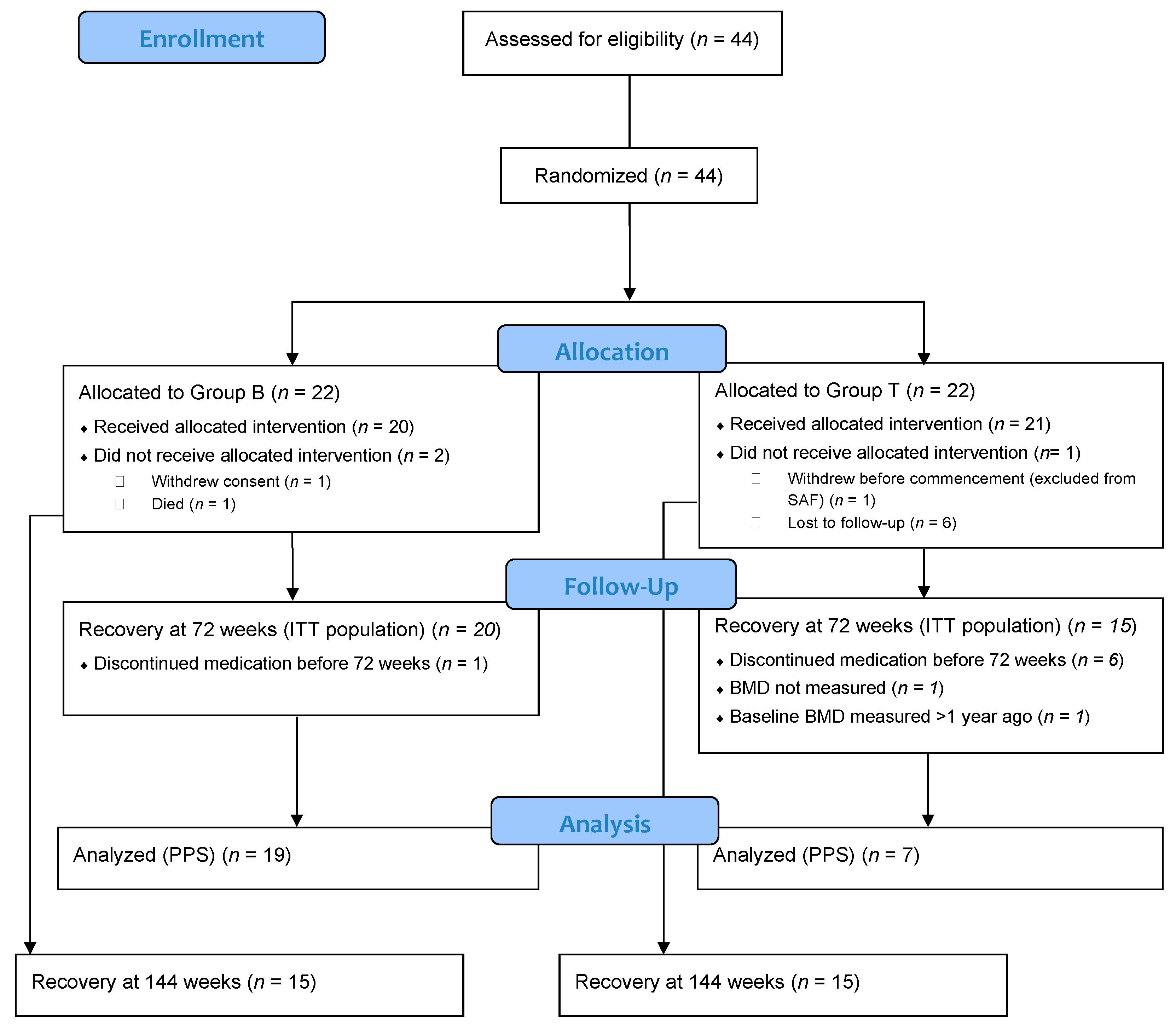

| All Patients (n = 43) | Group B (n = 22) | Group T (n = 21) | p Value | |
|---|---|---|---|---|
| Mean age, in years | 68.2 ± 12.6 (36–87) | 67.0 ± 13.7 (36–85) | 69.5 ± 11.5 (46–87) | 0.567 |
| Sex, M:F (with menstruation):F (after menopause) | 8:4:31 | 4:3:15 | 4:1:16 | 0.699 |
| Mean corticosteroid dose at enrollment (prednisolone equivalent), in mg/day | 6.5 ± 2.8 (5–20) | 5.9 ± 1.5 (5–10) | 7.1 ± 3.7 (5–20) | 0.550 |
| Mean duration of corticosteroid administration to date, in months | 90.0 ± 109.2 (7–516) | 95.9 ± 103.1 (7–384) | 83.8 ± 117.4 (9–516) | 0.402 |
| Mean duration of bisphosphonate administration to date, in months | 51.3 ± 40.7 (6–144) | 57.9 ± 43.2 (6–144) | 44.3 ± 37.7 (6–120) | 0.291 |
| Frequency of prior fragility fractures after previous steroid initiation | 22/43 (51.2%) | 11/22 (50%) | 11/22 (52.4%) | 1.000 |
| Mean lumbar spine (L1–L4) BMD, in g/cm2 | 0.827 ± 0.117 | 0.828 ± 0.117 | 0.826 ± 0.12 | 0.938 |
| Mean lumbar spine (L1–L4) BMD YAM, in % | 81.07 ± 11.436 | 81 ± 11.776 | 81.143 ± 11.359 | 0.890 |
| Proximal femur (total) BMD BMD, in g/cm2 | 0.676 ± 0.079 | 0.689 ± 0.085 | 0.661 ± 0.071 | 0.444 |
| Proximal femur (total) BMD YAM, in % | 76.674 ± 9.461 | 78.182 ± 10.595 | 75.095 ± 8.062 | 0.331 |
| Mean serum osteocalcin, in ng/mL | 8.124 ± 3.926 | 7.9 ± 3.144 | 8.37 ± 4.713 | 0.596 |
| Mean serum TRACP-5b, in mU/dL | 242.86 ± 106.902 | 246 ± 93.836 | 239.57 ± 121.373 | 0.985 |
| Mean urinary NTx (corrected for CRE), in mmolBCE/mmolCr | 18.98 ± 9.489 | 18.055 ± 8.382 | 19.95 ± 10.647 | 0.732 |
| Rate of Change | Week 72 | Week 144 | ||||
|---|---|---|---|---|---|---|
| Group B (n = 20) | Group T (n = 15) | p Value | Group B (n = 15) | Group T (n = 15) | p Value | |
| Lumbar spine (L1–L4) | ||||||
| BMD | 0.8 ± 5.3 | 4.7 ± 4.5 | 0.0143 | 2.5 ± 6.0 | 8.0 ± 10.9 | 0.126 |
| YAM | 0.5 ± 5.5 | 4.7 ± 4.5 | 0.0073 | 2.6 ± 6.4 | 8.3 ± 11.0 | 0.126 |
| Proximal femur (neck) | ||||||
| BMD | 1.3 ± 5.2 | 1.5 ± 5.1 | 0.8051 | 2.7 ± 6.3 | 3.1 ± 5.6 | 0.903 |
| YAM | 0.9 ± 5.5 | 1.5 ± 4.9 | 0.6988 | 2.6 ± 6.5 | 3.1 ± 5.6 | 0.927 |
| Proximal femur (trochanter) | ||||||
| BMD a | −0.9 ± 3.0 | 0.9 ± 4.9 | 0.3426 | −1.6 ± 4.2 | 3.2 ± 6.0 | 0.038 |
| YAM a | −1.1 ± 3.3 | 0.7 ± 5.2 | 0.394 | −1.0 ± 3.9 | 3.0 ± 6.2 | 0.055 |
| Proximal femur (total) | ||||||
| BMD | 0.5 ± 3.1 | 1.0 ± 4.2 | 0.5644 | 1.5 ± 5.6 | 2.4 ± 4.4 | 0.389 |
| YAM | 0.3 ± 3.4 | 0.8 ± 4.0 | 0.519 | 1.1 ± 5.7 | 2.3 ± 4.1 | 0.345 |
| Adverse Event | Total | Group B | Group T |
|---|---|---|---|
| At least one adverse event | 11 (26.2) | 2 (9.5) | 9 (42.9) |
| Nausea | 4 (9.5) | 0 (0.0) | 4 (19.0) |
| Headache | 3 (7.1) | 0 (0.0) | 3 (14.3) |
| Dizziness | 3 (7.1) | 0 (0.0) | 2 (9.5) |
| Fever | 2 (4.8) | 0 (0.0) | 2 (9.5) |
| Fatigue | 2 (4.8) | 0 (0.0) | 2 (9.5) |
| Back pain | 1 (2.4) | 0 (0.0) | 1 (4.8) |
| Loss of appetite | 1 (2.4) | 0 (0.0) | 1 (4.8) |
| Facial flushing | 1 (2.4) | 0 (0.0) | 1 (4.8) |
| Chest pain | 1 (2.4) | 0 (0.0) | 1 (4.8) |
| Ureteral stone | 1 (2.4) | 1 (4.8) | 0 (0.0) |
| Jaw pain | 1 (2.4) | 1 (4.8) | 0 (0.0) |
| Abdominal pain | 1 (2.4) | 0 (0.0) | 1 (4.8) |
| Hypercalcemia | 1 (2.4) | 0 (0.0) | 1 (4.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanki, T.; Kawazoe, M.; Uno, K.; Hirose, W.; Dobashi, H.; Kataoka, H.; Mimura, T.; Hagino, H.; Kono, H. Improvement in Glucocorticoid-Induced Osteoporosis on Switching from Bisphosphonates to Once-Weekly Teriparatide: A Randomized Open-Label Trial. J. Clin. Med. 2023, 12, 292. https://doi.org/10.3390/jcm12010292
Nanki T, Kawazoe M, Uno K, Hirose W, Dobashi H, Kataoka H, Mimura T, Hagino H, Kono H. Improvement in Glucocorticoid-Induced Osteoporosis on Switching from Bisphosphonates to Once-Weekly Teriparatide: A Randomized Open-Label Trial. Journal of Clinical Medicine. 2023; 12(1):292. https://doi.org/10.3390/jcm12010292
Chicago/Turabian StyleNanki, Toshihiro, Mai Kawazoe, Kiyoko Uno, Wataru Hirose, Hiroaki Dobashi, Hiroshi Kataoka, Toshihide Mimura, Hiroshi Hagino, and Hajime Kono. 2023. "Improvement in Glucocorticoid-Induced Osteoporosis on Switching from Bisphosphonates to Once-Weekly Teriparatide: A Randomized Open-Label Trial" Journal of Clinical Medicine 12, no. 1: 292. https://doi.org/10.3390/jcm12010292
APA StyleNanki, T., Kawazoe, M., Uno, K., Hirose, W., Dobashi, H., Kataoka, H., Mimura, T., Hagino, H., & Kono, H. (2023). Improvement in Glucocorticoid-Induced Osteoporosis on Switching from Bisphosphonates to Once-Weekly Teriparatide: A Randomized Open-Label Trial. Journal of Clinical Medicine, 12(1), 292. https://doi.org/10.3390/jcm12010292








