Abstract
This prospective, open-label clinical study was carried out to evaluate both the efficacy and safety of intramuscular paravertebral injections of an oxygen–ozone (O2–O3) mixture in patients with cervicobrachial pain. We enrolled 540 subjects affected by cervicobrachial pain referred to the Ozone Therapy Ambulatory at the Mater Domini Hospital of Catanzaro (Italy) and to the Center of Pain in Taurianova (Reggio Calabria, Italy). All the subjects (n = 540) completed the treatment and the follow-up visits. The subjects received a mean of 11 cervical intramuscular treatments with an O2–O3 mixture (5 mL) with an O3 concentration of 10 μg/mL bis a week. The improvement of pain was measured by a change in the mean of the Visual Analog Scale (VAS) score from baseline to the end of treatment and during follow-ups. Patient satisfaction was assessed at the end of treatment using the SF-36 Questionnaire. The development of adverse drug reactions was recorded. The mean (±standard deviation) VAS pain score at baseline, at the end of treatment, and during follow-ups showed a significant reduction in pain over time (p < 0.001). All the patients who were enrolled (n: 540) were pain-free after one year. According to the pain distribution, all subjects showed a significant reduction in pain over time in each group (p < 0.05). No significant differences were observed with respect to sex or age. No adverse events were observed during the study. In conclusion, we documented that the intramuscular injection of an O2–O3 mixture is an effective and safe treatment option for patients with cervicobrachial pain.
1. Introduction
Neck pain is a common and multifactorial disease, often associated with brachial pain [1]. Pharmacological and non-pharmacological therapies are commonly used to reduce pain and improve the quality of life [2]. Concerning new therapies, ozone (O3) could be an effective treatment option for musculoskeletal disorders and painful syndromes affecting muscles, tendons, and joints [3]. Ozone is a natural gas, first used in medicine for the antimicrobial treatment of wounds during the First World War, and nowadays, it is used in many areas of medicine [4]. Recently, several authors focused on the biological and therapeutic effects of oxygen–ozone (O2–O3) on inflammatory conditions and musculoskeletal diseases [5,6]. It has been reported that several proinflammatory mediators, e.g., prostaglandins (Pgs) and bradykinin, released by macrophages and leukocytes might be regulated by O2–O3 [7].
Moreover, O2–O3 decreases the production of reactive oxygen species (ROS) [8], and increases the release of nitric oxide (NO), reducing cell damage and improving tissue oxygenation [9,10].
In particular, the improvement of tissue oxygenation increases the use of glucose in cellular metabolism, protein metabolism, and erythrocyte activity. Taken together, it is easy to understand that O2–O3 has analgesic and anti-inflammatory activities also related to the increased stimulation of the secretion of both serotonin and endogenous opioids [11,12].
Moreover, other authors have suggested that after continuous administration O3 upregulates Nrf2, which reduces the intracellular signaling pathways of inflammation and increases antioxidant activity [13,14].
However, to date, the use of O2–O3 in the management of musculoskeletal or inflammatory pain has not been reported in the guidelines [15,16].
Therefore, in this study, we evaluated the efficacy and the safety of intramuscular paravertebral injections of an O2–O3 mixture in patients referred to medical care with cervicobrachial pain.
2. Materials and Methods
2.1. Study Design
We conducted an open-label prospective clinical study between 1 February 2021, and 31 August 2022, on patients with cervicobrachial pain referred to the Ambulatory of Pain Medicine of Mater Domini Hospital in Catanzaro (Calabria, Italy) and the Center of Pain Disease in Taurianova (Reggio Calabria, Italy). The study, approved by the Ethics Committee, was carried out according to the Good Clinical Practice guidelines and under the ethical principles of the Declaration of Helsinki. Before the beginning of the study, all participants signed a written informed consent form.
2.2. Experimental Protocol
At admission (T0), each patient, after medical history, underwent a physical examination, e.g., detection of vital parameters, thoracic and cardiac evaluation, abdominal palpation, and evaluation of osteotendinous and osteo-muscular activity. Particular attention was paid to the cervical brachial area to evaluate motility and the presence of points of greater pain. Radiological, ultrasound, and laboratory examinations were also employed. Pain severity was evaluated using the NRS (Numeric Rating Scale) score [17], while neuropathic pain was assessed using the DN4 (Neuropathic Pain 4 Questions) survey [18,19]. The SF-36 (Short Form Health Survey 36) survey was used to evaluate the quality of life [20].
Once the diagnosis of cervicobrachial pain was made or confirmed, patients were evaluated for appropriate prescriptions considering the Body Mass Index, age, gender, and comorbidities. Only patients whose drug treatments were not effective were scheduled for a cycle of O2–O3 cervical intramuscular infiltrative therapy, adding on to their current drug therapies. The follow-ups were performed after 8 (T1) and 12 (T2) sessions of topical O2–O3 therapy. Each patient was monitored using telemedicine, and patients with a new instance of brachial pain were immediately admitted to the hospital. We added only the data after 1 year because, until this period, we did not record any impairments due to symptoms. The development of adverse drug reactions (ADRs) during the treatment with O2–O3 was evaluated in agreement with our previous studies [21,22].
2.3. Local Infiltration
Subject to written informed consent, patients underwent cervical intramuscular injections of an O2–O3 mixture with an O3 concentration of 10 mcg/mL in the cervicobrachial tract (5 mL for each site of injection, usually in the paravertebral site in the region of the brachial plex and the median nerve, bilaterally, for a total of 30 mL) [23].
The injections were performed bilaterally to improve the oxygenation in the body region, bis a week for the first month and once a week for the second month. After the injections, the area was massaged with a topical compound to increase the activity of O2–O3 in the tissues. The gas mixture was obtained using an ozone generator (Ozo2 Alnitec, Cremosano (CR), Italy) connected to a pure O2 source.
2.4. Inclusion and Exclusion Criteria
We enrolled patients aged >18 years with clinical symptoms of cervicobrachial pain. Patients previously treated with ozone or patients with blood diseases (i.e., hemolytic anemia or glucose-6-phosphate dehydrogenase deficiency), pregnancy (a relative contraindication), uncontrolled hyperthyroidism, severe cardiovascular diseases, and heart failure were excluded.
2.5. Endpoints
The primary clinical endpoint was the statistically significant reduction in pain at T1 (end of the study) compared with T0 (admission) in terms of changes in NRS.
The secondary clinical endpoint was a significant change in the DN4 score and/or in the SF36 value for T2 vs. T1 vs. T0.
Finally, the primary safety endpoint was considered to be the absence of ADRs or drug–drug interactions (DDIs) related to ozone administration. The persistence of symptoms in T2 vs. T1 and/or the development of ADRs that could lead to the discontinuation of treatment were defined as clinical failures.
2.6. Statistical Analyses
Data are presented as mean ± standard deviation (SD). At baseline, the independent sample 2-tailed t-test was used to compare variables. For categorical parameters, the chi-square test was used. Changes from baseline to end of therapy were analyzed using ranked one-way analysis of variance (ANOVA) with a term for the treatment group. Both Kruskal–Wallis and sign tests were used for non-parametric variables. The Shapiro–Wilk test was used to evaluate the normality of distribution. To reduce the error related to false positives, our hypotheses were tested using Bonferroni-adjusted alpha levels of 0.025 per test. p-values < 0.05 were considered statistically significant. Statistical analysis was performed using SPSS 21.0 (International Business Machines Corporation, Armonk, NY, USA). To evaluate the effects of sex and age on both the efficacy and safety of the intramuscular administration of O2–O3, we performed a sub-analysis of the data.
3. Results
3.1. Patients’ Basic Characteristics
During the study, 828 patients were eligible. In total, 540 patients (65.2%) 34–87 years old (mean age 61.2 ± 11.7 years) were enrolled and completed the study (Figure 1). In particular, we enrolled 240 men and 300 women with a mean age of 62.6 ± 10.5 years and 59.2 ± 12.3 years, respectively (p = 0.2) The most common comorbidities were obesity and blood hypertension (37.8%), followed by type 2 diabetes (24.4%), with differences relating to both sex (Table 1) and age (Table 2).
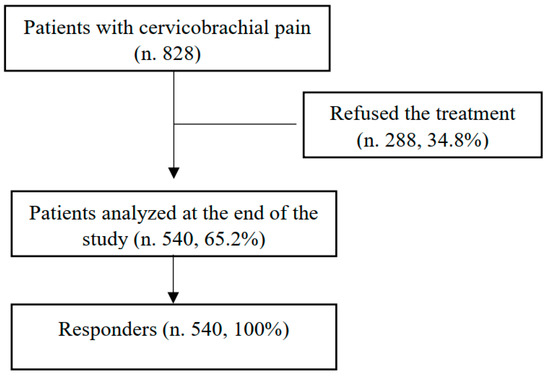
Figure 1.
Patient selection flow diagram.

Table 1.
Demographic characteristics of enrolled patients (n. 540) at the time of admission (T0). Data are expressed as absolute values with respect to the sex evaluation.

Table 2.
Demographic characteristics of enrolled patients (n. 540) evaluated considering the age (<65 and ≥65) at the time of admission (T0). Data are expressed as absolute values respect to the age evaluation.
3.2. Pain and Quality of Life
At admission (T0), an NRS questionnaire documented a severe pain level of 7.7 ± 1.3 (men: 7.75 ± 1.25; women: 7.72 ± 1.37; p = 0.939) without correlation with respect to age (men: r 0.079925; women: r −0.00887) or BMI (men: r 0.302446; women: r −0.10572); in 110 patients (20.4%), we documented neuropathic pain (DN-4: 5.8 ± 3.9) (Table 3); in 70 patients (13%), we documented nociplastic pain; and in 360 patients (66.7%), we documented a nociceptive pain. In all enrolled patients, an SF-36 questionnaire documented a low level of quality of life (Table 4). All patients documented a chronic use of at least one drug (range 1–5; mean 1.9 ± 1.06). The most common drugs used were acetaminophen and n-acetyl carnitine (Figure 2). All the patients received a mean of 11.7± 3 cycles of treatment (men: 12.5 ± 1.6; women: 11.1 ± 3.1; p = 0.107).

Table 3.
DN-4 scores. Data represent the mean ± standard deviation of the score achieved at admission (T0), at the end of the study (T1), and at the follow-up (T2).

Table 4.
SF-36 scores representing the percentage of the total possible scores achieved at admission (T0), at the end of the study (T1), and at the follow-up (T2).
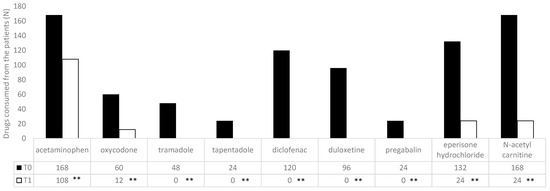
Figure 2.
Drugs most commonly used by the patients at the time of enrollment (T0) and after the treatment (T1). Data are expressed as the absolute value. ** p < 0.01, T1 vs. T0.
3.3. O2–O3 Treatment
As reported in Figure 1, all patients completed the treatment protocol. At T1, O2–O3 administration induced a statistically significant (p < 0.01) improvement in quality of life, recorded using an SF-36 score (Table 4), and this effect was maintained at the follow-up (T2) (Table 4). At T1, we recorded a statistically significant decrease (p < 0.01) in both the DN4 (T1: 4.04 ± 4.37) (Table 3) and NRS values (1.3 ± 1.5). An NRS questionnaire documented a mild pain of 1.3 ± 1.5 (men: 1 ± 1.26; women: 1.52 ± 1.64; p = 0.234543) without correlation with respect to age (men: r 0.302446; women: r 0.033841) or BMI (men: r −0.29639; women: r 0.122636). These effects were also maintained during the entire study until the follow-up (T2) (Table 3).
Patients subjected to the O2–O3 treatment significantly reduced (p < 0.01) the use of drugs considering both sex (men: 0.65 ± 0.81; women: 0.72 ± 0.74) (Table 5) and age (Figure 3).

Table 5.
Drug use at the time of admission (T0) and after the O2–O3 cycle of treatment (T1 and T2) in enrolled patients (N. 540). Data are expressed as the absolute value. Data are expressed as the mean ± standard deviation.
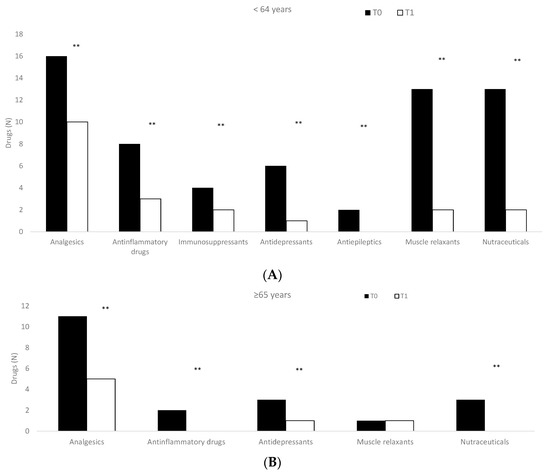
Figure 3.
Drugs used in enrolled patients considering age ((A): <64 years; (B): ≥65 years) at the time of enrollment (T0) and after the treatments (T1). ** p < 0.01, T0 vs. T1.
Finally, during the study, we did not record the development of serious adverse drug reactions able to stop the treatment or the use of other drugs.
4. Discussion
In this study, we evaluated the efficacy and safety of topical O2–O3 therapy in patients with cervicobrachial pain. Cervical pain is commonly due to cervical spondylolysis or osteoarthritis, a chronic degenerative condition inducing changes in bones, intervertebral discs, and/or joints connected to the neck.
We documented that O2–O3 therapy reduced pain and DN4, improving the quality of life in both sexes without differences considering age.
The clinical efficacy of O2–O3 therapy may be related to the mechanism of action of this compound. In fact, O2–O3 can elicit the upregulation of antioxidant enzymes such as superoxide dismutase (SOD), GSH-peroxidases (GSH-Px), GSH-reductase (GSH-Rd), and catalase (CAT), inducing an antioxidant response able to reduce the chronic oxidative stress [24].
Moreover, O2–O3 decreases the NF-kB pathway and inhibits the cascade of proinflammatory cytokines involved in the chronic inflammatory process and in pain [25].
Several studies highlight the efficacy of O2–O3 therapy in cervicobrachial pain; Alexandre et al. [26] assessed the efficacy of a single intradiscal injection of O2–O3 preceded and followed by 5 intramuscular paravertebral injections in the treatment of 252 patients with cervical disc herniation, documenting a significant decrease in pain in 79.3% and sensory dysfunction in 78.1%. In 61.9% of patients, the authors showed the complete regression of motor deficits.
Raeissadat et al. [27] documented that O2–O3 and lidocaine treatments showed superior, although not statistically different, results compared with a dry needling group. In a case series, Martinelli et al. [16] studied the safety and effectiveness of intramuscular–paravertebral injections of O2–O3 (O3 concentration of 16 mcg/mL once a week) in 168 patients affected by cervicobrachial pain, showing a significant pain reduction (p < 0.001) at follow-ups after 1, 2, 3, 4, and 5 years.
Beyaz et al. [28] investigated the 6-month efficacy and safety of intradiscal O2–O3 mixture therapy in 44 patients with cervical disc herniation and chronic neck pain. A 73.1% decrease in the average VAS score compared with the baseline values at the final follow-up was observed. In total, 88.6% of patients were satisfied, 9.1% were moderately satisfied, and 2.3% were poorly satisfied.
No data related to the effect of this treatment on drug use have been published. In the present study, we documented that all enrolled patients received a drug treatment to reduce pain. These treatments did not reduce the clinical symptoms. In contrast, intramuscular treatment with O2–O3 induced a time-dependent decrease in pain with an improvement in quality of life, as recorded using the SF-36 scale. Moreover, this treatment significantly reduced the use of drugs in both sexes and all ages. This point is very relevant because, in elderly patients, the high number of drugs increases the risk of drug interaction with a decrease in quality of life [29,30].
Low O2–O3 efficacy could be related to (i) an imprecise ozone generator; (ii) an imprecise gas volume; (iii) an undefined ozone concentration; or (iv) a nonoptimal dose for achieving a therapeutic effect.
Before the administration of O2–O3, the concentration should be set to a specific range to ensure safety; after injections, patients might feel a little burning and/or a sensation of heaviness at the injection site that spontaneously decreases in a few minutes. Adverse effects might be related to an incorrect administration technique, including pain, hematoma, infections in the injection site, vagal crisis, and even death [31]. In our study, among the enrolled patients, none had ADR even if they experienced a transient burning in the infiltration sites and redness, which disappeared after a few minutes.
In agreement with the statistical analyses, we can argue that, in neck and brachial pain, the intramuscular administration of O2–O3, as an add-on to pharmacological treatments, can favor an improvement in the clinical conditions of patients.
In our study, we documented that before the treatment the patients reported the presence of pain and fatigue with a decrease in physical functioning that induced, particularly in women, a decrease in social functioning with the presence of anxiety and depression. After the O2–O3 treatment, we documented a statistically significant reduction in the pain rating scales and a statistically significant improvement in quality of life. Subsequent studies are required to broaden the patient sample and to evaluate the efficacy of placebo-controlled oxygen–ozone infiltrative therapy alone. The present study has some limitations. The most important is the absence of a control group; it is impossible to use an intramuscular treatment with a placebo (it is not ethical) or other drugs (e.g., corticosteroids or anesthetics, poorer appropriate drugs).
In conclusion, we reported that intramuscular–paravertebral O2–O3 injections represent an effective, safe, conservative approach in patients affected by cervical pain, particularly in patients with comorbidities and polytherapy.
Author Contributions
Conceptualization, L.G.; Methodology, V.R., G.S., F.M. and L.G.; Software, G.S. and R.C.; Validation, G.D.S., F.M. and L.G.; Formal analysis, A.C.; Investigation, G.M., V.R., C.V., E.C. and F.M.; Resources, R.C. and G.D.S.; Data curation, A.C., C.P. and G.S.; Writing—original draft, G.M., V.R. and C.V.; Writing—review & editing, R.C., G.D.S., F.M. and L.G.; Supervision, L.G.; Project administration, F.M.; Funding acquisition, G.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study, approved by the Ethics Committee, was carried out according to the Good Clinical Practice guidelines and under the ethical principles of the Declaration of Helsinki. Calabria Centro number n. 156, 22 April 2021.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kazeminasab, S.; Nejadghaderi, S.A.; Amiri, P.; Pourfathi, H.; Araj-Khodaei, M.; Sullman, M.J.M.; Kolahi, A.-A.; Safiri, S. Neck pain: Global epidemiology, trends and risk factors. BMC Musculoskelet. Disord. 2022, 23, 1–13. [Google Scholar] [CrossRef]
- Skelly, A.C.; Chou, R.; Dettori, J.R.; Turner, J.A.; Friedly, J.L.; Rundell, S.D.; Fu, R.; Brodt, E.D.; Wasson, N.; Kantner, S.; et al. Noninvasive Nonpharmacological Treatment for Chronic Pain: A Systematic Review Update. In Comparative Effectiveness Review, No. 227; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2020. [Google Scholar]
- Khan, S.A.; Seyam, O.; Smith, N.L.; Reid, I.; Gandhi, J.; Jiang, W. Clinical utility of ozone therapy for musculoskeletal disorders. Med. Gas Res. 2018, 8, 103–110. [Google Scholar] [CrossRef]
- Stoker, G. The Surgical Uses of Ozone. Lancet 1916, 188, 712. [Google Scholar] [CrossRef]
- Özcan, Ç.; Polat, Ö.; Çelik, H.; Uçar, B.Y. The Effect of Paravertebral Ozone Injection in the Treatment of Low Back Pain. Pain Pract. 2019, 19, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Kadir, K.; Syam, Y.; Yusuf, S.; Zainuddin, M. Ozone Therapy on Reduction of Bacterial Colonies and Acceleration of Diabetic Foot Ulcer Healing. Home Healthc. Now 2020, 38, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.; Xaus, C.; Bartrons, R.; Leon, O.S.; Gelpi, E.; Rosello-Catafau, J. Effect of Ozone Treatment on Reactive Oxygen Species and Adenosine Production During Hepatic Ischemia-Reperfusion. Free Rad. Res. 2000, 33, 595–605. [Google Scholar] [CrossRef]
- Sagai, M.; Bocci, V. Mechanisms of Action Involved in Ozone Therapy: Is Healing Induced via a Mild Oxidative Stress? Med. Gas Res. 2011, 1, 29. [Google Scholar] [CrossRef]
- Clavo, B.; Pérez, J.L.; López, L.; Suárez, G.; Lloret, M.; Rodríguez, V.; Macías, D.; Santana, M.; Morera, J.; Fiuza, D.; et al. Effect of Ozone Therapy on Muscle Oxygenation. J. Altern. Complement. Med. 2003, 9, 251–256. [Google Scholar] [CrossRef]
- Clavo, B.; Pérez, J.L.; López, L.; Suárez, G.; Lloret, M.; Rodríguez, V.; Macías, D.; Santana, M.; Hernández, M.A.; Martín-Oliva, R.; et al. Ozone Therapy for Tumor Oxygenation: A Pilot Study. eCAM 2004, 1, 93–98. [Google Scholar] [CrossRef]
- Calderón Guzmá, D.; Islas, J.L.H.; Mejía, G.B.; del Angel, D.S.; García, E.H.; Olguín, H.J. Effect of nutritional status and ozone exposure on Na+/K+ ATPpase and lipid peroxidation in rat brain. Proc. West. Pharmacol. Soc. 2005, 48, 118–121. [Google Scholar]
- Barragán-Mejía, M.G.; Castilla-Serna, L.; Calderón-Guzmán, D.; Hernández-Islas, J.; Labra-Ruiz, N.A.; Rodríguez-Pérez, R.; Angel, D.S.-D. Effect of Nutritional Status and Ozone Exposure on Rat Brain Serotonin. Arch. Med. Res. 2002, 33, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.S.; Yu, S.; Kong, A.N. Activation of Nrf2-antioxidant signaling attenuates NFkappaB inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Mourkioti, F.; Kratsios, P.; Luedde, T.; Song, Y.-H.; Delafontaine, P.; Adami, R.; Parente, V.; Bottinelli, R.; Pasparakis, M.; Rosenthal, N. Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J. Clin. Investig. 2006, 116, 2945–2954. [Google Scholar] [CrossRef] [PubMed]
- Ulusoy, G.R.; Bilge, A.; Öztürk, Ö. Comparison of corticosteroid injection and ozone injection for relief of pain in chronic lateral epicondylitis. Acta Orthop. Belg. 2019, 85, 317–324. [Google Scholar] [PubMed]
- Martinelli, M.; Giovannangeli, F.; Venditto, T.; Travagli, V. The use of oxygen ozone therapy in the treatment of cervicobrachial pain: Case series study. J. Biol. Regul. Homeost. Agents 2020, 34, 47–55. [Google Scholar]
- Thong, I.S.K.; Jensen, M.P.; Miró, J.; Tan, G. The validity of pain intensity measures: What do the NRS, VAS, VRS, and FPS-R measure? Scand. J. Pain 2018, 18, 99–107. [Google Scholar] [CrossRef]
- VanDenKerkhof, E.G.; Stitt, L.; Clark, A.J.; Gordon, A.; Lynch, M.; Morley-Forster, P.K.; Nathan, H.J.; Smyth, C.; Toth, C.; Ware, M.A.; et al. Sensitivity of the DN4 in Screening for Neuropathic Pain Syndromes. Clin. J. Pain 2018, 34, 30–36. [Google Scholar] [CrossRef]
- Lin, S.H.; Wu, C.C.; Hung, C.J. Use dn4-t questionnaire to rule out non-neuropathic pain. J. Chin. Med. Assoc. 2020, 83, 510. [Google Scholar] [CrossRef]
- Ware, J.E.; Gandek, B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J. Clin. Epidemiol. 1998, 51, 903–912. [Google Scholar] [CrossRef]
- Gallelli, L.; Ferreri, G.; Colosimo, M.; Pirritano, D.; Flocco, M.A.; Pelaia, G.; De Sarro, G.B. Adverse drug reactions to antibiotics observed in two pulmonology divisions of Catanzaro, Italy: A six-year retrospective study. Pharmacol. Res. 2002, 46, 395–400. [Google Scholar] [CrossRef]
- Gallelli, L.; Ferreri, G.; Colosimo, M.; Pirritano, D.; Flocco, M.A.; Pelaia, G.; De Sarro, G.B. Retrospective analysis of adverse drug reactions to bronchodilators observed in two pulmonary divisions of Catanzaro, Italy. Pharmacol. Res. 2003, 47, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, M.; Cotticelli, B.; Albertini, F.; Brayda-Bruno, M.; Valdenassi, L.; Richelmi, P. Percutaneous Paravertebral Ozone Therapy. Rivista Neuroradiologia 2002, 15, 415–419. [Google Scholar] [CrossRef]
- Bocci, V.A. Scientific and medical aspects of ozone therapy. State of the art. Riv. Ital. Di Ossigeno-Ozonoterapia 2006, 5, 93–104. [Google Scholar] [CrossRef]
- Zeng, J.; Lei, L.; Zeng, Q.; Yao, Y.; Wu, Y.; Li, Q.; Gao, L.; Du, H.; Xie, Y.; Huang, J. Ozone therapy attenuates NF-ΚB–mediated local inflammatory response and activation of th17 cells in treatment for psoriasis. Int. J. Biol. Sci. 2020, 16, 1833–1845. [Google Scholar] [CrossRef]
- Alexandre, A.; Corò, L.; Azuelos, A.; Buric, J.; Salgado, H.; Murga, M.; Marin, F.; Giocoli, H. Intradiscal injection of oxygen-ozone gas mixture for the treatment of cervical disc herniations. Acta Neurochir. Suppl. 2005, 92, 79–82. [Google Scholar] [PubMed]
- Raeissadat, S.A.; Rayegani, S.M.; Sadeghi, F.; Rahimi-Dehgolan, S. Comparison of ozone and lidocaine injection efficacy vs dry needling in myofascial pain syndrome patients. J. Pain Res. 2018, 11, 1273. [Google Scholar] [CrossRef]
- Beyaz, S.G.; Sayhan, H. Six-Month Results of Cervical Intradiscal Oxygen-Ozone Mixture Therapy on Patients with Neck Pain: Preliminary Findings. Pain Physician 2018, 21, E449–E456. [Google Scholar]
- Caterina, P.; Di Paolo, A.; Giofrè, C.; Caglioti, C.; Leuzzi, G.; Siniscalchi, A.; De Sarro, G.; Gallelli, L. Pharmacokinetic drug-drug interaction and their implication in clinical management. J. Res. Med. Sci. 2013, 18, 601. [Google Scholar]
- Gallelli, L.; Siniscalchi, A.; Palleria, C.; Mumoli, L.; Staltari, O.; Squillace, A.; Maida, F.; Russo, E.; Gratteri, S.; De Sarro, G.; et al. Adverse Drug Reactions Related to Drug Administration in Hospitalized Patients. Curr. Drug Saf. 2017, 12, 171–177. [Google Scholar] [CrossRef]
- Madrid Declaration on Ozone Therapy, 3rd ed.; Official Document of ISCO3; Faculdade do Centro Oeste Paulista: Madrid, Spain, March 2020.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).