A Frail Hairy Cell Leukemia Patient Successfully Treated with Pegylated Interferon-α-2A
Abstract
1. Introduction
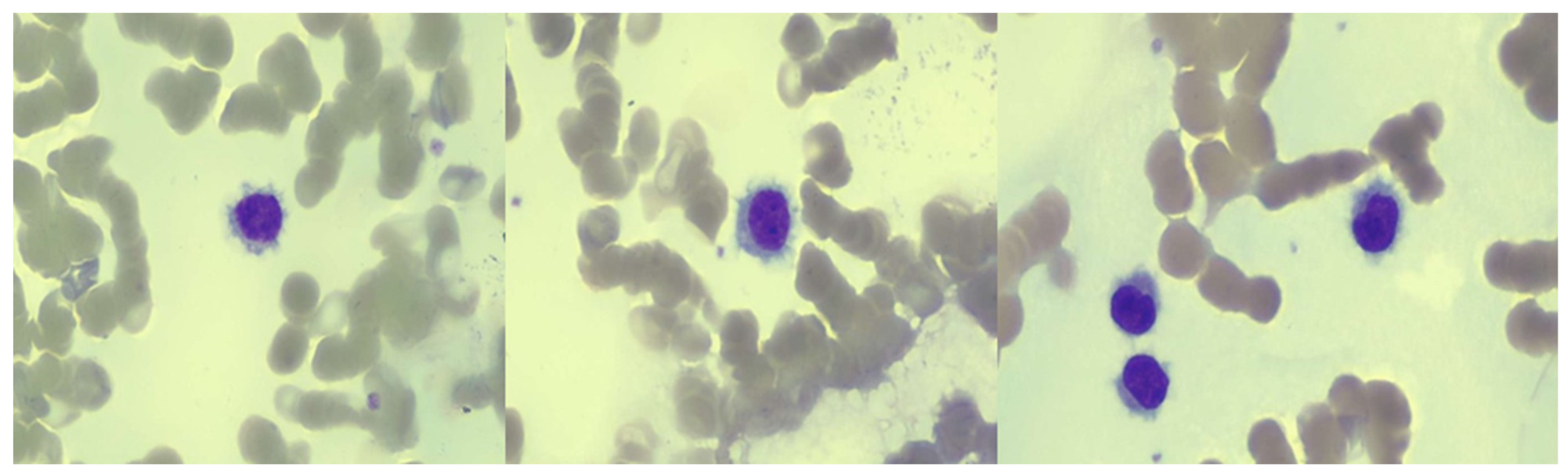
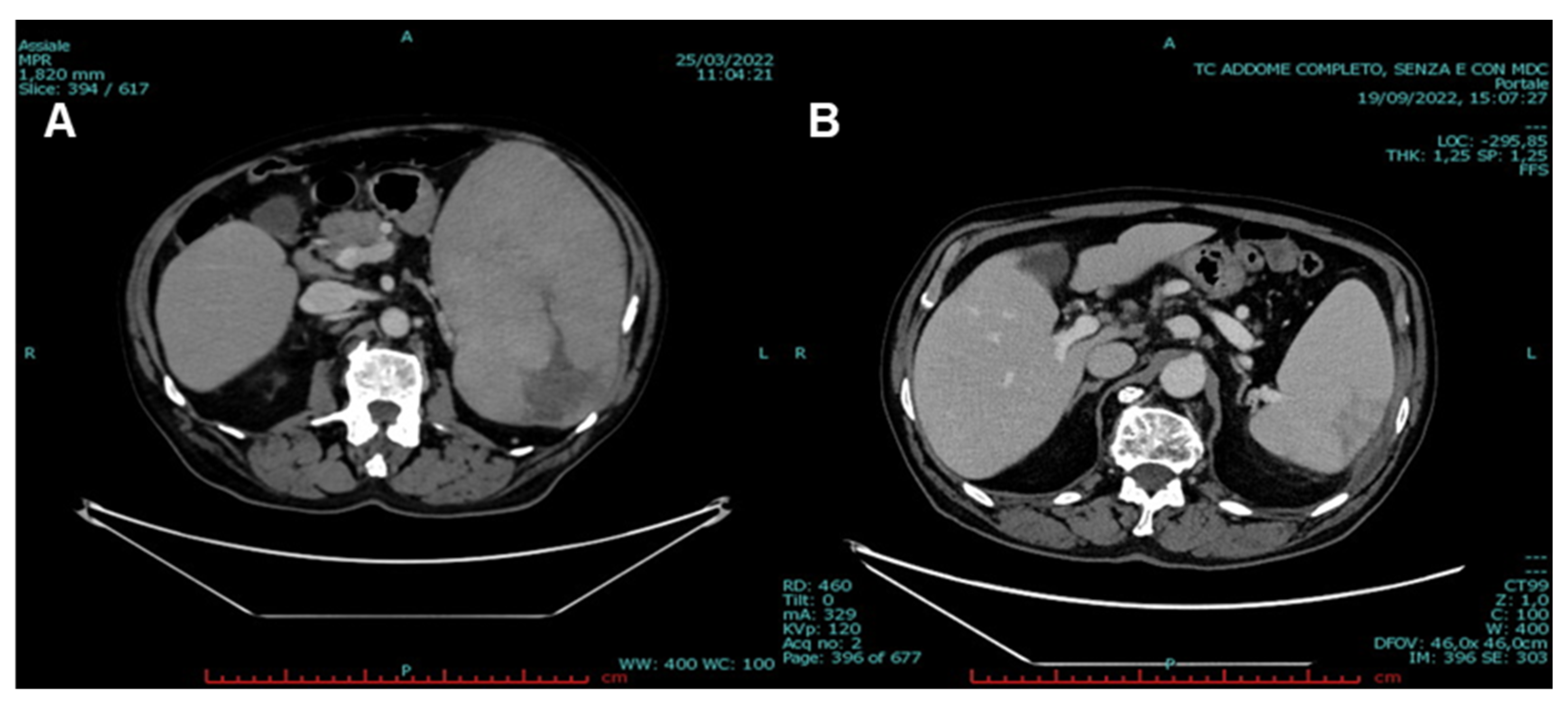
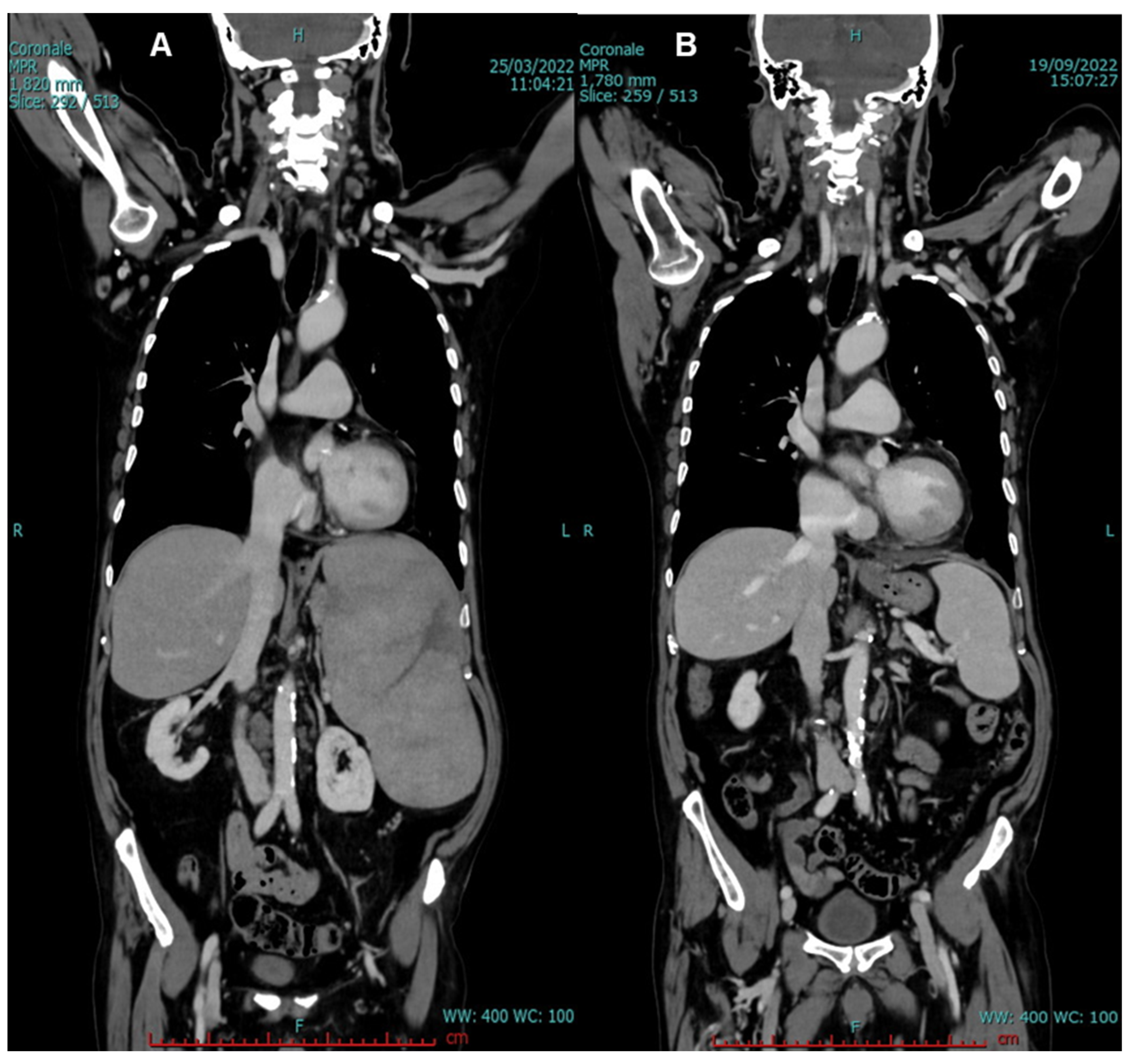
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maitre, E.; Cornet, E.; Troussard, X. Hairy cell leukemia: 2020 update on diagnosis, risk stratification, and treatment. Am. J. Hematol. 2019, 94, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Gorrese, M.; Guariglia, R.; Campana, A.; Bertolini, A.; Fresolone, L.; Martorelli, M.C.; Serio, B.; Selleri, C.; Giudice, V. Biclonality in hairy cell leukemia: Co-occurrence of CD10+ and CD10− clones with different surface membrane immunoglobulin expression. Cytom. B Clin. Cytom. 2022, 102, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Vittoria, L.; Bozzi, F.; Capone, I.; Carniti, C.; Lorenzini, D.; Gobbo, M.; Bolli, N.; Aiello, A. A rare biclonal Hairy Cell Leukemia disclosed by an integrated diagnostic approach: A case report. Cytom. B Clin. Cytom. 2021, 100, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Robak, T.; Matutes, E.; Catovsky, D.; Zinzani, P.L.; Buske, C.; ESMO Guidelines Committee. Hairy cell leukaemia: ESMO Clinical Practice Guidelines for diagnosis; treatment and follow-up. Ann. Oncol. 2015, 26, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Cornet, E.; Delmer, A.; Feugier, P.; Garnache-Ottou, F.; Ghez, D.; Leblond, V.; Levy, V.; Maloisel, F.; Re, D.; Zini, J.M.; et al. Recommendations of the SFH (French Society of Haematology) for the diagnosis; treatment and follow-up of hairy cell leukaemia. Ann. Hematol. 2014, 93, 1977–1983. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tiacci, E.; Schiavoni, G.; Martelli, M.P.; Boveri, E.; Pacini, R.; Tabarrini, A.; Zibellini, S.; Santi, A.; Pettirossi, V.; Fortini, E.; et al. Constant activation of the RAF-MEK-ERK pathway as a diagnostic and therapeutic target in hairy cell leukemia. Haematologica 2013, 98, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Habermann, T.M.; Rai, K. Historical treatments of in hairy cell leukemia; splenectomy and interferon: Past and current uses. Leuk. Lymphoma 2011, 52, 18–20. [Google Scholar] [CrossRef]
- Pagano, L.; Criscuolo, M.; Broccoli, A.; Piciocchi, A.; Varettoni, M.; Galli, E.; Anastasia, A.; Cantonetti, M.; Trentin, L.; Kovalchuk, S.; et al. Long-term follow-up of cladribine treatment in hairy cell leukemia: 30-year experience in a multicentric Italian study. Blood Cancer J. 2022, 12, 109. [Google Scholar] [CrossRef]
- Kreitman, R.J. Hairy cell leukemia: Present and future directions. Leuk. Lymphoma 2019, 60, 2869–2879. [Google Scholar] [CrossRef]
- D’Addona, M.; Giudice, V.; Pezzullo, L.; Ciancia, G.; Baldi, C.; Gorrese, M.; Bertolini, A.; Campana, A.; Fresolone, L.; Manzo, P.; et al. Hodgkin Lymphoma and Hairy Cell Leukemia Arising from Chronic Lymphocytic Leukemia: Case Reports and Literature Review. J. Clin. Med. 2022, 11, 4674. [Google Scholar] [CrossRef]
- Grever, M.; Kopecky, K.; Foucar, M.K.; Head, D.; Bennett, J.M.; Hutchison, R.E.; Corbett, W.E.; Cassileth, P.A.; Habermann, T.; Golomb, H.; et al. Randomized comparison of pentostatin versus interferon alfa-2a in previously untreated patients with hairy cell leukemia: An intergroup study. J. Clin. Oncol. 1995, 13, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.A.; O’Brien, S.; Bueso-Ramos, C.; Faderl, S.; Keating, M.J.; Giles, F.J.; Cortes, J.; Kantarjian, H.M. Rituximab in relapsed or refractory hairy cell leukemia. Blood 2003, 102, 3906–3911. [Google Scholar] [CrossRef] [PubMed]
- Tiacci, E.; De Carolis, L.; Simonetti, E.; Capponi, M.; Ambrosetti, A.; Lucia, E.; Antolino, A.; Pulsoni, A.; Ferrari, S.; Zinzani, P.L.; et al. Vemurafenib plus Rituximab in Refractory or Relapsed Hairy-Cell Leukemia. N. Engl. J. Med. 2021, 384, 1810–1823. [Google Scholar] [CrossRef]
- Yurkiewicz, I.R.; Coutre, S.; Ghesquieres, H.; Pastan, I.; Kreitman, R.J. Moxetumomab pasudotox as re-treatment for heavily-pretreated relapsed hairy cell leukemia. Leuk. Lymphoma 2021, 62, 2812–2814. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Moreau, P.; Ravandi, F.; Hutchings, M.; Gazzah, A.; Michallet, A.S.; Wainberg, Z.A.; Stein, A.; Dietrich, S.; de Jonge, M.J.A.; et al. Dabrafenib plus trametinib in patients with relapsed/refractory BRAF V600E mutation-positive hairy cell leukemia. Blood 2022, 2021013658. [Google Scholar] [CrossRef]
- Rogers, K.A.; Andritsos, L.A.; Wei, L.; McLaughlin, E.M.; Ruppert, A.S.; Anghelina, M.; Blachly, J.S.; Call, T.; Chihara, D.; Dauki, A.; et al. Phase 2 study of ibrutinib in classic and variant hairy cell leukemia. Blood 2021, 137, 3473–3483. [Google Scholar] [CrossRef]
- Maevis, V.; Mey, U.; Schmidt-Wolf, G.; Schmidt-Wolf, I.G. Hairy cell leukemia: Short review; today’s recommendations and outlook. Blood Cancer J. 2014, 4, e184. [Google Scholar] [CrossRef]
- Damasio, E.E.; Clavio, M.; Masoudi, B.; Isaza, A.; Spriano, M.; Rossi, E.; Casciaro, S.; Cerri, R.; Risso, M.; Nati, S.; et al. Alpha-interferon as induction and maintenance therapy in hairy cell leukemia: A long-term follow-up analysis. Eur. J. Haematol. 2000, 64, 47–52. [Google Scholar] [CrossRef]
- Kojima, G.; Iliffe, S.; Walters, K. Frailty index as a predictor of mortality: A systematic review and meta-analysis. Age Ageing 2018, 47, 193–200. [Google Scholar] [CrossRef]
- Peña, F.G.; Theou, O.; Wallace, L.; Brothers, T.D.; Gill, T.M.; Gahbauer, E.A.; Kirkland, S.; Mitnitski, A.; Rockwood, K. Comparison of alternate scoring of variables on the performance of the frailty index. BMC Geriatr. 2014, 14, 25. [Google Scholar] [CrossRef]
- Giudice, V.; Pezzullo, L.; Ciancia, G.; D’Addona, M.; D’Alto, F.; Gorrese, M.; Cuffa, B.; Selleri, C. Post-therapy B Regulatory Cells Might early Predict Relapse in Hodgkin Lymphoma. Mediterr. J. Hematol. Infect. Dis. 2022, 14, e2022042. [Google Scholar] [CrossRef]
- Grever, M.R.; Abdel-Wahab, O.; Andritsos, L.A.; Banerji, V.; Barrientos, J.; Blachly, J.S.; Call, T.G.; Catovsky, D.; Dearden, C.; Demeter, J.; et al. Consensus guidelines for the diagnosis and management of patients with classic hairy cell leukemia. Blood 2017, 129, 553–560. [Google Scholar] [CrossRef]
- Kiladjian, J.J.; Mesa, R.A.; Hoffman, R. The renaissance of interferon therapy for the treatment of myeloid malignancies. Blood 2011, 117, 4706–4715. [Google Scholar] [CrossRef]
- Chawla-Sarkar, M.; Lindner, D.J.; Liu, Y.F.; Williams, B.R.; Sen, G.C.; Silverman, R.H.; Borden, E.C. Apoptosis and interferons: Role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 2003, 8, 237–249. [Google Scholar] [CrossRef]
- D’Addona, M.; Pezzullo, L.; Giudice, V.; Serio, B.; Baldi, C.; Zeppa, P.; Selleri, C. Kaposi’s sarcoma associated with chronic myeloid leukemia and imatinib mesylate therapy. Clin. Case Rep. 2022, 10, e05919. [Google Scholar] [CrossRef]
- Vedantham, S.; Gamliel, H.; Golomb, H.M. Mechanism of interferon action in hairy cell leukemia: A model of effective cancer biotherapy. Cancer Res. 1992, 52, 1056–1066. [Google Scholar]
- Furlan, A.; Rossi, M.C.; Gherlinzoni, F.; Scotton, P. Prompt Hematological Recovery in Response to a Combination of Pegylated Interferon α-2a and Rituximab in a Profoundly Immuno-Suppressed Hairy Cell Leukemia Patient with a Mycobacterial Infection at Onset: Benefits and Drawbacks of Rapid Immune Reconstitution. Hematol. Rep. 2022, 14, 135–142. [Google Scholar]
- Assanto, G.M.; Riemma, C.; Malaspina, F.; Perrone, S.; De Luca, M.L.; Pucciarini, A.; Annechini, G.; D’Elia, G.M.; Martelli, M.; Foà, R.; et al. The current role of interferon in hairy cell leukaemia: Clinical and molecular aspects. Br. J. Haematol. 2021, 194, 78–82. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Lauria, F.; Salvucci, M.; Rondelli, D.; Raspadori, D.; Bendandi, M.; Magagnoli, M.; Tura, S. Hairy-cell leukemia and alpha-interferon treatment: Long-term responders. Haematologica 1997, 82, 152–155. [Google Scholar]
- Benz, R.; Siciliano, R.D.; Stussi, G.; Fehr, J. Long-term follow-up of interferon-alpha induction and low-dose maintenance therapy in hairy cell leukemia. Eur. J. Haematol. 2009, 82, 194–200. [Google Scholar] [CrossRef]
- Ratain, M.J.; Golomb, H.M.; Bardawil, R.G.; Vardiman, J.W.; Westbrook, C.A.; Kaminer, L.S.; Lembersky, B.C.; Bitter, M.A.; Daly, K. Durability of responses to interferon alfa-2b in advanced hairy cell leukemia. Blood 1987, 69, 872–877. [Google Scholar] [CrossRef]
- Ahmed, S.; Rai, K.R. Interferon in the treatment of hairy-cell leukemia. Best Pract. Res. Clin. Haematol. 2003, 16, 69–81. [Google Scholar] [CrossRef]
- Gidlund, M.; Orn, A.; Wigzell, H.; Senik, A.; Gresser, I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature 1978, 273, 759–761. [Google Scholar] [CrossRef]
- Sacchi, S.; Federico, M.; Vitolo, U.; Boccomini, C.; Vallisa, D.; Baldini, L.; Petrini, M.; Rupoli, S.; Di Raimondo, F.; Merli, F.; et al. Clinical activity and safety of combination immunotherapy with IFN-alpha 2a and Rituximab in patients with relapsed low grade non-Hodgkin’s lymphoma. Haematologica 2001, 86, 951–958. [Google Scholar]
- Bohn, J.P.; Gastl, G.; Steurer, M. Long-term treatment of hairy cell leukemia with interferon-α: Still a viable therapeutic option. Memo 2016, 9, 63–65. [Google Scholar] [CrossRef]
- Bohn, J.P.; Pircher, A.; Wanner, D.; Vill, D.; Foeger, B.; Wolf, D.; Steurer, M. Low-dose vemurafenib in hairy cell leukemia patients with active infection. Am. J. Hematol. 2019, 94, E180–E182. [Google Scholar] [CrossRef]
- Levavi, H.; Lancman, G.; Gabrilove, J. Impact of rituximab on COVID-19 outcomes. Ann. Hematol. 2021, 100, 2805–2812. [Google Scholar] [CrossRef]
- Gowin, K.; Jain, T.; Kosiorek, H.; Tibes, R.; Camoriano, J.; Palmer, J.; Mesa, R. Pegylated interferon alpha—2a is clinically effective and tolerable in myeloproliferative neoplasm patients treated off clinical trial. Leuk Res. 2017, 54, 73–77. [Google Scholar] [CrossRef]
- Glue, P.; Fang, J.W.; Rouzier-Panis, R.; Raffanel, C.; Sabo, R.; Gupta, S.K.; Salfi, M.; Jacobs, S. Pegylated interferon-alpha2b: Pharmacokinetics; pharmacodynamics; safety; and preliminary efficacy data. Hepatitis C Intervention Therapy Group. Clin. Pharmacol. Ther. 2000, 68, 556–567. [Google Scholar] [CrossRef]
- Sleijfer, S.; Bannink, M.; Van Gool, A.R.; Kruit, W.H.; Stoter, G. Side effects of interferon-alpha therapy. Pharm. World Sci. 2005, 27, 423–431. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Pharmacokinetic consequences of pegylation. Drug. Deliv. 2006, 13, 399–409. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Novellis, D.; Giudice, V.; Ciccone, V.; Erra, P.; De Vita, A.; Picone, F.; Serio, B.; Selleri, C. A Frail Hairy Cell Leukemia Patient Successfully Treated with Pegylated Interferon-α-2A. J. Clin. Med. 2023, 12, 193. https://doi.org/10.3390/jcm12010193
De Novellis D, Giudice V, Ciccone V, Erra P, De Vita A, Picone F, Serio B, Selleri C. A Frail Hairy Cell Leukemia Patient Successfully Treated with Pegylated Interferon-α-2A. Journal of Clinical Medicine. 2023; 12(1):193. https://doi.org/10.3390/jcm12010193
Chicago/Turabian StyleDe Novellis, Danilo, Valentina Giudice, Vincenzo Ciccone, Paola Erra, Alba De Vita, Francesca Picone, Bianca Serio, and Carmine Selleri. 2023. "A Frail Hairy Cell Leukemia Patient Successfully Treated with Pegylated Interferon-α-2A" Journal of Clinical Medicine 12, no. 1: 193. https://doi.org/10.3390/jcm12010193
APA StyleDe Novellis, D., Giudice, V., Ciccone, V., Erra, P., De Vita, A., Picone, F., Serio, B., & Selleri, C. (2023). A Frail Hairy Cell Leukemia Patient Successfully Treated with Pegylated Interferon-α-2A. Journal of Clinical Medicine, 12(1), 193. https://doi.org/10.3390/jcm12010193





