ABO Blood Groups in Systemic Sclerosis: Distribution and Association with This Disease’s Characteristics
Abstract
1. Introduction
2. Materials and Methods
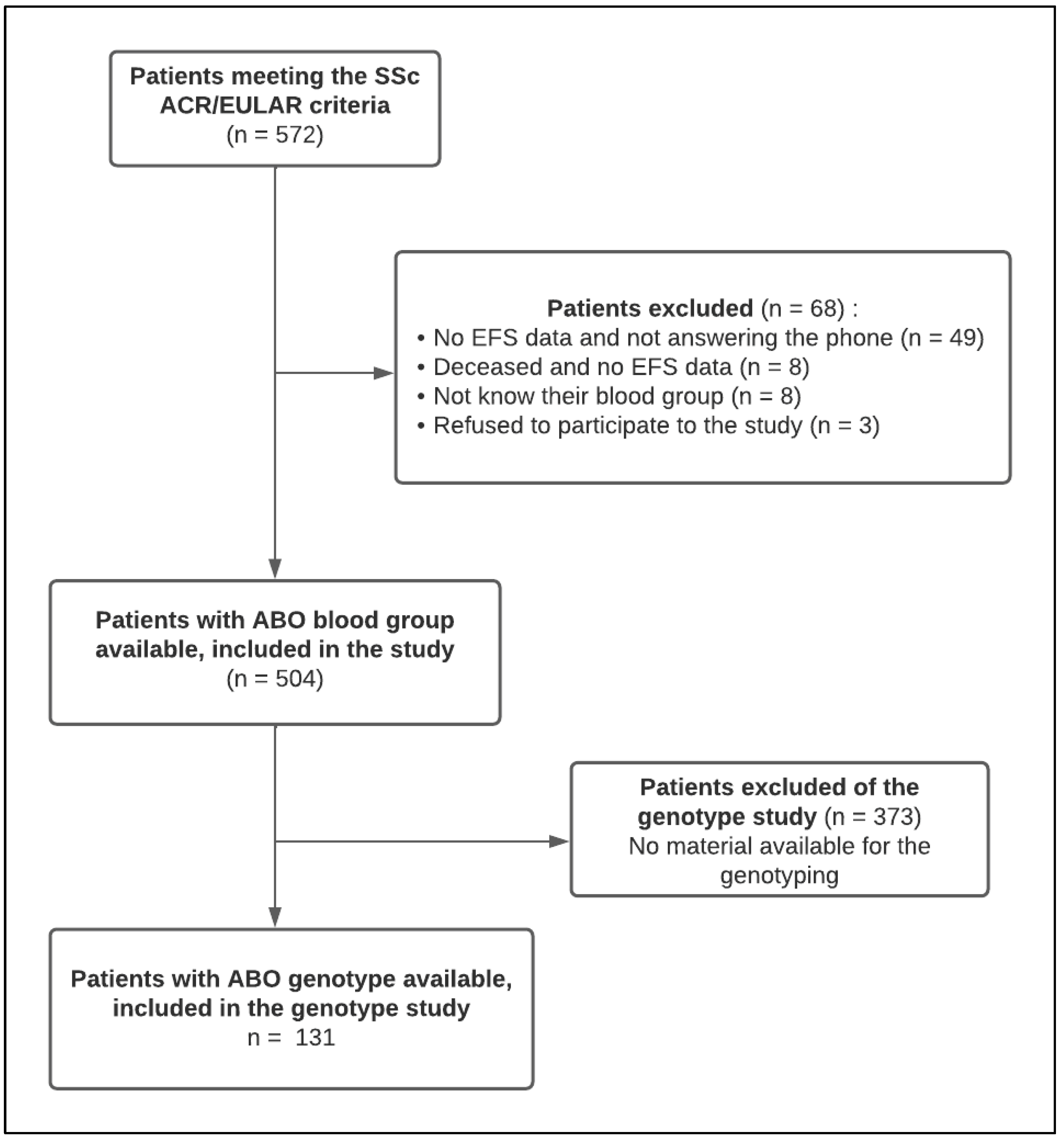
2.1. Patient Selection
2.2. Collected Data and Methods
2.3. Ethics
2.4. Statistical Methods
3. Results
3.1. Patients’ Characteristics
3.2. ABO Blood Group Repartition in the SSc Population and Comparison with the General Population
3.2.1. Phenotypical Study
3.2.2. Genotyping Study
3.3. Association between Phenotypical ABO Blood Group and SSc Characteristics
3.4. Association between Haemostasis and Endothelial Activation Parameters and SSc Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bailly, P.; Chiaroni, J.; Roubinet, F. Les Groupes Sanguins Erythrocytaires; John Libbey Eurotext: Montrouge, France, 2015. [Google Scholar]
- Franchini, M.; Mannucci, P.M. ABO blood group and thrombotic vascular disease. J. Thromb. Haemost. 2014, 112, 1103–1109. [Google Scholar]
- O’Donnell, J.; Laffan, M.A. The relationship between ABO histo-blood group, factor VIII and von Willebrand factor. Transfus. Med. 2001, 11, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Morelli, V.M.; De Visser, M.C.H.; Vos, H.L.; Bertina, R.M.; Rosendaal, F.R. ABO blood group genotypes and the risk of venous thrombosis: Effect of factor V Leiden. J. Thromb. Haemost. 2005, 3, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Goumidi, L.; Thibord, F.; Wiggins, K.L.; Li-Gao, R.; Brown, M.R.; van Hylckama Vlieg, A.; Souto, J.C.; Soria, J.M.; Ibrahim-Kosta, M.; Saut, N.; et al. Association between ABO haplotypes and the risk of venous thrombosis: Impact on disease risk estimation. Blood 2021, 137, 2394–2402. [Google Scholar] [CrossRef] [PubMed]
- Poujol-Robert, A.; Boëlle, P.Y.; Wendum, D.; Poupon, R.; Robert, A. Association Between ABO Blood Group and Fibrosis Severity in Chronic Hepatitis C Infection. Dig. Dis. Sci. 2006, 51, 1633–1636. [Google Scholar] [CrossRef]
- Shaheen, A.; Khaddam, J.; Kesh, F. Risk factors of keloids in Syrians. BMC Dermatol. 2016, 16, 13. [Google Scholar] [CrossRef]
- Acar, E.; İzci, S.; Inanir, M.; Yılmaz, M.; Kılıçgedik, A.; Güler, Y.; Izgi, I.A.; Kirma, C. Non-O-blood types associated with higher risk of high-grade atrioventricular block. Arch. Med. Sci. Atheroscler. Dis. 2019, 4, 243–247. [Google Scholar] [CrossRef]
- Cildag, S.; Kara, Y.; Senturk, T. ABO blood groups and rheumatic diseases. Eur. J. Rheumatol. 2017, 4, 250–253. [Google Scholar] [CrossRef]
- Karadağ, A. Comparison of distribution of blood groups in inflammatory rheumatic diseases and healthy subjects. Cumhur. Med. J. 2019, 41, 516–523. Available online: https://dergipark.org.tr/tr/doi/10.7197/cmj.vi.588232 (accessed on 24 April 2020). [CrossRef]
- Di Battista, M.; Barsotti, S.; Orlandi, M.; Lepri, G.; Codullo, V.; Della Rossa, A.; Guiducci, S.; Del Galdo, F. One year in review 2021: Systemic sclerosis. Clin. Exp. Rheumatol. 2021, 39, S3–S12. [Google Scholar] [CrossRef]
- Jerjen, R.; Nikpour, M.; Krieg, T.; Denton, C.P.; Saracino, A.M. Systemic sclerosis in adults. Part I: Clinical features and pathogenesis. J. Am. Acad. Dermatol. 2022, 87, 937–954. [Google Scholar] [CrossRef] [PubMed]
- Kavian, N.; Batteux, F. Macro-and microvascular disease in systemic sclerosis. Vasc. Pharmacol. 2015, 71, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.; Allanore, Y.; Chung, L.; Pauling, J.D.; Denton, C.P.; Matucci-Cerinic, M. Raynaud phenomenon and digital ulcers in systemic sclerosis. Nat. Rev. Rheumatol. 2020, 16, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Stafford, L.; Englert, H.; Gover, J.; Bertouch, J. Distribution of macrovascular disease in scleroderma. Ann. Rheum. Dis. 1998, 57, 476–479. [Google Scholar] [CrossRef]
- Cutolo, M.; Soldano, S.; Smith, V. Pathophysiology of systemic sclerosis: Current understanding and new insights. Expert Rev. Clin. Immunol. 2019, 15, 753–764. [Google Scholar] [CrossRef]
- Fabianne, Z.; Nordin, A. SSc and the Significance of Blood Group Antigens. In Proceedings of the ACR/ARHP Annual Meeting, Washington, DC, USA, 11–16 November 2016. [Google Scholar]
- Van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef]
- Nishimukai, H.; Okiura, T.; Shinomiya, T.; Fukumori, Y.; Ohnoki, S.; Shibata, H.; Okiura, T.; Yuasa, I.; Vogt, U. Genotyping of the ABO blood group system: Analysis of nucleotide position 802 by PCR-RFLP and the distribution of ABO genotypes in a German population. Int. J. Leg. Med. 1996, 109, 90–93. [Google Scholar] [CrossRef]
- Germain, M.; Chasman, D.I.; de Haan, H.; Tang, W.; Lindström, S.; Weng, L.C.; de Andrade, M.; de Visser, M.C.H.; Wiggins, K.L.; Suchon, P.; et al. Meta-analysis of 65,734 Individuals Identifies TSPAN15 and SLC44A2 as Two Susceptibility Loci for Venous Thromboembolism. Am. J. Hum. Genet. 2015, 96, 532–542. [Google Scholar] [CrossRef]
- Macafee, A.L. Blood groups and diabetes mellitus. J. Clin. Pathol. 1964, 17, 39–41. [Google Scholar] [CrossRef]
- Lopetegi, I.; Muñoz-Lopetegi, A.; Arruti, M.; Prada, A.; Urcelay, S.; Olascoaga, J.; Otaegui, D.; Castillo-Trivino, T. ABO blood group distributions in multiple sclerosis patients from Basque Country; O− as a protective factor. Mult. Scler. J. Exp. Transl. Clin. 2019, 5, 205521731988895. [Google Scholar] [CrossRef]
- Simonneau, G.; Torbicki, A.; Dorfmüller, P.; Kim, N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur. Respir. Rev. 2017, 26, 160112. [Google Scholar] [CrossRef] [PubMed]
- Bonderman, D.; Wilkens, H.; Wakounig, S.; Schafers, H.J.; Jansa, P.; Lindner, J.; Simkova, I.; Martsching, A.M.; Dudczak, J.; Sadushi, R.; et al. Risk factors for chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2008, 33, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Nik, A.; Mirfeizi, Z.; Rezaieyazdi, Z.; Khodashahi, M.; Danevash, S.; Sheikh Andalibi, M.S.; Abassi, M.; Sahebari, M. ABO and Rh blood groups in patients with Lupus and Rheumatoid Arthritis. Casp. J. Intern. Med. 2021, 12, 568–572. [Google Scholar] [CrossRef]
- Gong, P.; Li, S.; Luo, S.H.; Zhang, Y.; Li, X.L.; Guo, Y.L.; Zhu, C.G.; Xu, R.X.; Dong, Q.; Liu, G.; et al. High-sensitivity C-reactive protein mediates in part the impact of ABO blood group on coronary artery disease. Int. J. Cardiol. 2014, 177, 641–643. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Zabaneh, D.; Gaunt, T.; Swerdlow, D.I.; Shah, S.; Talmud, P.J.; Day, I.N.; Whittaker, J.; Holmes, M.V.; Sofat, E.; et al. Gene-centric analysis identifies variants associated with interleukin-6 levels and shared pathways with other inflammation markers. Circ. Cardiovasc. Genet. 2013, 6, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Melzer, D.; Perry, J.R.B.; Hernandez, D.; Corsi, A.M.; Stevens, K.; Rafferty, I.; Lauretani, F.; Murray, A.; Gibbs, J.R.; Paolisso, G.; et al. A Genome-Wide Association Study Identifies Protein Quantitative Trait Loci (pQTLs). Cheung VG, éditeur. PLoS Genet. 2008, 4, e1000072. [Google Scholar] [CrossRef] [PubMed]
- Chikhoune, L.; Brousseau, T.; Morell-Dubois, S.; Farhat, M.M.; Maillard, H.; Ledoult, E.; Lambert, M.; Yelnik, C.; Sanges, S.; Sobanski, V.; et al. Association between Routine Laboratory Parameters and the Severity and Progression of Systemic Sclerosis. JCM 2022, 11, 5087. [Google Scholar] [CrossRef]
- Jick, H.; Slone, D.; Westerholm, B.; Inman, W.H.W.; Vessey, M.P.; Shapiro, S.; Worcester, J. Venous thromboembolic disease and abo blood type. A cooperative study. Obstet. Gynecol. Surv. 1969, 24, 1154–1157. [Google Scholar] [CrossRef] [PubMed]
- Wu, O.; Bayoumi, N.; Vickers, M.A.; Clark, P. ABO(H) blood groups and vascular disease: A systematic review and meta-analysis: ABO groups and thrombosis. J. Thromb. Haemost. 2007, 6, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Liumbruno, G.M.; Franchini, M. Beyond immunohaematology: The role of the ABO blood group in human diseases. Blood Transfus. 2013, 11, 491. [Google Scholar] [CrossRef]
- Nauffal, V.; Achanta, A.; Goldhaber, S.Z.; Piazza, G. Association of ABO blood group type with cardiovascular events in COVID-19. J. Thromb. Thrombolysis 2021, 51, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.L.; Chen, M.H.; Dehghan, A.; Strachan, D.P.; Basu, S.; Soranzo, N.; Hayward, C.; Rudan, I.; Sabater-Llealm, M.; Bis, J.C.; et al. Novel associations of multiple genetic loci with plasma levels of factor VII, factor VIII, and von Willebrand factor: The CHARGE (Cohorts for Heart and Aging Research in Genome Epidemiology) Consortium. Circulation 2010, 121, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Van Alsten, S.C.; Aversa, J.G.; Santo, L.; Camargo, M.C.; Kemp, T.; Liu, J.; Huang, W.Y.; Sampson, J.; Rabkin, C.S. Association between ABO and Duffy blood types and circulating chemokines and cytokines. Genes Immun. 2021, 22, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Kahaleh, M.B. Increased Factor VIII/von Willebrand Factor Antigen and von Willebrand Factor Activity in Scleroderma and in Raynaud’s Phenomenon. Ann. Intern. Med. 1981, 94, 482. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M. Biomarkers in systemic sclerosis: Their potential to predict clinical courses. J. Dermatol. 2016, 43, 29–38. [Google Scholar] [CrossRef]
- James, J.P.; Stevens, T.R.J.; Hall, N.D.; Maddison, P.J.; Goulding, N.J.; Silman, A.; Holligan, S.; Black, C. Factor VIII Related Antigen in Connective Tissue Disease Patients and Relatives. Rheumatology 1990, 29, 6–9. [Google Scholar] [CrossRef]
- Scheja, A.; Akesson, A.; Geborek, P.; Wildt, M.; Wollheim, C.B.; Wollheim, F.A.; Vischer, U.M. Von Willebrand factor propeptide as a marker of disease activity in systemic sclerosis (scleroderma). Arthritis Res. 2001, 3, 178–182. [Google Scholar] [CrossRef]
- Kumánovics, G.; Minier, T.; Radics, J.; Pálinkás, L.; Berki, T.; Czirják, L. Comprehensive investigation of novel serum markers of pulmonary fibrosis associated with systemic sclerosis and dermato/polymyositis. Clin. Exp. Rheumatol. 2008, 26, 414–420. [Google Scholar]
- Barnes, T.; Gliddon, A.; Doré, C.J.; Maddison, P.; Moots, R.J.; The QUINs Trial Study Group. Baseline vWF factor predicts the development of elevated pulmonary artery pressure in systemic sclerosis. Rheumatology 2012, 51, 1606–1609. [Google Scholar] [CrossRef]
- Utsunomiya, A.; Oyama, N.; Hasegawa, M. Potential Biomarkers in Systemic Sclerosis: A Literature Review and Update. JCM 2020, 9, 3388. [Google Scholar] [CrossRef]
- Habe, K.; Wada, H.; Higashiyama, A.; Akeda, T.; Tsuda, K.; Mori, R.; Kakeda, M.; Matsumoto, T.; Ohishi, K.; Yamanaka, K.; et al. The Plasma Levels of ADAMTS-13, von Willebrand Factor, VWFpp, and Fibrin-Related Markers in Patients with Systemic Sclerosis Having Thrombosis. Clin. Appl. Thromb. Hemost. 2018, 24, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Kuszmiersz, P.; Pacholczak-Madej, R.; Siwiec, A.; Celińska-Lowenhoff, M.; Iwaniec, T.; Kosałka-Węgiel, J.; Zaręba, L.; Bazan-Socha, S.; Dropiński, J. Thrombin generation potential is enhanced in systemic sclerosis: Impact of selected endothelial biomarkers. Clin. Exp. Rheumatol. 2021, 39, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Garratty, G.; Glynn, S.A.; McEntire, R.; Retrovirus Epidemiology Donor Study. ABO and Rh(D) phenotype frequencies of different racial/ethnic groups in the United States: ABO/Rh phenotypes in the United States. Transfusion 2004, 44, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Pasko, B.E.; Abbott, D.; Bocsi, G.T.; Draper, N.L. ABO Blood Groups Are Not Associated With COVID-19 Disease Incidence and Severity When Correcting for Ethnicity Differences in Blood Type. Am. J. Clin. Pathol. 2022, 158, 249–253. [Google Scholar] [CrossRef] [PubMed]

| Allele | SNP |
|---|---|
| O1 | rs8176719 |
| A1/O2 | rs579459 |
| B | rs8176749 |
| A1 | rs2519093 |
| A1/A2 | rs1053878 |
| O2 | rs41302905 |
| Demographic Parameters | |
|---|---|
| Age—median (Q1; Q3), year | 64 (53; 73) |
| Women—n (%) | 414 (82.1) |
| SSc Clinical Characteristics | |
| SSc subtype | |
| dcSSc—n (%) | 110 (21.8) |
| lcSSc—n (%) | 336 (66.7) |
| SSc sine scleroderma—n (%) | 58 (11.5) |
| Time since SSc diagnosis—median (Q1; Q3), year | 10 (6; 19) |
| VT history—n (%) | 73 (14.5) |
| AT history—n (%) | 57 (11.3) |
| ILD—n (%) | 204 (41.8) |
| ILD extension | |
| Limited—n (% among ILD) | 140 (68.6) |
| Extensive—n (% among ILD) | 52 (25.5) |
| ND—n (% among ILD) | 12 (5.9) |
| PAH—n (%) | 62 (12.3) |
| Digital ulcer, past or present—n (%) | 245 (49.1) |
| Calcinosis—n (%) | 88 (17.8) |
| Digestive tract involvement—n (%) | 60 (11.9) |
| Renal crisis—n (%) | 7 (1.4) |
| mRSS—median (Q1; Q3) | 3.0 (1.0; 8.0) |
| Telangiectasias—n (%) | 378 (75.8) |
| NYHA score | |
| I—n (%) | 232 (46.4) |
| II—n (%) | 135 (27.0) |
| III—n (%) | 92 (18.4) |
| IV—n (%) | 41 (8.2) |
| Biological Parameters | |
| CRP—median (Q1; Q3), mg/L | 0.0 (0.0; 5.0) |
| NT-pro-BNP—median (Q1; Q3), pg/mL | 107.0 (52.0; 252.0) |
| ANA specificity | |
| ACA—n (%) | 234 (53.4) |
| Scl70—n (%) | 85 (19.4) |
| RNAP3—n (%) | 13 (3.0) |
| RNP—n (%) | 9 (2.1) |
| Hemostasis and Endothelial Activation Parameters | |
| FVIII:C—mean ± sd (%) | 191.8 ± 54.5 |
| VWF:Ag—mean ± sd (%) | 182.4 ± 73.6 |
| VWF:Act—mean ± sd (%) | 165.3 ± 69.7 |
| D dimers—median (Q1; Q3), ng/mL | 360.0 (270.0; 590.0) |
| Fibrinogen—mean ± sd (g/L) | 3.6 ± 0.9 |
| Cardiopulmonary Involvement Evaluation | |
| Tricuspid regurgitant velocity—mean ± sd (m/s) | 2.6 ± 0.6 |
| FVC—mean ± sd (% of predicted value) | 100.3 ± 22.1 |
| FEV/FVC—mean ± sd | 77.5 ± 8.1 |
| DLCO—mean ± sd (% of predicted value) | 67.6 ± 19.7 |
| Distance at 6 MWT—mean ± sd (% of predicted value) | 75.5 ± 19.0 |
| Activity and Severity of the Disease | |
| EUSTAR 2011 score—median (Q1; Q3) | 1.0 (0.5; 2.0) |
| EUSTAR 2016 score—median (Q1; Q3) | 1.2 (0.2; 1.9) |
| Medsger score—median (Q1; Q3) | 0.0 (0.0; 1.0) |
| Death—n (%) | 7 (1.4) |
| Non-O Blood Group (n = 294) | O Blood Group (n = 210) | p | |
|---|---|---|---|
| Demographic Parameters | |||
| Age—median (IQR), year | 64 (53–73) | 64 (54–74) | 0.30 |
| Women—n (%) | 238 (81) | 176 (83) | 0.41 |
| SSc Clinical Characteristics | |||
| SSc subtype | 0.77 | ||
| dcSSc—n (%) | 61 (20.7) | 49 (23.3) | |
| lcSSc—n (%) | 198 (67.3) | 138 (65.7) | |
| SSc sine scleroderma—n (%) | 35 (11.9) | 23 (11.0) | |
| Delay since SSc diagnosis—median (Q1; Q3), year | 10.0 (6.0; 18.0) | 11.0 (6.0; 19.0) | 0.41 |
| VT history—n (%) | 48 (16.3) | 25 (11.9) | 0.16 |
| AT history—n (%) | 32 (10.9) | 25 (11.9) | 0.72 |
| ILD—n (%) | 122 (42.8) | 82 (40.4) | 0.59 |
| ILD extension | 0.11 | ||
| Limited—n (% among ILD) | 90 (31.1) | 50 (24.0) | |
| Extensive—n (% among ILD) | 25 (8.7) | 27 (13.0) | |
| PAH—n (%) | 41 (15.3) | 21 (11.2) | 0.21 |
| Digital ulcer—n (%) | 151 (51.7) | 84 (45.4) | 0.17 |
| Calcinosis—n (%) | 55 (19.2) | 33 (15.9) | 0.36 |
| Organic microangiopathy—n (%) | 88 (62.8) | 17 (70.8) | 0.49 |
| Digestive tract involvement—n (%) | 38 (12.9) | 22 (10.5) | 0.40 |
| Renal crisis—n (%) | 4 (1.4) | 3 (1.5) | NA |
| mRSS—median (Q1; Q3) | 4.0 (2.0; 8.0) | 3.0 (0.0; 7.0) | 0.44 |
| Telangiectasias—n (%) | 224 (77.2) | 154 (73.7) | 0.36 |
| NYHA score | 0.20 | ||
| I—n (%) | 128 (44.1) | 104 (49.5) | |
| II—n (%) | 88 (30.3) | 47 (22.4) | |
| III—n (%) | 49 (16.9) | 43 (20.5) | |
| IV—n (%) | 25 (8.6) | 16 (7.6) | |
| Biological Parameters | |||
| CRP—median (Q1; Q3), mg/L | 0.0 (0.0; 5.0) | 0.0 (0.0; 4.0) | 0.021 |
| NT-pro-BNP—median (Q1; Q3), pg/mL | 103.0 (51.0; 248.0) | 115.0 (58.0; 259.0) | 0.52 |
| ANA specificity | |||
| ACA—n (%) | 139 (54.7) | 95 (51.6) | 0.52 |
| Scl70—n (%) | 52 (20.5) | 33 (17.9) | 0.51 |
| RNAP3—n (%) | 4 (1.6) | 9 (4.9) | 0.04 |
| RNP—n (%) | 3 (1.2) | 6 (3.3) | 0.18 |
| Hemostasis and Endothelial Activation Parameters | |||
| FVIII:C—mean ± sd (%) | 206.5 ± 53.0 | 168.1 ± 48.6 | <0.001 |
| VWF:Ag—mean ± sd (%) | 196.2 ± 73.5 | 160.4 ± 68.6 | <0.001 |
| VWF:Act—mean ± sd (%) | 181.4 ± 74.3 | 141.1 ± 54.1 | <0.001 |
| D dimers—median (Q1; Q3), ng/mL | 350 (270.0; 540.0) | 370.0 (270.0; 620.0) | 0.28 |
| Fibrinogen—mean ± sd (g/L) | 3.6 ± 0.8 | 3.6 ± 1.0 | 0.93 |
| Cardiopulmonary Involvement Evaluation | |||
| Tricuspid leak—mean ± sd (m/s) | 2.7 ± 0.6 | 2.6 ± 0.5 | 0.098 |
| FVC—mean ± sd (% of predicted value) | 100.7 ± 22.3 | 99.8 ± 21.7 | 0.65 |
| FEV/FVC—mean ± sd | 76.9 ± 7.8 | 78.5 ± 8.6 | 0.039 |
| DLCO—mean ± sd (% of predicted value) | 68.1 ± 20.0 | 67.0 ± 19.3 | 0.54 |
| Distance at 6 MWT—mean ± sd (% of predicted value) | 75.8 ± 19.2 | 75.1 ± 18.9 | 0.72 |
| Severity of the Disease | |||
| EUSTAR 2011 score—median (Q1; Q3) | 1.0 (0.5; 2.0) | 1.0 (0.5; 2.0) | 0.43 |
| EUSTAR 2016 score—median (Q1; Q3) | 1.2 (0.3; 1.9) | 1.2 (0.2; 2.0) | 0.73 |
| Medsger score | 0.051 | ||
| 0—n (%) | 186 (70.7) | 142 (78.5) | |
| 1—n (%) | 27 (10.3) | 17 (9.4) | |
| 2—n (%) | 17 (6.5) | 10 (5.5) | |
| 3—n (%) | 16 (6.1) | 3 (1.7) | |
| 4—n (%) | 5 (1.5) | 4 (2.2) | |
| 5—n (%) | 2 (0.8) | 1 (0.6) | |
| 6—n (%) | 3 (1.1) | 2 (1.1) | |
| 7—n (%) | 4 (1.5) | 1 (0.6) | |
| 8—n (%) | 1 (0.4) | 0 (0.0) | |
| 9—n (%) | 1 (0.4) | 0 (0.0) | |
| 10—n (%) | 2 (0.8) | 0 (0.0) | |
| 13—n (%) | 0 (0.0) | 1 (0.6) | |
| VWF:Ag % n = 219 | VWF:Act % n = 171 | FVIII:C % n = 226 | |
|---|---|---|---|
| Age (year) Pearson correlation coefficient p | 0.18 0.006 | 0.17 0.02 | 0.30 <0.0001 |
| Sex (Women vs. Men) p | 179.7 ± 75.8 vs. 193.4 ± 63.0 0.27 | 159.7 ± 69.8 vs. 186.0 ± 65.8 0.04 | 192.4 ± 56.6 vs. 189.4 ± 45.7 0.73 |
| SSc subtype (lcSSc vs. dcSSc vs. sine scleroderma) p | 175.9 ± 66.5 vs. 195.6 ± 72.3 vs. 189.5 ± 101.9 0.22 | 156.3 ± 67.67 vs. 186.7 ± 63.6 vs. 165.8 ± 83.3 0.05 | 185.6 ± 52.19 vs. 198.3 ± 46.4 vs. 210.27 ± 73.03 0.05 |
| VT history (Presence vs. Absence) p | 197.0 ± 96.0 vs. 180.3 ± 69.9 0.38 | 190.2 ± 79.3 vs. 161.5 ± 67.6 0.07 | 201.3 ± 59.1 vs. 190.5 ± 53.9 0.33 |
| AT history (Presence vs. Absence) p | 244.0 ± 120.2 vs. 176.5 ± 64.9 0.02 | 213.3 ± 143.2 vs. 161.3 ± 58.9 0.21 | 239.2 ± 81.7 vs. 187.4 ± 49.4 0.01 |
| ILD (Presence vs. Absence) p | 193.4 ± 78.2 vs. 176.1 ± 69.3 0.08 | 177.6 ± 79.2 vs. 156.4 ± 59.5 0.05 | 199.0 ± 54.8 vs. 187.7 ± 53.1 0.12 |
| PAH (Presence vs. Absence) p | 195.0 ± 61.8 vs. 182.8 ± 76.0 0.48 | 188.6 ± 63.8 vs. 163.2 ± 71.6 0.17 | 195.2 ± 45.7 vs. 192.9 ± 55.8 0.86 |
| Digital ulcer (Presence vs. Absence) p | 183.0 ± 84.1 vs. 182.0 ± 62.6 0.92 | 165.8 ± 80.0 vs. 165.0 ± 57.8 0.94 | 187.2 ± 54.0 vs. 196.9 ± 54.9 0.18 |
| mRSS Pearson correlation coefficient p | 0.16 0.02 | 0.23 0.003 | 0.10 0.13 |
| Telangiectasias (Presence vs. Absence) p | 182.7 ± 73.6 vs. 183.0 ± 75.1 0.98 | 168.6 ± 72.4 vs. 156.3 ± 60.8 0.34 | 193.1 ± 55.2 vs. 189.1 ± 53.6 0.64 |
| ACA specificity (Presence vs. Absence) p | 177.0 ± 59.7 vs. 189.0 ± 82.3 0.25 | 159.8 ± 70.4 vs. 172.3 ± 68.7 0.28 | 194.5 ± 54.3 vs. 190.7 ± 53.6 0.62 |
| Anti Scl70 specificity (Presence vs. Absence) p | 187.1 ± 71.7 vs. 182.0 ± 72.2 0.69 | 171.6 ± 65.1 vs. 164.4 ± 71.0 0.61 | 200.8 ± 54.7 vs. 190.4 ± 53.6 0.28 |
| Tricuspid leak (m/s) Pearson correlation coefficient p | 0.16 0.04 | 0.17 0.05 | 0.15 0.05 |
| FVC (% of predicted value) Pearson correlation coefficient p | −0.16 0.02 | −0.12 0.12 | −0.08 0.22 |
| DLCO (% of predicted value) Pearson correlation coefficient p | −0.19 0.005 | −0.19 0.01 | −0.12 0.08 |
| CRP (mg/L) Pearson correlation coefficient p | 0.24 0.0004 | 0.19 0.01 | 0.24 0.0004 |
| Nt-pro-BNP (pg/mL) Pearson correlation coefficient p | 0.23 0.0008 | 0.19 0.01 | 0.12 0.08 |
| Medsger score Pearson correlation coefficient p | 0.06 0.40 | 0.02 0.80 | 0.09 0.22 |
| EUSTAR 2011 score Pearson correlation coefficient p | 0.09 0.19 | 0.18 0.03 | −0.04 0.52 |
| EUSTAR 2016 score Pearson correlation coefficient p | 0.20 0.01 | 0.26 0.003 | 0.11 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collet, A.; Zawadzki, C.; Jeanpierre, E.; Kitel, C.; Dubucquoi, S.; Hachulla, E.; Susen, S.; Launay, D. ABO Blood Groups in Systemic Sclerosis: Distribution and Association with This Disease’s Characteristics. J. Clin. Med. 2023, 12, 148. https://doi.org/10.3390/jcm12010148
Collet A, Zawadzki C, Jeanpierre E, Kitel C, Dubucquoi S, Hachulla E, Susen S, Launay D. ABO Blood Groups in Systemic Sclerosis: Distribution and Association with This Disease’s Characteristics. Journal of Clinical Medicine. 2023; 12(1):148. https://doi.org/10.3390/jcm12010148
Chicago/Turabian StyleCollet, Aurore, Christophe Zawadzki, Emmanuelle Jeanpierre, Caroline Kitel, Sylvain Dubucquoi, Eric Hachulla, Sophie Susen, and David Launay. 2023. "ABO Blood Groups in Systemic Sclerosis: Distribution and Association with This Disease’s Characteristics" Journal of Clinical Medicine 12, no. 1: 148. https://doi.org/10.3390/jcm12010148
APA StyleCollet, A., Zawadzki, C., Jeanpierre, E., Kitel, C., Dubucquoi, S., Hachulla, E., Susen, S., & Launay, D. (2023). ABO Blood Groups in Systemic Sclerosis: Distribution and Association with This Disease’s Characteristics. Journal of Clinical Medicine, 12(1), 148. https://doi.org/10.3390/jcm12010148






