Etiology of Pneumoparotid: A Systematic Review
Abstract
1. Introduction
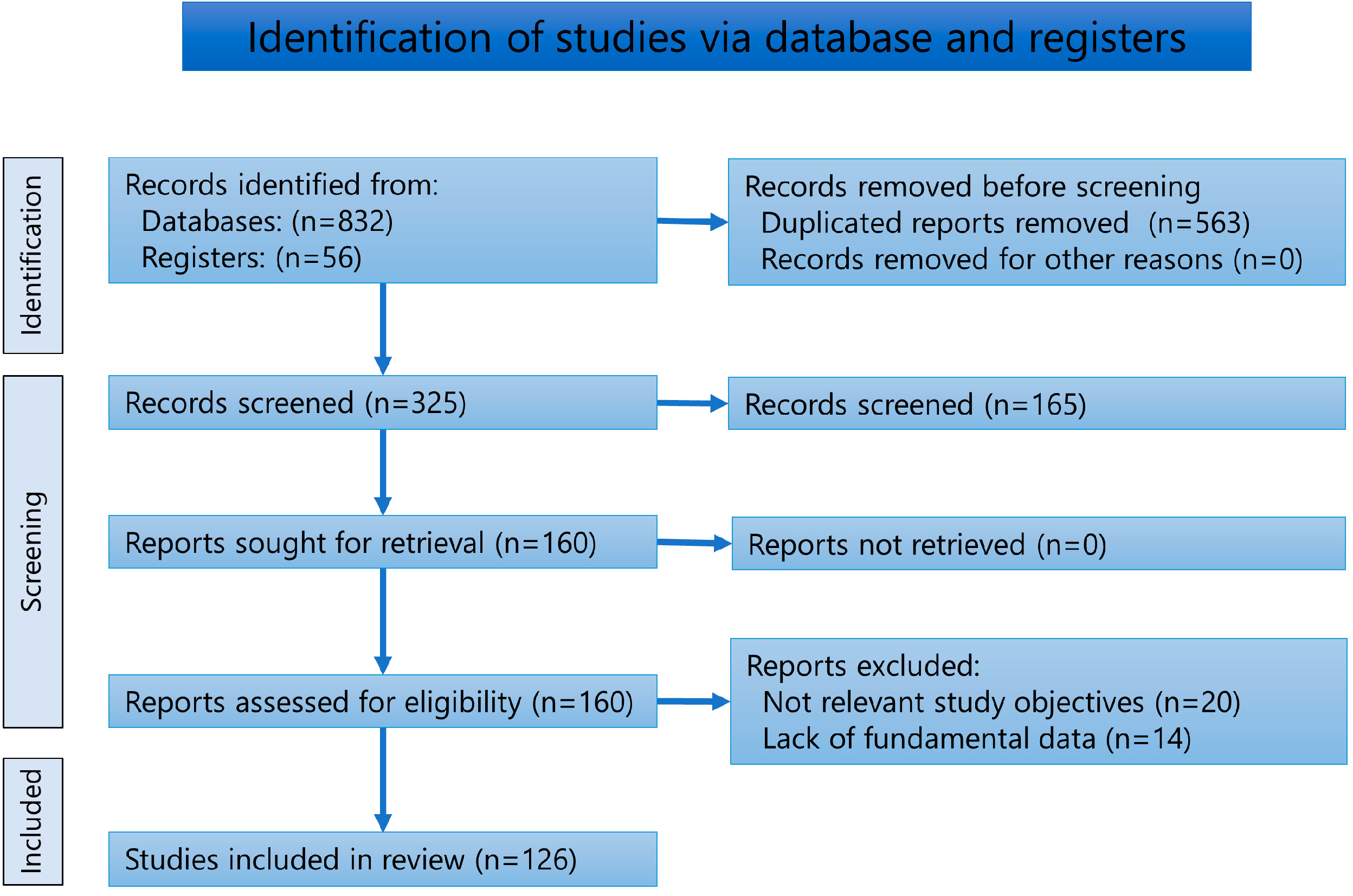
2. Materials and Methods
2.1. Review of Literature
2.2. Analysis
3. Results
3.1. Symptoms and Diagnoses
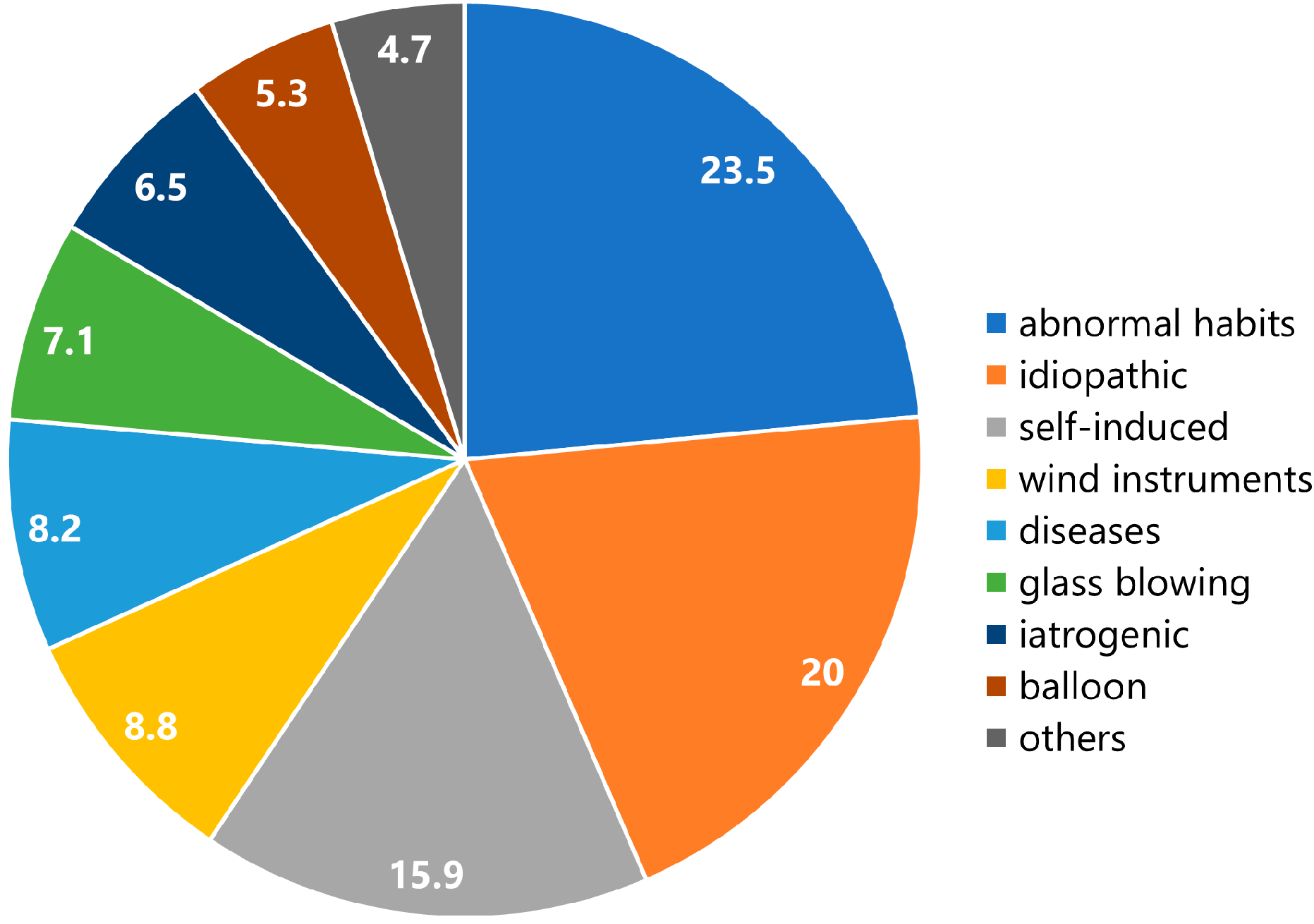
3.2. Etiology
3.3. Treatments and Sequelae
4. Discussion
4.1. Protective Mechanism of the Orifice of the Stensen’s Duct
4.2. Pathology of Pneumoparotid
4.3. Predisposing Factors of Pneumoparotid
4.4. Diagnosis of Pneumoparotid
4.5. Etiology of Pneumoparotid
4.6. Treatment of Pneumoparotid
4.7. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deichmüller. Luftgeschwulst der Wange (Pneumatozele). Berl. Klin. Wochenschr. 1890, 54, 1226. [Google Scholar]
- Scheele. Über Glasbläsern und seine Komplikationen. Berl. Klin. Wochenschr. 1900, 10, 207–210, 241–244. [Google Scholar]
- Scheier, M. Über die Krankheiten der Mundhöhle bei Glasbläsern. Arch. f. Laryngol. 1907, 19, 472–496. [Google Scholar]
- Narath, A. Über operative Eingriffe bei der Pneumatozele der Parotis und des Ductus Stenoianus (Glasbläsergeschwulst). Dtsch. Zeitschr. Chir. 1912, 119, 201–220. [Google Scholar] [CrossRef]
- Gaus, W. Seltene Spätfolge nach Parotis epidemica. Zeitschr. F. Hals- Nasen Ohrenheilkd. 1941, 47, 97–102. [Google Scholar]
- Rysenaer, L.; Van Deinse, J.; Stuyt, L.B. Pneumo-parotidite récidivante. Pr. Otorhinolaryngol. 1963, 25, 128–131. [Google Scholar] [CrossRef]
- Rupp, R.N. Pneumoparotid. Arch. Otolaryngol. 1963, 77, 111. [Google Scholar] [CrossRef]
- Reitlinger, A. Parotisveränderung bei Musikern. Monatschr. Ohrenheilkd. Laryngo-Rhinol. 1964, 98, 101–103. [Google Scholar]
- Greisen, O. Pneumatocele glandulae parotis. J. Laryngol. Otol. 1968, 82, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Yoel, J.; Alberti, C.D.; Cignetti, J.M. Neumoparotiditis. Pren. Med. Argent. 1970, 57, 423–428. [Google Scholar]
- Rosefsky, J.B. Parotid swelling and school phobia. Arch. Otolaryngol. 1970, 92, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Calcaterra, T.C.; Lowe, J. Pneumoparotiditis. An unusual case of parotid gland swelling. Arch. Otolaryngol. 1973, 97, 468–469. [Google Scholar] [CrossRef] [PubMed]
- Saunders, H.F. Wind parotitis. N. Engl. J. Med. 1973, 289, 698. [Google Scholar] [CrossRef] [PubMed]
- O’hara, A.E.; Keohane, R.B. Sialography in an unusual case of subcutaneous emphysema of the neck. Arch. Otolaryngol. 1973, 98, 354–355. [Google Scholar] [CrossRef]
- Watt, J. Benign parotid swellings: A review. Proc. Soc. Med. 1977, 70, 483–486. [Google Scholar] [CrossRef]
- Sánchez, M.L.; Millán Núñez-Cortés, J.; Calvo Manuel, E.; Espinos Perez, D.; Perez Rubio, P. Crisis de tumefaccion parotidea recurrente y enfisema subcutaneo cervico-facial facticio. Rev. Clín. Esp. 1980, 57, 197–199. [Google Scholar]
- Hadas, E.; Leventon, G.; Lerner, K.; Zimin, R.J. Pneumoparotitis: Psychosomatic aspects. Harefuah 1982, 102, 104–105. [Google Scholar]
- Aristy, F.R. Un caso de tumefaccion parotidea con enfisema cervicofacial. Rev. Med. Panama 1982, 7, 49–52. [Google Scholar]
- Byard, R. Acute parotid swelling with rapid subsidence in childhood. J. Otolaryngol. 1986, 15, 67. [Google Scholar]
- Garber, M.W. Pneumoparotitis: An unusual manifestation of hay fever. Am. J. Emerg. Med. 1987, 5, 40–41. [Google Scholar] [CrossRef]
- Markowitz-Spence, L.; Brodsky, L.; Seidell, G.; Stanievich, J.F. Self-induced pneumoparotitis in an adolescent. Report of a case and review of the literature. Int. J. Pediatr. Otorhinolaryngol. 1987, 14, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Sato, O.; Mori, Y.; Ishii, M.; Enomoto, S. Emphysema of the parotid gland induced by abnormal habit: Report of a case. J. Jpn. Stomatol. Soc. 1988, 37, 696–701. [Google Scholar]
- David, M.L.; Kanga, J.F. Pneumoparotid. In cystic fibrosis. Clin. Pediatr. 1988, 27, 506–508. [Google Scholar] [CrossRef]
- Brodie, H.A.; Chole, R.A. Recurrent pneumosialadenitis: A case presentation and new surgical intervention. Otolaryngol. Head. Neck Surg. 1988, 98, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Telfer, M.R.; Irvine, G.H. Pneumoparotitis. Br. J. Surg. 1989, 76, 978. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Oishi, K.; Sawaki, S.; Furukawa, M. A case of pneumoparotid. Stomato-Pharyngology 1990, 3, 58. [Google Scholar]
- Mandel, L.; Kaynar, A.; Wazen, J. Pneumoparotid: A case report. Oral Surg. Oral Med. Oral Pathol. 1991, 72, 22–24. [Google Scholar] [CrossRef]
- Piette, E.; Walker, R.T. Pneumoparotid during dental treatment. Oral Surg. Oral Med. Oral Pathol. 1991, 72, 415–417. [Google Scholar] [CrossRef]
- Takenoshita, Y.; Kawano, Y.; Oka, M. Pneumoparotis, an unusual occurrence of parotid gland swelling during dental treatment. Report of a case with a review of the literature. J. Craniomaxillofac. Surg. 1991, 19, 362–365. [Google Scholar] [CrossRef]
- Krief, O.; Gomori, J.M.; Gay, I. CT of pneumoparotitis. Comput. Med. Imaging Graph. 1992, 16, 39–41. [Google Scholar] [CrossRef]
- Curtin, J.J.; Ridley, N.T.; Cumberworth, V.L.; Glover, G.W. Pneumoparotitis. J. Laryngol. Otol. 1992, 106, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Ferlito, A.; Andretta, M.; Baldan, M.; Candiani, F. Non-occupational recurrent bilateral pneumoparotitis in an adolescent. J. Laryngol. Otol. 1992, 106, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Brown, F.H.; Ogletree, R.C.; Houston, G.D. Pneumoparotitis associated with the use of an air-powder prophylaxis unit. J. Periodontol. 1992, 63, 642–644. [Google Scholar] [CrossRef] [PubMed]
- Terahara, E.; Jitosho, T.; Ninomiya, M.; Umemoto, M.; Minamishima, I.; Take, H.; Kiyota, R. A case of self-induced pneumoparotitis. J. Pediatr. Pract. 1992, 145, 1705–1708. [Google Scholar]
- Yonetsu, K.; Miwa, K.; Kanda, S.; Oobu, K.; Shiratsuchi, Y. Pneumoparotid. Oral Radiol. 1993, 9, 41–42. [Google Scholar] [CrossRef]
- Birzgalis, A.R.; Curley, J.W.; Camphor, I. Pneumoparotitis, subcutaneous emphysema and pleomorphic adenoma. J. Laryngol. Otol. 1993, 107, 349–351. [Google Scholar] [CrossRef]
- McDuffie, M.W.; Brown, F.H.; Raines, W.H. Pneumoparotitis with orthodontic treatment. Am. J. Orthod. Dentofac. Orthop. 1993, 103, 377–379. [Google Scholar] [CrossRef]
- Cook, J.N.; Layton, S.A. Bilateral parotid swelling associated with chronic obstructive pulmonary disease. A case of pneumoparotid. Oral Surg. Oral Med. Oral Pathol. 1993, 76, 157–158. [Google Scholar] [CrossRef]
- Nassimbeni, G.; Ventura, A.; Boehm, P.; Guastalla, P.; Zocconi, E. Self-induced pneumoparotitis. Clin. Pediatr. 1995, 34, 160–162. [Google Scholar] [CrossRef]
- Goguen, L.A.; April, M.M.; Karmody, C.S.; Carter, B.L. Self-induced pneumoparotitis. Arch. Otolaryngol. Head Neck Surg. 1995, 121, 1426–1429. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ino, Y.; Morita, T. Recurrent parotid swelling due to psychologic stress -Report of a case. Otolaryngol. Head Neck Surg. 1996, 68, 166–170. [Google Scholar]
- Ros, S.P.; Tamayo, R.C. A case of swollen parotid gland. Pediatr. Emerg. Care 1996, 12, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Eligi, C.; Scasso, C.A.; Bruschini, P.; Neri, E.; Dotti, S. Pneumoparotite da Pseudomonas aeruginosa. Descrizione di un caso. Radiol. Med. 1997, 94, 108–110. [Google Scholar] [PubMed]
- Barthold, U. Luftansammlungen in der Regio parotidea und den Halsweichteilen bds. durch Selbstinsufflation. HNO 1998, 46, 64–65. [Google Scholar] [CrossRef]
- Gudlaugsson, Ó.; Geirsson, Á.J.; Benediktsdóttir, K. Pneumoparotitis: A new diagnostic technique and a case report. Ann. Otol. Rhinol. Laryngol. 1998, 107, 356–358. [Google Scholar] [CrossRef]
- Alcalde, R.E.; Ueyama, Y.; Lim, D.J.; Matsumura, T. Pneumoparotid: Report of a case. J. Oral Maxillofac. Surg. 1998, 56, 676–680. [Google Scholar] [CrossRef]
- Leuwer, A.; Greess, H. Schmerzlose rezidivierende Wangenschwellung. Parotisemphysem (Pneumoparotis). HNO 1998, 46, 766–767. [Google Scholar] [CrossRef]
- Golz, A.; Joachims, H.Z.; Netzer, A.; Westerman, S.T.; Gilbert, L.M. Pneumoparotitis: Diagnosis by computed tomography. Am. J. Otolaryngol. 1999, 20, 68–71. [Google Scholar] [CrossRef]
- Sittel, C.; Jungehülsing, M.; Fischbach, R. High-resolution magnetic resonance imaging of recurrent pneumoparotitis. Ann. Otol. Rhinol. Laryngol. 1999, 108, 816–818. [Google Scholar] [CrossRef]
- Kirsch, C.M.; Shinn, J.; Porzio, R.; Trefelner, E.; Kagawa, F.T.; Wehner, J.H.; Jensen, W.A. Pneumoparotid due to spirometry. Chest 1999, 116, 1475–1478. [Google Scholar] [CrossRef]
- Martín-Granizo, R.; Herrera, M.; García-González, D.; Mas, A. Pneumoparotid in childhood: Report of two cases. J. Oral Maxillofac. Surg. 1999, 57, 1468–1471. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.C.; Schuster, D.; Misko, G. Pneumoparotid: A case report and review of its pathogenesis, diagnosis, and management. Ear Nose Throat J. 2000, 79, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Nonaka, S.; Takahara, M.; Harabuchi, Y. A case of pneumoparotitis. Pract. Otol. 2001, 94, 33–37. [Google Scholar] [CrossRef][Green Version]
- Cho, Y.S.; Seo, I.S.; Na, D.G.; Chu, K.C. A case of recurrent pneumoparotitis in a wind instrumentalist. Korean J. Otolaryngol. 2001, 44, 330–332. [Google Scholar]
- Franco, V.; Houliat, T.; Devars, F.; Traissac, L. Pneumoparotide: À propos d’un cas, revue de la littérature. Rev. Laryngol. Otol. Rhinol. 2002, 123, 149–151. [Google Scholar]
- Brasseur, P.; Peché, R.; Sukkarieh, F.; Van Meerhaeghe, A. Emphysème sous-cutané et troubles de la personnalité. Rev. Pneumol. Clin. 2003, 59, 149–153. [Google Scholar]
- Orabi, A.A.; Nigam, A. Bilateral longstanding self-induced pneumoparotitis. Aust. J. Otolaryng. 2004, 7, 43–46. [Google Scholar]
- Han, S.; Isaacson, G. Recurrent pneumoparotid: Cause and treatment. Otolaryngol. Head Neck Surg. 2004, 131, 758–761. [Google Scholar] [CrossRef]
- Apaydin, M.; Sarsilmaz, A.; Calli, C. Giant pneumoparotitis. Eur. J. Radiol. Extra 2004, 52, 17–20. [Google Scholar] [CrossRef]
- Maehara, M.; Ikeda, K.; Ohmura, N.; Sugimoto, T.; Harima, K.; Ino, C.; Sawada, S. Multislice computed tomography of pneumoparotid: A case report. Radiat. Med. 2005, 23, 147–150. [Google Scholar]
- De Meerleer, K.; Hermans, R. Pneumoparotitis. JBR-BTR 2005, 88, 248. [Google Scholar] [PubMed]
- Grainger, J.; Saravanappa, N.; Courteney-Harris, R.G. Bilateral pneumoparotid. Otolaryngol. Head Neck Surg. 2006, 134, 531–532. [Google Scholar] [CrossRef] [PubMed]
- Paksoy, G.; Oğuz, H.; Arslan, N.; Demirci, M.; Şafak, M.A. Idiopathic recurrent pneumoparotitis: Case report. KBB-Forum 2006, 5, 161–163. [Google Scholar]
- Scherr, M.K.; Schmitz, S.; Wirth, S. Pneumoparotis und zervikales Emphysem als seltene Komplikation des Arzteprotestes. Rofo 2006, 178, 816–818. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M. A Case of parotid emphysema in a severely intellectually retarded patient with repeated mouth blowing habits. J. Sev. Mot. Intellect. Disabil. 2006, 31, 299–303. [Google Scholar]
- Yang, S.M.; Park, C.H.; Hong, S.J.; Kim, H.C. Case of bilateral pneumoparotitis in the children. Korean J. Otolaryngol. 2007, 50, 366–368. [Google Scholar]
- Chun, J.H.; Kim, H.Y.; Kwon, S.J.; Nam, S.Y. A case of self-induced pneumoparotitis. Korean J. Otorhinolaryngol. Head Neck Surg. 2007, 50, 726–728. [Google Scholar]
- Balasubramanian, S.; Srinivas, S.; Aparna, K.R. Pneumoparotitis with subcutaneous emphysema. Indian Pediatr. 2008, 45, 58–60. [Google Scholar]
- Núñez, M.J.H.; Navarro, J.R.B.; Fernández-Freire, A.R.; Pérez, M.A.R. Parotid pneumocele in Down’s syndrome. Acta Otorrinolaringol. Esp. 2008, 59, 41–42. [Google Scholar]
- Luaces, R.; Ferreras, J.; Patiño, B.; Garcia-Rozado, A.; Vázquez, I.; López-Cedrún, J.L. Pneumoparotid: A case report and review of the literature. J. Oral Maxillofac. Surg. 2008, 66, 362–365. [Google Scholar] [CrossRef]
- Joo, Y.H.; Shin, J.H.; Park, S.N.; Sun, D.I. A case of pneumoparotid treated by ligation of Stensen’s duct. Korean J. Otorhinolaryngol. Head Neck Surg. 2008, 51, 643–645. [Google Scholar]
- Prabhu, S.P.; Tran, B. Pneumoparotitis. Pediatr. Radiol. 2008, 38, 1144. [Google Scholar] [CrossRef] [PubMed]
- Faure, F.; Gaudon, I.P.; Tavernier, L.; Ayari-Khalfallah, S. A rare presentation of recurrent parotid swelling: Self-induced parotitis. Int. J. Pediatr. Otorhinolaryngol. Extra 2009, 4, 29–31. [Google Scholar] [CrossRef]
- Mukundan, D.; Jenkins, O. Images in clinical medicine. A tuba player with air in the parotid gland. N. Engl. J. Med. 2009, 360, 710. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.I.; Jung, H.W.; Joo, J.; Cho, J.E. A case of self-induced pneumoparotid. J. Clin. Otolaryngol. 2009, 20, 272–276. [Google Scholar] [CrossRef]
- Moënne, K.B.; Cordero, J.T.; Poli, C.H. Neumoparotiditis o neumoparótida en el niño: Un diagnóstico diferencial a considerar. Rev. Chil. Infectol. 2009, 26, 555–559. [Google Scholar] [CrossRef][Green Version]
- Kyung, S.K.; Heurtebise, F.; Godon, A.; Rivière, M.F.; Coatrieux, A. Head-neck and mediastinal emphysema caused by playing a wind instrument. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2010, 127, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, Y.; Fukuta, K.; Takeda, Y. Pneumoparotitis induced by an abnormal habit: Report of an adult case. Jpn. J. Oral Diag. 2011, 24, 26–29. [Google Scholar]
- Kolti, M. Blowing up a balloon leads to a blown-up face. Oral Surg. 2011, 4, 56. [Google Scholar]
- Van Ardenne, N.; Kurotova, A.; Boudewyns, A. Pneumoparotid: A rare cause of parotid swelling in a 7-year-old child. B-ENT 2011, 7, 297–300. [Google Scholar]
- Iwaki, H.; Suzuki, S.; Shinogami, M. A case of pneumoparotid due to habit of blowing the cheeks. Pract. Otol. 2011, 160, 131. [Google Scholar]
- Vasi, A.Z.; Hoskins, W.P. Radiology quiz case Bilateral self-induced pneumoparotitis. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 1041–1042. [Google Scholar] [CrossRef] [PubMed]
- Zuchi, D.F.; Silveira, P.C.; Cardoso, C.d.O.; De Almeida, W.M.; Feldman, C.J. Pneumoparotitis. Braz. J. Otorhinolaryngol. 2011, 77, 806. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, M.; Brown, J.; McGurk, M. Pneumoparotitis: A diagnostic challenge. Int. J. Oral Maxillofac. Surg. 2012, 41, 774–776. [Google Scholar] [CrossRef] [PubMed]
- Tekelioglu, U.Y.; Akkaya, A.; Apuhan, T.; Demirhan, A.; Bayır, H.; Kocoglu, H. A case of anesthesia mumps after general anesthesia. J. Anesth. 2012, 26, 130–131. [Google Scholar] [CrossRef]
- Tachibana, T.; Ogawara, Y.; Matsuyama, Y.; Abe, I.; Hase, S. A case of pediatric pneumoparotitis. Pract. Otol. 2012, 105, 567–570. [Google Scholar] [CrossRef]
- Li, F.; Ji, Y.X.; Zhu, S.R. A case report of pneumoparotid and review of the literature. China J. Oral Maxillofac. Surg. 2012, 10, 347–349. [Google Scholar]
- McCormick, M.E.; Bawa, G.; Shah, R.K. Idiopathic recurrent pneumoparotitis. Am. J. Otolaryngol. 2013, 34, 180–182. [Google Scholar] [CrossRef]
- Potet, J.; Arnaud, F.X.; Valbousquet, L.; Ukkola-Pons, E.; Donat-Weber, G.; Thome, A.; Peroux, E.; Teriitehau, C.; Baccialone, J. Pneumoparotid, a rare diagnosis to consider when faced with unexplained parotid swelling. Diagn. Interv. Imaging 2013, 94, 95–97. [Google Scholar] [CrossRef][Green Version]
- McGreevy, A.E.; O’Kane, A.M.; McCaul, D.; Basha, S.I. Pneumoparotitis: A case report. Head Neck 2013, 35, E55–E59. [Google Scholar] [CrossRef]
- Pillay, Y.; Goh, K.L. Transient unilateral pneumoparotid following upper endoscopy. JUMMEC 2014, 17, 12–13. [Google Scholar]
- Watanabe, S.; Hata, S.; Nakajima, J.; Yokoe, H.; Sato, Y. A case of pneumoparotid caused by strong pressure with an air syringe. J. Jpn. Stomatol. Soc. 2014, 63, 106–107. [Google Scholar]
- Nicot, R.; Myon, L.; Konopnicki, S.; Ferri, J.; Raoul, G. Pneumoparotide: Une étiologie rare de tumefaction parotidienne récidivante. Rev. Stomatol. Chir. Maxillofac. Chir. Orale 2014, 115, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Almario Hernández, A.F.; Trenchs Sainz de la Maza, V.; Sangorrin, A.; Luaces, C. Neumoparótida, a propósito de un caso. An. Pediatr. 2014, 81, e42–e43. [Google Scholar] [CrossRef]
- Konstantinidis, I.; Chatziavramidis, A.; Constantinidis, J. Conservative management of bilateral pneumoparotitis with sialendoscopy and steroid irrigation. BMJ Case Rep. 2014, bcr2013201429. [Google Scholar] [CrossRef]
- Ino, C.; Tada, N.; Minami, T.; Ino, M.; Tanabe, M. Pneumoparotid. Pract. Otol. 2015, 61, 155–169. [Google Scholar]
- Dietrich, U.; Holtmann, L.; Weller, P.; Ringelstein, A. Plötzliche Wangenschwellung nach Valsalva-Manöver. Laryngo-Rhino-Otol. 2015, 94, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Bowden, B.K.; Bowden, S.A. Cervicofacial subcutaneous emphysema in a 4-year-old boy. BMJ Case Rep. 2015, bcr2015210223. [Google Scholar] [CrossRef]
- Cabello, M.; Macias, E.; Fernández-Flórez, A.; Martínez-Martínez, M.; Cobo, J.; De Carlos, F. Pneumoparotid associated with a mandibular advancement device for obstructive sleep apnea. Sleep Med. 2015, 16, 1011–1013. [Google Scholar] [CrossRef]
- Osawa, Y.; Kaneko, M.; Takano, Y.; Maeda, S. Recurrent pneumoparotitis with pneumomediastinum. J. Jpn. Pediatr. Soc. 2015, 119, 1386–1390. [Google Scholar]
- Shibata, D.; Harada, T. Two cases of pneumoparotis. Kawasaki Med. J. 2016, 41, 25–31. [Google Scholar]
- Abdullayev, R.; Saral, F.C.; Kucukebe, O.B.; Sayiner, H.S.; Bayraktar, C.; Akgun, S. Bilateral parotitis in a patient under continuous positive airway pressure treatment. Braz. J. Anesth. Engl. Ed. 2016, 66, 661–663. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lagunas, J.G.; Fuertes, A.F. Self-induced parapharyngeal and parotid emphysema: A case of pneumoparotitis. Oral Maxillofac. Surg. Cases 2017, 3, 81–85. [Google Scholar] [CrossRef]
- Alnæs, M.; Furevik, L.L. Pneumoparotitis. Tidsskr. Nor. Laegeforen 2017, 137, 544. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oh, Y.S.; Kim, J.M.; Jung, H.J.; Shim, W.S. A case of bilateral pneumoparotid improved with conservative treatment. Korean J. Head Neck Surg. 2017, 33, 43–45. [Google Scholar] [CrossRef]
- Lee, K.P.; James, V.; Ong, Y.K.G. Emergency department diagnosis of idiopathic pneumoparotitis with cervicofacial subcutaneous emphysema in a pediatric patient. Clin. Pract. Cases Emerg. Med. 2017, 1, 399–402. [Google Scholar] [CrossRef][Green Version]
- Kwon, H.K.; Kim, D.J.; Chon, K.M.; Lee, B.J. A case of recurrent self-induced pneumoparotid. J. Clin. Otolaryngol. 2017, 28, 307–310. [Google Scholar]
- Goates, A.J.; Lee, D.J.; Maley, J.E.; Lee, P.C.; Hoffman, H.T. Pneumoparotitis as a complication of long-term oronasal positive airway pressure for sleep apnea. Head Neck 2018, 40, E5–E8. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Kojima, R.; Nakanishi, Y.; Kaneko, A. A case of early pneumoparotid presenting with oral noises. J. Oral Maxillofac. Surg. 2018, 76, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Paterson, T.; Maini, N.; Ganesh, V.; Newman, L. Pneumoparotid: An unusual case of intermittent unilateral cheek swelling. Int. J. Surg. 2018, 55, S27. [Google Scholar] [CrossRef]
- House, L.K.; Lewis, A.F. Pneumoparotitis. Clin. Exp. Emerg. Med. 2018, 5, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Miłoński, J.; Kolary, K.; Spencer, S.; Olszewski, J. A rare case of a pneumoparotid. J. Hear. Sci. 2019, 9, 46–50. [Google Scholar]
- Ambrosino, R.; Lan, R.; Romanet, I.; Le Roux, M.-K.; Gallucci, A.; Graillon, N. Severe idiopathic pneumoparotitis: Case report and study review. Int. J. Pediatr. Otorhinolaryngol. 2019, 125, 196–198. [Google Scholar] [CrossRef]
- Basha, M.S. A rare case of unilateral pnemoparotid. Sci. Arch. Dent. Sci. 2019, 2, 13–15. [Google Scholar]
- Raczkowska-Łabuda, K.; Jabłońska-Jesionowska, M.; Jadczyszyn, J.; Frąckiewicz, M.; Pilch, M.; Zawadzka-Glos, L. Self-induced pneumoparotitis—A rare case report. New Med. 2019, 2, 60–67. [Google Scholar] [CrossRef]
- Kim, H.Y.; Park., K.S.; Jung, S.H.; Lee, D.H. Incidentally diagnosed asymptomatic pneumoparotid. Korean J. Head Neck Oncol. 2019, 35, 81–83. [Google Scholar] [CrossRef][Green Version]
- Enami, S.; Sato, A.; Myers, M.; Maruoka, Y. A case of emphysema of the parotid gland caused by dental treatment. Jpn. J. Oral Diag. 2020, 33, 39–42. [Google Scholar] [CrossRef]
- Yang, Z.; Bundrick, P.E. Subcutaneous emphysema of the neck with pneumomediastinum. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 306–307. [Google Scholar] [CrossRef]
- Sinha, D.; Brown, J.; Fan, K. Bubbling parotitis. Clin. Surg. 2020, 5, 2812. [Google Scholar]
- Gazia, F.; Freni, F.; Galletti, C.; Bruno, R.; Galletti, C.; Meduri, A.; Galletti, F. Pneumoparotid and pneumoparotitis: A literary review. Int. J. Environ. Res. Public Health 2020, 17, 3936. [Google Scholar] [CrossRef]
- Gray, S.C.; Pienaar, J.A.; Sofianos, Z.; Varghese, J. Complicated spontaneous pneumoparotid mimicking a neck mass in a child with Down’s syndrome. SA J. Radiol. 2020, 24, 1883. [Google Scholar] [CrossRef] [PubMed]
- Aljeaid, D.; Mubarak, A.; Imarli, Y.; Alotaibi, O. Pneumoparotid: A rare but well-documented cause of parotid gland swelling. Egypt J. Otolaryngol. 2020, 36, 46. [Google Scholar] [CrossRef]
- Al Ohali, S.; Al-Qahtani, M.; Al Shahrani, M. Self-induced pneumoparotid: Case report of a rare cause. Oral Sci. Int. 2020, 17, 179–182. [Google Scholar] [CrossRef]
- Fernandez, S.; Garaycochea, O.; Prieto-Matos, C.; De Linera, M.A.; Alcalde, J. Inflating parotids with air: A case of pneumoparotid and review of the literature. A case of pneumoparotid. Otolaryngol. Case Rep. 2020, 17, 100227. [Google Scholar] [CrossRef]
- Yoshida, K. Pneumoparotid related to obstructive sleep apnea syndrome treated by oral appliance with anterior opening to reduce intraoral pressure. Clin. Case Rep. 2022, 10, e05816. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.; Sievert, M.; Mantsopoulos, K.; Schapher, M.L.; Mueller, S.K.; Iro, H.; Koch, M. Pneumoparotid: Practical impact of surgeon performed ultrasound in an effective diagnostic approach. Oral Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Hyrtl, J. Vordere Mundhöhle. Handbuch der Topographischen Anatomie und Ihrer Praktisch Medicinisch-Chirurgischen Anwendungen, 5th ed.; Braumüller: Wien, Austria, 1865; p. 390. [Google Scholar]
- Guinand. Plaques opalines professionelles de la bouche chez les souffleurs de verse. Lyon Med. 1880, 34, 303–308. [Google Scholar]
- Reichert, H. Berufsschädigungen bei Gläsern in der Mundhöhle und am Zahnsystem. Zent. Gewerbehyg. 1922. [Google Scholar]
- Wizgall, E. Die Pneumatocele der Parotis als Berufskrankheit der Glasbläser; Eisele: Munich, Germany, 1932. [Google Scholar]
- Trémollieres, F.; Caussade, L. Simulation des oreillons. Presse Méd. 1918, 192, 334. [Google Scholar]
- Medical News. Factitious Mumps. JAMA 1918, 71, 679. [Google Scholar]
- The simulation of mumps. Lancet 1918, 192, 363. [CrossRef]
- Office of the Surgeon General. Simulation of mumps. In Survey of Head Surgery; Division of Surgery of the Head in the Office of the Surgeon General: Washington, DC, USA, 1919; Volume 1, p. 171. [Google Scholar]
- Reilly, D.J. Benign transient swelling of the parotid glands following general anaesthesia: “anaesthesia mumps”. Anesth. Analg. 1973, 49, 560–563. [Google Scholar]
- Adachi, Y.U.; Matsuda, N. Is it fluid or air causing anesthesia mumps? J. Anesth. 2012, 26, 638–639. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Banks, P. Nonneoplastic parotid swellings: A review. Oral Surg. 1968, 25, 732–745. [Google Scholar] [CrossRef]
- Mandel, L.; Surattanont, F. Bilateral parotid swelling: A review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 93, 221–237. [Google Scholar] [CrossRef]
- Berkovitz, B.K.B. Parotid salivary gland. In Gray’s Anatomy, 41st ed.; Standring, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 504–505. [Google Scholar]
- Zenk, J.; Zikarsky, B.; Hosemann, W.; Iro, H. Die Durchmesser des Stenon- und Wharton-Ganges Bedeutung für Diagnostik und Therapie. HNO 1998, 46, 980–985. [Google Scholar] [CrossRef]
- Amano, K.; Shiga, H.; Sukekawa, R.; Itoh, I. Morphological study of the parotid duct -The running course in the buccal muscle and the morphology of the orifice of the parotid duct. Jpn. J. Oral Biol. 2002, 44, 515–521. [Google Scholar] [CrossRef]
- Amano, K.; Moriyama, H.; Shimada, K.; Matsumura, G. Study of human adult parotid duct in the area of penetration through buccinator muscle and their functional relationship as a sphincter. Ital. J. Anat. Embryol. 2013, 118, 6–18. [Google Scholar]
- Donders, F.C. Druckverhältnisse beim Athmen. In Physiologie des Menschen, 2nd ed.; Donders, F.C., Bauduin, A.F., Eds.; Hirzel: Leipzig, Germany, 1875; pp. 414–420. [Google Scholar]
- Bouhuys, A. Lung volumes and breathing patterns in wind-instrument players. J. Appl. Physiol. 1964, 19, 967–975. [Google Scholar] [CrossRef]
- Schwab, B.; Schultze-Florey, A. Intraorale Druckentwicklung bei Holz- und Blechbläsern. Musikphysiol. Musikermred. 2004, 4, 183–194. [Google Scholar]
- Kreuter, M.; Kreuter, C.; Herth, F. Pneumologische Aspekte des Musizierens auf einem Blasinstrument—Physiologische, pathophysiologische und therapeutische Gesichtspunkte. Pneumologie 2008, 62, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.M.; Van Zundert, A.A.J. Intraoperative Valsalva maneuver: A narrative review. Can. J. Anaesth. 2018, 65, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.W.; Devine, K.D.; Beahrs, O.H. Acute postoperative parotitis (“surgical mumps“). Plast. Reconstr. Surg. 1960, 25, 51–58. [Google Scholar] [CrossRef]
- Mundé, P.F. A case of ovariotomy during subacute peritonitis and suppuration of the cyst following aspiration: With remarks. Am. J. M. Sc. 1878, 75, 100–108. [Google Scholar] [CrossRef]
- Bonchek, L.I. Salivary gland enlargement during induction of anesthesia. JAMA 1969, 209, 1716–1718. [Google Scholar] [CrossRef]
- Sarr, M.G.; Frey, H. A unique case of benign postoperative parotid swelling. John Hopk. Med. J. 1980, 146, 11–12. [Google Scholar]
- Attas, M.; Sabawala, P.B.; Keats, A.S. Acute transient sialadenopathy during induction of anesthesia. Anesthesiology 1968, 29, 1050–1052. [Google Scholar] [CrossRef]
- Couper, J.L. Benign transient enlargement of the parotid glands associated with anesthesia. South Afr. Med. J. 1973, 47, 316–318. [Google Scholar]
- Shields, H.M.; Soloway, R.D.; Long, W.B.; Weiss, J.B. Bilateral recurrent parotid gland swelling after endoscopy. Gastroenterology 1977, 73, 164–165. [Google Scholar] [CrossRef]
- Palmer, E.D.; Boyce, H.V., Jr. Manual of Gastrointestinal Endoscopy; The Williams & Wilkins Company: Baltimore, MD, USA, 1964; p. 74. [Google Scholar]
- Slaughter, R.L.; Boyce, H.V., Jr. Submaxillary salivary gland swelling development during peroral endoscopy. Gastroenterology 1969, 57, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, T. Compton’s pouch. Stomach Intest. 1971, 6, 1404. [Google Scholar]
- Maruyama, M.; Endo, M.; Takemoto, T. Swelling of the neck during peroral endoscopy. Gastroenterol. Endosc. 1972, 14, 82–86. [Google Scholar]
- Fujita, K.; Yamamura, M. Compton’s pouch: Report of a case. Jpn. J. Oral Maxillofac. Surg. 1982, 28, 1755–1757. [Google Scholar] [CrossRef]
- Kuriyama, A. Compton’s pouch. J. Med. J. 1981, 2990, 70. [Google Scholar]
- Koch, M.; Iro, H. Salivary duct stenosis: Diagnosis and treatment. Acta Otorhinolaryngol. Ital. 2017, 37, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Blatt, I.M. The parotid-masseter hypertrophy-traumatic occlusion syndrome. Laryngoscope 1969, 79, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Aghaei Lasboo, A.; Nemeth, A.J.; Russell, E.J.; Siegel, G.J.; Karagianis, A. The use of the “puffed-cheek” computed tomography technique to confirm the diagnosis of pneumoparotitis. Laryngoscope 2010, 120, 967–969. [Google Scholar] [CrossRef]
- Asher, R. Munchausen’s syndrome. Lancet 1951, 1, 339–341. [Google Scholar] [CrossRef]
- Tatu, L.; Aybek, S.; Bogousslavsky, J. Munchausen Syndrome and the Wide Spectrum of Factitious Disorders. Front. Neurol. Neurosci. 2018, 42, 81–86. [Google Scholar] [CrossRef]
- Sousa Filho, D.; Kanomata, E.Y.; Feldman, R.J.; Maluf Neto, A. Munchausen syndrome and Munchausen syndrome by proxy: A narrative review. Einstein 2017, 15, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Treatment and research of sleep apnea syndrome from clinical and neurophysiological aspects in the stomatognathic system. Int. J. Med. Biol. Front. 2011, 17, 893–980. [Google Scholar]
- Yoshida, K. Treatment and Research of Sleep Apnea Syndrome from Clinical and Neurophysiological Aspects in the Stomatognathic System. Available online: https://sites.google.com/site/sleepapneasyndrome/ (accessed on 1 November 2022).
- Ahuja, C.K.; Yadav, M.K.; Gupta, V.; Khandelwal, N. Incidental pneumoparotid detected on computed tomography: Should it raise an alarm. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.; Kuppuswamy, M.; Santosh Kumar, D.G.; Bhat, V.; Karthik, G.A. Pneumoparotid in “puffed cheek” computed tomography: Incidence and relation to oropharyngeal conditions. Br. J. Oral Maxillofac. Surg. 2015, 53, 239–243. [Google Scholar] [CrossRef]
- Kaye, B.I. Discussion on “Rupture of the orbicularis oris in trumpet plyers (Satchmo’s syndrome)”. Planus J. 1982, 69, 696–697. [Google Scholar]
- Wilkie, T.F.; Brody, G.S. The surgical treatment of drooling. A ten-year review. Plast. Reconstr. Surg. 1977, 59, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Chitre, V.V.; Premchandra, D.J. Recurrent parotitis. Arch. Dis. Child 1997, 77, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Prosthetic therapy for sleep apnea syndrome. J. Prosthet. Dent. 1994, 72, 296–302. [Google Scholar] [CrossRef]
- Yoshida, K. Influence of sleep posture on response to oral appliance therapy for sleep apnea syndrome. Sleep 2001, 24, 538–544. [Google Scholar] [CrossRef]
- Yoshida, K. Effect on blood pressure of oral appliance therapy for sleep apnea syndrome. Int. J. Prosthodont. 2006, 19, 61–66. [Google Scholar]
- Ramar, K.; Dort, L.C.; Katz, S.G.; Lettieri, C.J.; Harrod, C.G.; Thomas, S.M.; Chervin, R.D. Clinical Practice Guideline for the Treatment of Obstructive Sleep Apnea and Snoring with Oral Appliance Therapy: An Update for 2015. J. Clin. Sleep. Med. 2015, 11, 773–827. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.; McEvoy, R.D.; Banks, S.; Tarquinio, N.; Murray, C.G.; Vowles, N.; Pierce, R.J. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2004, 170, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Hoekema, A.; Stegenga, B.; Wijkstra, P.J.; Van Der Hoeven, J.H.; Meinesz, A.F.; De Bont, L.G. Obstructive sleep apnea therapy. J. Dent. Res. 2008, 87, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.L.; Grunstein, R.R.; Darendeliler, M.A.; Mihailidou, A.S.; Srinivasan, V.K.; Yee, B.J.; Marks, G.B.; Cistulli, P.A. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: A randomized controlled trial. Am. J. Respir. Crit. Care Med. 2013, 187, 879–887. [Google Scholar] [CrossRef]
- Marklund, M.; Carlberg, B.; Forsgren, L.; Olsson, T.; Stenlund, H.; Franklin, K.A. Oral appliance therapy in patients with daytime sleepiness and snoring or mild to moderate sleep apnea: A randomized clinical trial. JAMA Intern. Med. 2015, 175, 1278–1285. [Google Scholar] [CrossRef]
- Sharples, L.D.; Clutterbuck-James, A.L.; Glover, M.J.; Bennett, M.S.; Chadwick, R.; Pittman, M.A.; Quinnell, T.G. Meta-analysis of randomised controlled trials of oral mandibular advancement devices and continuous positive airway pressure for obstructive sleep apnoea-hypopnoea. Sleep Med. Rev. 2016, 27, 108–124. [Google Scholar] [CrossRef]
- Gagnadoux, F.; Pépin, J.L.; Vielle, B.; Bironneau, V.; Chouet-Girard, F.; Launois, S.; Meslier, N.; Meurice, J.C.; Nguyen, X.L.; Paris, A.; et al. Impact of mandibular advancement therapy on endothelial function in severe obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2017, 195, 1244–1252. [Google Scholar] [CrossRef]
- Yoshida, K. Effect of a prosthetic appliance for treatment of sleep apnea syndrome on masticatory and tongue muscle activity. J. Prosthet. Dent. 1998, 79, 537–544. [Google Scholar] [CrossRef]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Hemphill, R.A. Wind parotitis. N. Engl. J. Med. 1973, 289, 1094–1095. [Google Scholar]
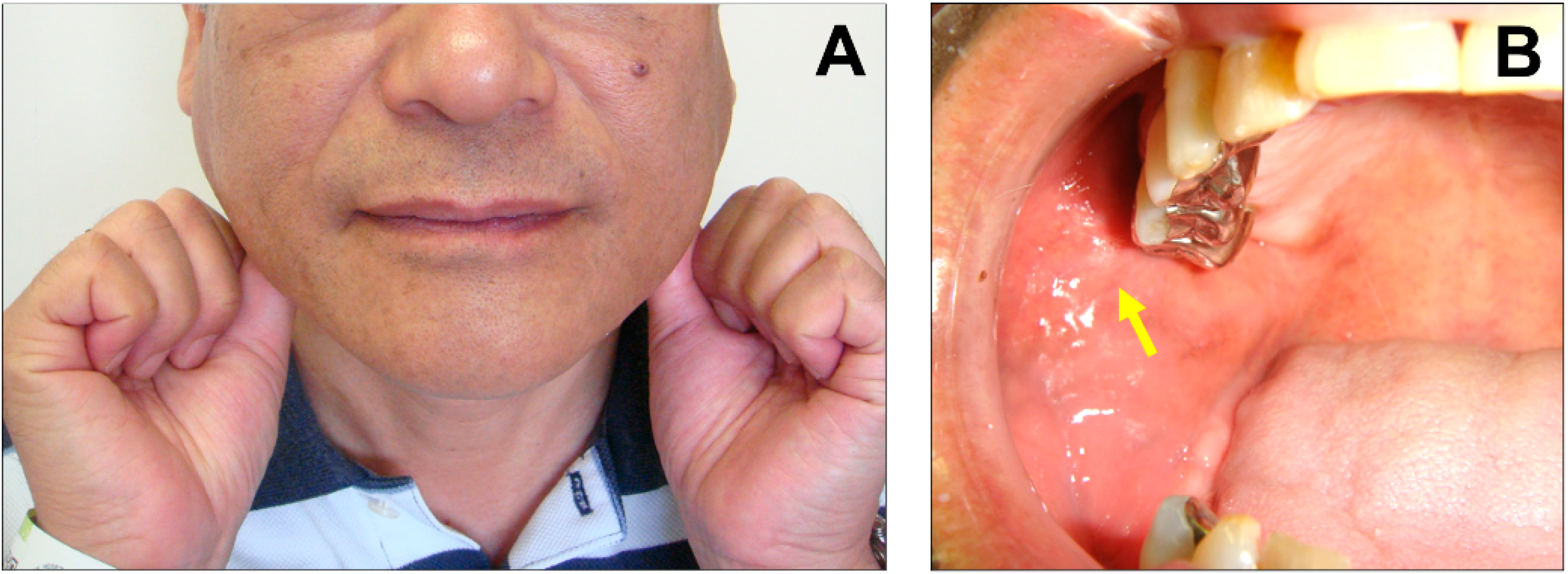
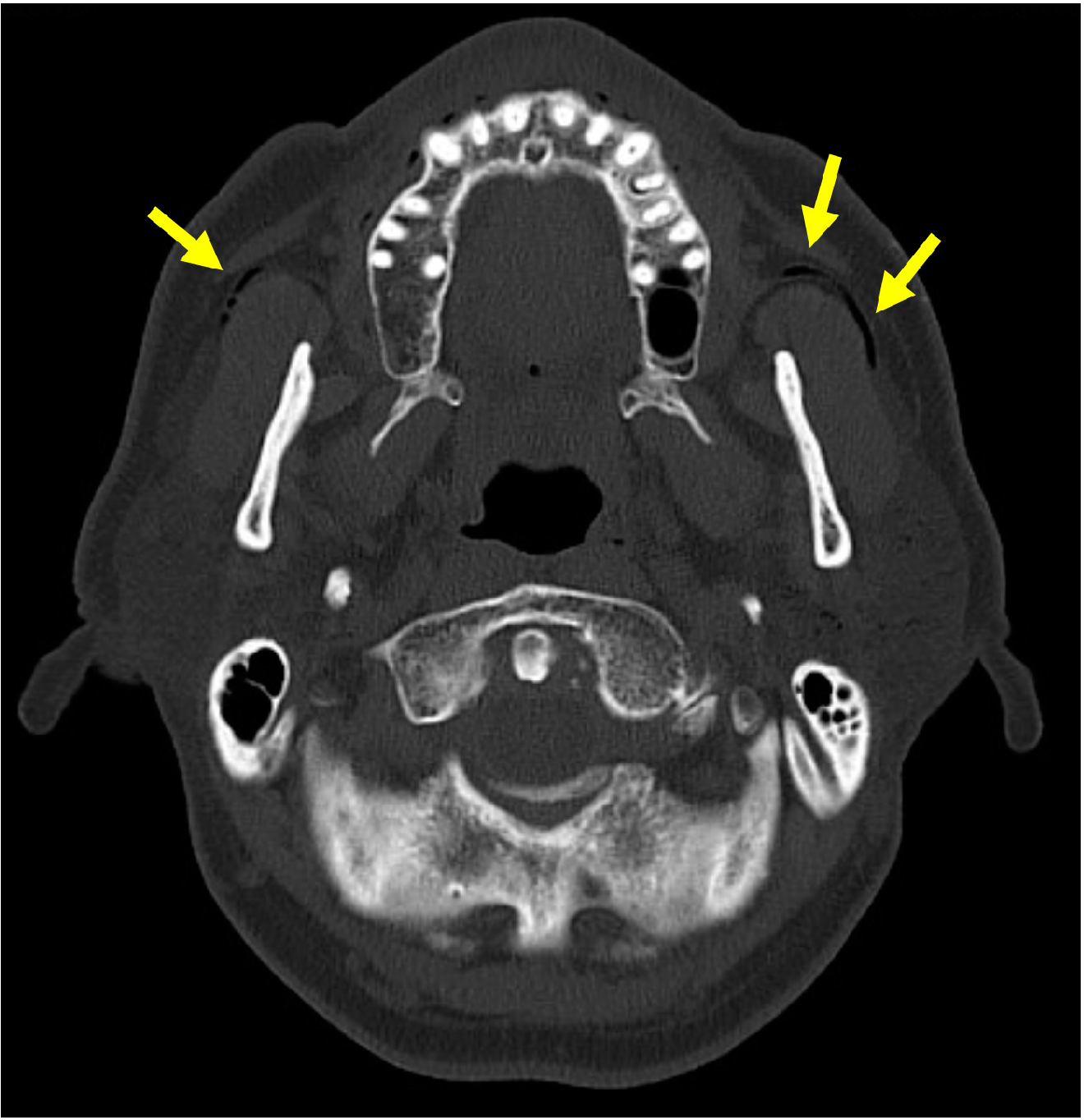

| Affected side, [n (%)] | Bilateral: 59 (34.7%), left: 56 (32.9%), right: 47 (27.6%), NR: 8 (4.7%) |
| Crepitus on the parotid region, [n (%)] | Yes: 68 (40%), No: 18 (10.6%), NR: 84 (49.4%) |
| Bubbles from the orifice of the duct, [n (%)] | Yes: 67 (39.4%), No: 33 (19.4%), NR: 70 (41.2%) |
| Symptoms, [n (%)] | Swelling: 144 (84.7%), pain: 61 (35.9%), discomfort: 8 (4.7%), noise 6 (3.5%) |
| Emphysema, [n (%)] | Face: 30 (17.6%), neck: 23 (13.5%), mediastinum: 6 (3.5%) |
| Diagnostic images, [n (%)] | CT: 95 (55.9%), US: 47 (27.6%), sialography: 35 (20.6%), radiography: 29 (17.1%), sialendoscopy: 13 (7.6%), MRI: 6 (3.5%), fluoroscopy: 1 (0.6%), NR: 26 (15.3%) |
| Imaging findings, [n (%)] | Air in the gland: 100: (58.8%), air in the duct: 55 (32.4%), enlarged duct: 27 (15.9%), emphysema: 19 (11.2%), mass: 14 (8.2%), enlarged gland: 11 (6.5%) |
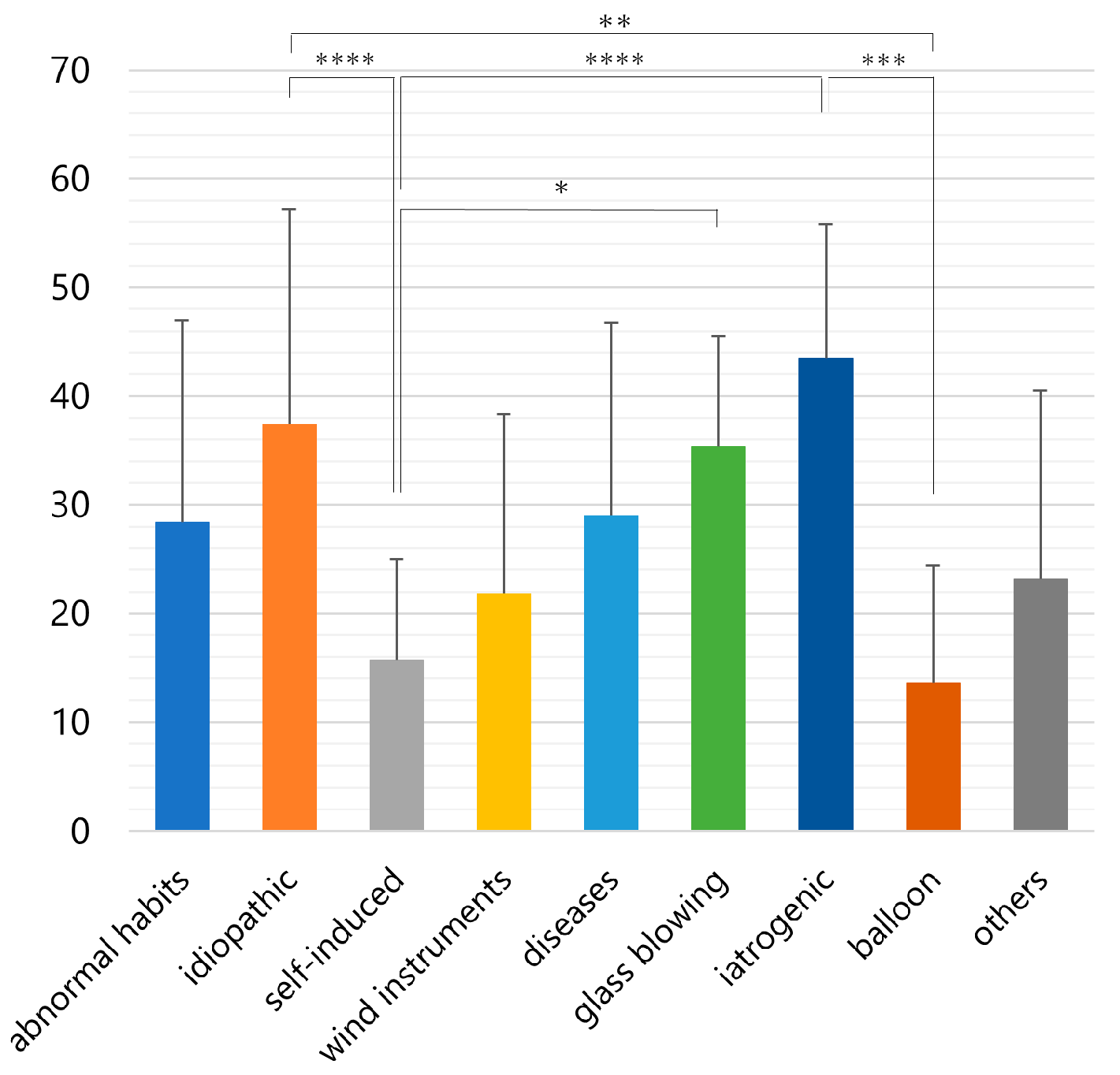
| Abnormal habits, n = 40 women; 12, men; 28 | Blowing out the cheeks; n = 35, puffing out of the cheeks to prevent irritation by orthodontic braces; n = 2, biting the lower lip and whistling with a high-frequency sound; n = 1, blowing out the cheek to stop aphthous ulcer pain while eating; n = 1, puffing the cheek during unbearable itching; n = 1 |
| Idiopathic, n = 34 women; 8, men; 26 | No causative factors were identified; n = 34 |
| Self-induced, n = 27 women; 7, men; 20 | Blowing out the cheeks with closed mouth; n = 26, self-injury into the Stensen’s duct with pins; n = 1 |
| Wind instruments, n = 15 women; 4, men; 7, NR; 4 | Trumpet; n = 4, flute; n = 2, clarinet; n = 1, horn; n = 1, tuba; n = 1, fanfare; n = 1, paper trumpet; n = 1, recorder; n = 1, NR; n = 3 |
| Diseases, n = 14 women; 4, men; 9 | Coughing attack; n = 4, nervous tic; n = 3; obstructive sleep apnea syndrome; n = 2, sneezing crisis; n = 1, mental disorder; n = 1, clearing nares during hay fever attack; n = 1, head and maxillofacial trauma; n = 1, vomiting; n = 1 |
| Glass blowing, n = 12 women; 0, men; 12 | Professional glass blower; n = 12 |
| Iatrogenic, n = 11 women; 4, men; 6, NR; 1 | Dental air syringe; n = 4, continuous positive airway pressure; n = 2, air powder prophylaxis unit; n = 1, spirometry; n = 1, general anesthesia; n = 1, upper endoscopy; n = 1, noninvasive positive pressure ventilation; n = 1 |
| Balloon, n = 9 women; 0, men; 8, NR; 1 | Balloon blowing; n = 7, balloon blowing for their children; n = 2 |
| Others, n = 8 women; 2, men; 6 | Decompression after diving; n = 1, watchkeeping in a compartment; n = 1, massage in the periauricular region; n = 1, lifting heavy luggage; n = 1, diving with air in the oral cavity; n = 1, facial trauma; n = 1, diving while holding breath; n = 1, radiation therapy; n = 1 |
| Treatment, [n (%)] | Antibiotics; n = 51 (30%) |
| Behavioral therapy; n = 44 (25.9%) | |
| Psychiatric therapy; n = 14 (8.2%) | |
| Analgesics; n = 10 (5.9%) | |
| Aspiration; n = 8 (4.7%) | |
| Massage; n = 4 (2.4%) | |
| Parotidecomy; n = 4 (2.4%) | |
| Rerouting; n = 4 (2.4%) | |
| Continuous positive airway pressure; n = 3 (1.8%) | |
| Incision; n = 3 (1.8%) | |
| Ductal ligation; n = 2 (1.2%) | |
| Irrigation; n = 2 (1.2%) | |
| Oral appliance; n = 1 (0.6%) | |
| NR; n = 45 (27.1%) | |
| Resolution, [n (%), mean ± SD] | Yes; n = 87 (51.2%), 9.9 ± 26.5 (days), range (0–180), |
| No; n = 10 (5.9%) | |
| NR; n = 73 (42.9%) | |
| Relapse, [n (%)] | Yes, n = 29 (17.1%) |
| No; n = 47 (27.6%) | |
| NR; n = 94 (55.3%) | |
| Follow-up, (months) [n (%), mean ± SD] | Yes; n = 42 (24.7%), 20 ± 27.4, range (1 week–10 years), |
| No; n = 7 (4.1%) | |
| NR; n = 121 (71.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K. Etiology of Pneumoparotid: A Systematic Review. J. Clin. Med. 2023, 12, 144. https://doi.org/10.3390/jcm12010144
Yoshida K. Etiology of Pneumoparotid: A Systematic Review. Journal of Clinical Medicine. 2023; 12(1):144. https://doi.org/10.3390/jcm12010144
Chicago/Turabian StyleYoshida, Kazuya. 2023. "Etiology of Pneumoparotid: A Systematic Review" Journal of Clinical Medicine 12, no. 1: 144. https://doi.org/10.3390/jcm12010144
APA StyleYoshida, K. (2023). Etiology of Pneumoparotid: A Systematic Review. Journal of Clinical Medicine, 12(1), 144. https://doi.org/10.3390/jcm12010144






