Abstract
Immunotherapy with immune checkpoint inhibitors (ICIs) have been reported to induce de novo or exacerbate pre-existing Myasthenia Gravis (MG). We present a single center case series of patients who developed an immune-related myasthenia gravis (irMG) related with ICIs. We performed a retrospective chart review of the electronic medical records between 1 September 2017 and 2022. We report the clinical features, presentation forms, diagnostic workflows, general management and outcomes of six patients who received ICIs for different solid organ malignancies and developed an irMG frequently overlapping with immune-related myocarditis and/or myositis. The aim of the article is to describe the clinical features, treatment and outcomes of this challenging and potentially life-threating syndrome, comparing our data with those described in the literature. Differences between irMG and classic MG are highlighted.
1. Introduction
Immune checkpoint inhibitors (ICIs) are a type of passive immunotherapy that have become part of the standard of care of many cancer types including lung, liver, pancreas, renal, breast, melanoma and lymphoma [1]. The increasing use of ICIs in patients with cancer, either in monotherapy or in combination with chemotherapy or other ICI, has led to markedly improved survival rates and longer remission periods [2]. To date, the ICIs approved by the European Medicines Agency (EMA) and Food and Drug Administration (FDA) include monoclonal antibodies targeting immune checkpoint molecules programmed cell death protein-1 (PD-1) (pembrolizumab, nivolumab, cemiplimab), or its ligand (PD-L1) (atezolizumab, avelumab, durvalumab), and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) (ipilimumab, tremelimumab) [3,4]. In addition, a new ICI (relatlimab) has been recently approved against a novel target: lymphocyte-activation gene 3 (LG3) [5].
ICIs induce the immune system, blocking the co-inhibitory T-cell signals that, under normal conditions, prevent the chronic activation of the immune system. Thus, immune checkpoint inhibition can enhance the antitumor activity of T cells stimulating the destruction of the cancer cells [6]. Despite its effectiveness, several immune-related adverse events (irAEs) associated to ICI therapy have been described and any system can be affected [7]. The most commonly involved organs are the gastrointestinal tract, endocrine glands, skin and liver [8]. Neurological immune related adverse events (nrl-irAEs) are uncommon presenting an estimated overall incidence of 7.2% [9]; with severe forms occurring in up to 3% of patients receiving ICIs [10]. Nrl-irAEs include a wide spectrum of manifestations involving the entire neuroaxis, i.e., muscle, neuromuscular junction, peripheral nerve and central nervous system (CNS) [11,12,13]. Neuromuscular disorders are more frequent and have an earlier presentation than those involving the CNS [3,12]. Among neuromuscular irAEs, myasthenia gravis (MG) is the third in frequency after myositis and peripheral neuropathies including Guillain-Barré syndrome, and cranial neuropathies. However, irMG is associated with the highest morbidity and mortality rates [3,11,14].
Immune-related myasthenia gravis (irMG) can occur as an exacerbation of pre-existing MG or de novo in patients with no previous MG diagnosis. The clinical picture is usually characterized by progressive weakness affecting the extraocular, bulbar and limb muscles, progressing to respiratory failure in 40–65% of reported cases [13]. Some patients have mild symptoms such as ptosis while other patients may present with rapidly progressive respiratory failure with fatal outcome [15]. The presence of myositis and myocarditis overlapping irMG is a very common association. This syndrome, also named “3M triad”, is especially challenging and life-threatening [13,15,16,17].
An approach involving experienced neurologists in specialized multidisciplinary care teams is of great importance in the management of these patients [13,18]. In the present retrospective case-series study, we describe our experience in an oncologic center with six patients diagnosed of irMG due to ICI. Clinical, biological, radiological, electrophysiological and outcome data are described, with the objective of contributing to the knowledge of irMG and their overlaps.
2. Materials and Methods
Consecutive patients with suspected ICI-related neuromuscular irAE assessed at the Neuro-Oncology Unit of Catalan Institute of Oncology-Bellvitge University Hospital (Hospitalet de Llobregat, Barcelona, Spain) on dates between 1 September 2017 and 1 September 2022, were reviewed. We included patients who met the diagnostic criteria for irMG described by the “Consensus Disease Definitions for nrl-irAEs of ICI” published on May 2021 [19]. irMG diagnosis is definite when the patient has symptoms, electrodiagnostic studies (EDX) and positive antibodies (Ab) (acetylcholine receptor (anti-R Ach) or Musk). A probable diagnosis is considered when, in the clinical context, the patient has compatible EDX or positive Ab or unequivocal clinical response with cholinesterase inhibitors. The classification includes the possible category when the Ab are negative (or not performed), the EDX does not show disorders of the neuromuscular junction (without irritative myopathy) but the patient has a clinical picture compatible with normal creatin kinase (CK) serum levels. We assessed the clinical severity of irMG using the Myasthenia Gravis Foundation of America (MGFA) classification. Briefly, MGFA class I is defined as ocular muscle weakness, MGFA classes II as mild weakness involving any other than ocular muscles. MGFA class III and IV are defined by moderate and severe muscle weakness, respectively. MGFA class V is defined as myasthenic crisis with respiratory failure requiring endotracheal intubation or non-invasive mechanical ventilation [20]. Additionally, the severity of irMG was classified according to the adapted the Common Terminology Criteria for Adverse Events Criteria (CTCAE) for nrl-irAEs [19]. Standard grading (severity) CTCAE scale was used for non-neurological irAE. Briefly, CTCAE displays grades 1 (mild) through 5 (death due to the AE) with unique clinical descriptions of severity [21]. Collected data from our own files included patient´s demographics and baseline characteristics (age, gender, type of cancer, ICI therapy), non-neurological associated irAEs, clinical course, serological and EDX results, treatments received, evolution and subsequent outcome. The whole of patients were assessed by neurooncologists and neurologists specialized in neuromuscular diseases, who underwent EDX studies. This retrospective study was launched after having obtained approval from the Institutional Ethics Review Board (PR309/22). Written informed consent was taken from living patients and waiver of consent from deceased patients. We excluded patients who did not meet the Guidon diagnostic criteria irMG or those for whom we did not have sufficient clinical or EDX data to be able to evaluate them properly.
3. Results
3.1. Patients
Six patients with ICI-related MG diagnosis were included (all men); the median age at symptom onset was 74 years old (range, 65–85). No patients reported history of previous MG, thymoma, positive AntiR-Ach or anti-Musk Ab or other autoimmune diseases. All less one patient developed irMG with anti-PD1 treatment. The median follow-up from symptom onset to the last visit (or death) was 196 days [range 30–487]. Demographic, clinical, and electrodiagnostic data are summarized in Table 1.

Table 1.
Characteristics of patients with irMG.
3.2. Clinical Features irMG, Laboratory and Radiological Results
In our series, three (50%) patients met the MG diagnostic criteria of definitive, two (33%) of probable and one (17%) of possible irMG according to the recently established diagnosis immune related MG [19]. Five patients (83%) developed moderate to severe disease (MGFA III-V). Regarding the irMG onset, we can differentiate two onset patterns: those who developed symptoms early after the first ICI cycles (patients 1, 2, 3 and 6) with an early onset (median 33 days; range 10–60), and those with a delayed irMG onset (patients 4 and 5), presenting with irMG after receiving more than 30 cycles, with a median onset of 804 days [623–986 days] after first ICI dose. Noteworthy, both patients with late-onset irMG were on chronic low-dose corticosteroid treatment for previous adrenal insufficiency related to ICIs. However, no recent changes in corticosteroid doses had been done before irMG onset.
Clinical manifestations initially were mainly cranial symptoms, including: diplopia (n = 5, 83%), dysphonia (n = 4, 67%), ptosis (n = 4, 67%), and dysphagia (n = 5, 83%). Limb weakness was present in four out of six patients (67%), three of whom had concurrent myalgia. Four patients (67%) associated both ir-myocarditis and ir-myositis.
Blood CKs levels and troponin T were markedly increased in half of patients, with a mean of 13-fold (range 8–18) the normal value of our laboratory reference value. AntiR Ach were identified in all but one patient (83%), and titles were variable. None of the patients of the present series had anti-Musk positivity neither had Antinuclear Ab. Anti-titin antibodies were positive in two of the three (67%) patients tested.
Patient 2 had concurrent hepatitis with elevated alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT) and coagulation disorder at irMG diagnosis. Two patients (Patient 3 and 6) had elevated ALT/AST, associated with CK peak and no other signs of hepatic failure.
3.3. Electrophysiological Tests
Electroneuromyography including repetitive nerve stimulation (RNS) at 3 Hz and postexercise facilitation were performed in the whole series of patients. Additionally, single fiber electromyography (SFEMG) was done in four out of six patients. RNS at 3 Hz was normal in all muscles tested (nasalis, trapezius and adductor digiti minimi) in our six patients (Figure 1). Postexercise facilitation was also normal in all of them. Two out of four patients who underwent single fiber study had an increased jitter. Patient 3 showed an unstable motor unit potential (increase jiggle) [19,22], which can be observed in Figure 1. On needle electromyography (EMG) most patients (5/6, 83%) showed a myopathic pattern, characterized by the presence of mild spontaneous activity and myopathic recruitment (polyphasic, short-duration, or low amplitude motor unit action potential with normal or early recruitment) in proximal muscles of upper and lower extremities. The patient with an ocular form of irMG had normal EMG.
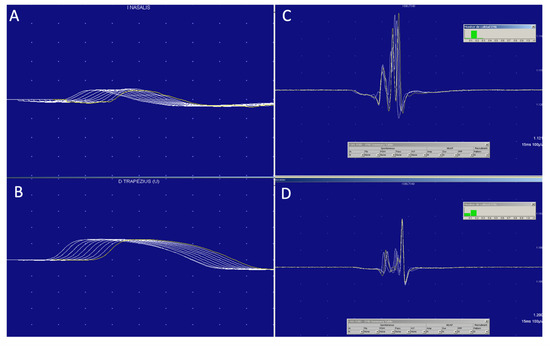
Figure 1.
MG diagnosis is clinical, supported by antibodies and neurophysiological studies, which do not always show the classic drop in potential in 3 Hz repetitive stimulation (A,B), but, in which, the variability or the Jiggle of the motor unit (C,D) can be a very useful sign demonstrating impaired neuromuscular transmission.
3.4. Treatment and Outcomes
ICI therapy was discontinued after irMG diagnosis in all patients, and none of them have restarted it to date. The average time from symptom onset to irMG suspicion or diagnosis and therefore treatment initiation was 28 days [4–69 days]. All but one of our patients (patient 4) required hospitalization. Corticosteroids and pyridostigmine were initially administered to all patients. In total, five patients received intravenous immunoglobulins (IGIV), in four of them administered at diagnosis concomitantly with corticoids (patient 2, 3, 4, and 6) and in one patient (patient 1) IGIV was administered due to lack of improvement. Plasma exchange (PEX) was performed in two patients due to the absence of improvement after corticosteroids and immunoglobulins. Third-line therapy with immunosuppressants like Rituximab and cyclophosphamide was considered in two patients.
In our series, irMG presented with associated myositis and myocarditis, in the setting of the overlap syndrome called “3M triad”, in four (67%) patients. Most of them (3/4, 75%) required admission to the intensive care unit and mechanical ventilation. In two of them (patient 1 and 2) the indication for ventilation was related to respiratory failure due to the irMG and the median time between the onset of symptoms and the start of mechanical ventilation was 12 days [range 9–15]. In a third patient, mechanical ventilation was due to SARS-COV2 bilateral pneumonia (patient 3) during irMG recovery. All patients diagnosed of “3M triad” died. Their deaths were related to complications associated with the severity of their condition and not exclusively to irMG. Conversely, two patients recovered from irMG. However, very recently, patient 5 presented an irMG relapse concurrently with corticosteroids tapering. Of the two patients who survived the irMG (patient 4 and 5), both have had a partial response to the cancer and no further oncospecific treatment has been restarted to date.
Patient 3, who had concurrent ir-myositis, presented a spontaneous intramuscular bleeding in two different localizations (brachioradialis and adductor magnus) that led to a hypovolemic shock requiring blood transfusions and admission to the intensive care unit. No coagulation alteration or low platelets were detected.
4. Discussion
This study presents a single center experience of a rare neurological complication due to ICI. Over a 5-year observation period, we have diagnosed six patients meeting the recently established consensus diagnostic criteria for immune-related MG in a university cancer center that covers 45% of the adult cancer population in Catalonia, Spain. The rate of ICI-treated patients who developed MG in our series is in line with previous literature, where irMG is an unusual complication, accounting for 0.5% of all irAes and 13.5% of all nrl-irAEs [3]. In recent years, some single-center case series have been published showing a cumulative incidence similar to ours. In 2018, Safa et al. published the largest case series described to date, with 14 patients from the MD Anderson Center over a 7-year observation period (2011–2018) [23], followed by Shi et al. [24] with six patients over two years (2019–2021) and Wong et al., with four patients [17]. At our center, we have observed an upward trend in the number of cases, which could be explained by the increasing use of these treatments.
Unlike classic MG, all our patients were elderly males. The predominance in this population has been previously described [23,25] and may be explainable by the target population for these treatments. Additionally, none of our patients had thymoma. In contrast to classic MG [26], thymoma does not appear to play a role in the pathogenesis of irMG [25]. Most of our patients had irMG in association with PD1 and only one with anti PDL1 agents. To date, we have not identified this type of neurotoxicity following CTLA-4 treatment. These data are similar to those published in reviews describing a higher incidence of irMG in relation to PD1/ PDL1 (86%) compared to CTLA4 alone (5%) or in combination with PD1 or PDL1 (9%) [3].
irMG is a complication that usually appears early after initiating ICI, within the first four cycles [23], as is the case of in most of our patients (67%). However, irMG can occur at any time during ICI treatment [27,28]. It is noteworthy that we have detected two patients with a very late onset, who started the disease after receiving more than 30 cycles of ICIs. Interestingly, both were on chronic corticosteroids treatment for adrenal insufficiency secondary to immunotherapy. However, none of our patients were on a tapering schedule, which precludes us from establishing an association with the late onset. Further research on whether this observation in causal is needed.
The clinical picture of irMG is characterized by progressive muscle weakness, with ocular, bulbar and proximal limb involvement that can progress to respiratory failure in about half of the patients [7]. Most of our patients (83%) developed moderate to severe muscle weakness (MGFA III–V) at onset, with need of mechanical ventilation in 50% of the cases. Larger case series are consistent with this feature that differentiates it from classic MG [23,29]. MG usually manifests as a milder disease and most patients fall into MGFA classes I and II at onset, with a death rate of 8% due to respiratory failure [26,30]. In Table 2 we summarize the main differences between irMG and classic MG.

Table 2.
Differences between immune-related and idiopathic forms of MG.
In our study, we identified that 67% of our patients presented ir-myositis associated with irMG. The coexistence of myositis is frequent, and it has been reported in up to two thirds of cases in the literature [25]. In series in which a lower incidence was reported, underdiagnosis has been suggested [23]. It is noteworthy that two thirds of our patients were diagnosed with ICI-related myocarditis, which is higher than expected (estimated prevalence varying from 13% [29] to 31% [25]). Overall, the ratio of patients with the “3M triad” in our series (67%) is also higher than that described in the literature, with a reported estimated prevalence of 8% [23]. A higher clinical suspicion and an active search for the concurrent syndromes could explain our results. Regarding the EDX results, half of our patients showed findings compatible with neuromuscular junction impairment with pathological jitter to jiggle, but none had RNS with pathological decrement of amplitude. These studies are in line with previously published data describing findings compatible with neuromuscular junction involvement in up to 50% rate of the cases [7,13]. Remarkably, all the patients with generalized myasthenia in our series displayed a myopathic pattern with mild spontaneous activity in proximal muscles, which is a finding also described in classic MG [31,32]. These findings can be difficult to differentiate from myositis, and for this reason we have not relied solely on EDX findings to diagnose an immune-mediated myopathy. We have taken into consideration the clinical picture, CK levels and the presence of moderate spontaneous activity together with a myopathic pattern. A limitation this report is the absence of muscle biopsy or muscle image to confirm the diagnosis. Importantly, the diagnostic criteria reported by Guidon et al., rely on EDX findings for diagnosis, but they are not essential to classify MG as probable. [19]. As in classic MG, EDX findings are not required to confirm the diagnosis [30]. This facilitates the diagnosis of irMG but may lead to a misdiagnosis of ir-myositis in patients with cranial or respiratory involvement without elevated CK [15]. The combination of these syndromes (irMG and ir-myositis) confers the patient a different prognosis than presenting them separately, highlighting importance of investigating their concomitant presence [25].
In a case of isolated ocular symptoms, it is important to make a broad differential diagnosis (thyroid eye disease, ocular myositis, myasthenia gravis, tumor or vascular compression, etc.). In our patient (patient 4), the acute onset without pain, the clinical fluctuation, the fatigue and the normal brain MRI were the key to the diagnosis.
Most of our patients (83%) had positive AChR Ab, which is a slightly higher rate than that published in previous cases series or reviews [7,23], where AChR Ab positivity varied, ranging from 50% [25] to 66.7% [29]. None of our patients had anti-MUSK antibodies. Its positivity has been reported anecdotally in irMG [25].
Therapeutic management included ICI withdrawal in all cases; initiation of acetylcholinesterase inhibitors; early use of corticosteroids; and IVIG and/or PEX in case of non-response or worsening [33,34,35]. A very recent study showed that patients with irMG may benefit from initial therapy with IVIG or PEX regardless of initial severity [23], which was applied in the most recently diagnosed patients of ours series. Unlike the idiopathic form, irMG can be monophasic [36], so additional corticosteroid sparing agents may not be always necessary. Refractory cases have been reported and the use of mycophenolate mophetil or rituximab has been required [13].
IrMG can occur associated with other neurological or non-neurological irAEs. As it has been widely described the association of irMG with other irAEs are highly common [23,37]. Among our patients, myocarditis and myositis were the most frequently observed (67%), followed by hepatitis (17%). Importantly, the elevation of ALT/AST without elevation of GGT should be interpreted carefully [35] as it may be elevated due to rhabdomyolysis and not to hepatitis. Furthermore, irMG relapses could be associated to the management with corticosteroids in other irAEs. In our series, two patients developed irMG and one patient relapsed in the setting of corticosteroids tapering.
Unfortunately, four out of six (66.7%) patients had a fatal outcome in our series, reaching with 100% mortality rate in patients with the 3M triad. It is noteworthy that not all deaths were directly related to respiratory insufficiency, some of there were related to other systemic complications like SARS-CoV-2 or faecaloid peritonitis (see outcome). Our results contrast with the outcome in other series where partial or full recovery of irMG was observed in 70% of the cases that received adequate and prompt treatment [3]. However, in the literature, mortality due to irMG is much higher than expected in classic MG (28–30% vs. 6%), as a consequence of respiratory failure [13]. The coexistence of other irAES increases the risk of mortality, being 35% in patients with ir-myopathy and 60% in cases with ir-myocarditis [25]. irMG presenting with both myocarditis and myositis is known to carry the highest death rate (5/8, 62.5%) [37].
Some limitations should be acknowledged from the present study. The small sample size and the retrospective nature of the study design limit the reliability of our results. Furthermore, one of the six patients was categorized as possible irMG. However, all of them fulfilled the recently proposed diagnostic criteria for irMG. However, specialized and multidisciplinary evaluation limiting bias (neurooncologist and neurologist) and a long follow-up must be highlighted.
5. Conclusions
ICI-related MG is a rare but often life-threatening complication, especially in those patients presenting with the 3M triad. The frequency of irMG will likely increase as the use of ICI becomes more common. Importantly, some differences with classic MG syndromes regarding clinical presentation, management and outcome have been observed, highlighting the need for detailed descriptions of this challenging entity. Clinicians should be aware of this complication having a high index of clinical suspicion and they should perform a prompt and thorough investigation, including an active search for the most frequently associated syndromes: myositis and myocarditis. Early involvement of experienced neurologists in the oncologic multidisciplinary team, initial discontinuation of ICI and treatment with corticosteroids and immunomodulators are key aspects in the management of this neurological complication.
Author Contributions
All authors made substantial contributors to design, and/or acquisition of data, and/or analysis and interpretation of data. Individual contributors: conceived of study and design: R.V. and C.M. Performed research: C.M., M.S., M.A., C.C., R.D., N.V., M.C., J.M.-L., J.B. (Jesús Brenes), J.S.-R., J.B. (Jordi Bruna) and R.V. Acquisition, analysis, and interpretation data: C.M., M.S., M.A., C.C., R.D., N.V., M.C., J.M.-L., J.B. (Jesús Brenes), J.S.-R., J.B. (Jordi Bruna) and R.V. Manuscript writing: R.V. and C.M. Revising the manuscript for important intellectual content: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by a grant from Instituto de Salud Carlos III through the project PI20/00283 (Co-funded by European Regional Development Fund (ERDF)). We also thank CERCA Programme/Generalitat de Catalunya for institutional support.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of University Hospital of Bellvitge (PR309/22, 20 October 2022 (Act 23/22).
Informed Consent Statement
Informed consent was obtained from all alive subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
C.M., M.A., M.S., R.D. and J.B. have no conflict of interest. C.C. declares funding for advisory boards from Alexion, AstraZeneca Rare Disease, Alnylam Pharmaceuticals Inc., CSL Behring, Pfizer Inc., Argnex Inc. and PharmaNext, and speaker funding from Alexion, AstraZeneca Rare Disease, Sobi, Alnylam Pharmaceuticals Inc., CSL Behring and Pfizer Inc. and research support from Pfizer Inc. M.C. declares consulting and advisory role or speaker with Roche, Eisai, MSD, Astrazeneca, and Bayer; and safety monitoring board with Nerviano. J.M.-L. has received lecture fees from Astellas, Bristol-Myers Squibb, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Sanofi; advisory fees from Bristol-Myers Squibb, Highlight Therapeutics, Novartis, Pierre Fabre, Roche, Sanofi; research grants from Sanofi; and travel grants from Bristol-Myers Squibb, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Ipsen. R.V. declares fundings for advisory board Novartis, Seagen, Lab. Esteve, Gilea, and speaker funding from Takeda, Gilead-Kite, Eisai, Janssen.
References
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [PubMed]
- Haslam, A.; Gill, J.; Prasad, V. Estimation of the Percentage of US Patients with Cancer Who Are Eligible for Immune Checkpoint Inhibitor Drugs. JAMA Netw. Open 2020, 3, e200423. [Google Scholar] [CrossRef] [PubMed]
- Marini, A.; Bernardini, A.; Gigli, G.L.; Valente, M.; Muñiz-Castrillo, S.; Honnorat, J.; Vogrig, A. Neurologic Adverse Events of Immune Checkpoint Inhibitors: A Systematic Review. Neurology 2021, 96, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, R.; Stelitano, B.; Fraenza, F.; di Mauro, G.; Scavone, C.; Sportiello, L.; Rafaniello, C.; Di Napoli, R.; Danesi, R.; Del Re, M.; et al. Neurological Manifestations Related to Immune Checkpoint Inhibitors: Reverse Translational Research by Using the European Real-World Safety Data. Front. Oncol. 2022, 12, 824511. [Google Scholar] [CrossRef] [PubMed]
- FDA approves anti-LAG3 checkpoint. Nat. Biotechnol. 2022, 40, 625. [CrossRef]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Psimaras, D.; Velasco, R.; Birzu, C.; Tamburin, S.; Lustberg, M.; Bruna, J.; Argyriou, A.A. Immune Checkpoint Inhibitors-induced Neuromuscular Toxicity: From Pathogenesis to Treatment. J. Peripher. Nerv. Syst. 2019, 24, S74–S85. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Mikami, T.; Liaw, B.; Asada, M.; Niimura, T.; Zamami, Y.; Green-LaRoche, D.; Pai, L.; Levy, M.; Jeyapalan, S. Neuroimmunological Adverse Events Associated with Immune Checkpoint Inhibitor: A Retrospective, Pharmacovigilance Study Using FAERS Database. J. Neurooncol. 2021, 152, 135–144. [Google Scholar] [CrossRef]
- Dubey, D.; David, W.S.; Reynolds, K.L.; Chute, D.F.; Clement, N.F.; Cohen, J.V.; Lawrence, D.P.; Mooradian, M.J.; Sullivan, R.J.; Guidon, A.C. Severe Neurological Toxicity of Immune Checkpoint Inhibitors: Growing Spectrum. Ann. Neurol. 2020, 87, 659–669. [Google Scholar] [CrossRef]
- Fellner, A.; Makranz, C.; Lotem, M.; Bokstein, F.; Taliansky, A.; Rosenberg, S.; Blumenthal, D.T.; Mandel, J.; Fichman, S.; Kogan, E.; et al. Neurologic Complications of Immune Checkpoint Inhibitors. J. Neurooncol. 2018, 137, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Bruna, J.; Argyriou, A.A.; Anastopoulou, G.G.; Alemany, M.; Nadal, E.; Kalofonou, F.; Piulats, J.M.; Simó, M.; Velasco, R.; Kalofonos, H.P. Incidence and Characteristics of Neurotoxicity in Immune Checkpoint Inhibitors with Focus on Neuromuscular Events: Experience beyond the Clinical Trials. J. Peripher. Nerv. Syst. 2020, 25, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Villagrán-García, M.; Velasco, R. Neurotoxicity and Safety of the Rechallenge of Immune Checkpoint Inhibitors: A Growing Issue in Neuro-Oncology Practice. Neurol. Sci. 2022, 43, 2339–2361. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Mano, T.; Iwata, A.; Toda, T. Neurological and Related Adverse Events in Immune Checkpoint Inhibitors: A Pharmacovigilance Study from the Japanese Adverse Drug Event Report Database. J. Neurooncol. 2019, 145, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Ishikawa, N.; Konoeda, F.; Seki, N.; Fukushima, S.; Takahashi, K.; Uhara, H.; Hasegawa, Y.; Inomata, S.; Otani, Y.; et al. Nivolumab-Related Myasthenia Gravis with Myositis and Myocarditis in Japan. Neurology 2017, 89, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Katel, A.; Massarelli, E.; Villaflor, V.M.; Sun, V.; Salgia, R. Immune Checkpoint Inhibitor–Induced Myocarditis with Myositis/Myasthenia Gravis Overlap Syndrome: A Systematic Review of Cases. Oncologist 2021, 26, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.Y.T.; Yong, M.H.; Yong, K.P.; Tan, E.H.; Toh, C.K.; Kanesvaran, R.; Takano, A.; Ng, Q.S. Immune Checkpoint Inhibitor-associated Myositis and Myasthenia Gravis Overlap: Understanding the Diversity in a Case Series. Asia-Pac. J. Clin. Oncol. 2021, 17, e262–e267. [Google Scholar] [CrossRef]
- Zubiri, L.; Molina, G.E.; Mooradian, M.J.; Cohen, J.; Durbin, S.M.; Petrillo, L.; Boland, G.M.; Juric, D.; Dougan, M.; Thomas, M.F.; et al. Effect of a Multidisciplinary Severe Immunotherapy Complications Service on Outcomes for Patients Receiving Immune Checkpoint Inhibitor Therapy for Cancer. J. Immunother. Cancer 2021, 9, e002886. [Google Scholar] [CrossRef]
- Guidon, A.C.; Burton, L.B.; Chwalisz, B.K.; Hillis, J.; Schaller, T.H.; Amato, A.A.; Betof Warner, A.; Brastianos, P.K.; Cho, T.A.; Clardy, S.L.; et al. Consensus Disease Definitions for Neurologic Immune-Related Adverse Events of Immune Checkpoint Inhibitors. J. Immunother. Cancer 2021, 9, e002890. [Google Scholar] [CrossRef]
- Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America; Jaretzki, A.; Barohn, R.J.; Ernstoff, R.M.; Kaminski, H.J.; Keesey, J.C.; Penn, A.S.; Sanders, D.B. Myasthenia Gravis: Recommendations for Clinical Research Standards. Neurology 2000, 55, 16–23. [Google Scholar] [CrossRef]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. CTCAE versión 5.0. Evaluación de la gravedad de los eventos adversos dermatológicos de las terapias antineoplásicas. Actas Dermo-Sifiliográficas 2021, 112, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Juel, V.C. Clinical Neurophysiology of Neuromuscular Junction Disease. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 161, pp. 291–303. ISBN 978-0-444-64142-7. [Google Scholar]
- Safa, H.; Johnson, D.H.; Trinh, V.A.; Rodgers, T.E.; Lin, H.; Suarez-Almazor, M.E.; Fa’ak, F.; Saberian, C.; Yee, C.; Davies, M.A.; et al. Immune Checkpoint Inhibitor Related Myasthenia Gravis: Single Center Experience and Systematic Review of the Literature. J. Immunother. Cancer 2019, 7, 319. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Tan, Y.; Huang, Y.; Li, K.; Yan, J.; Guan, Y.; Zhang, L. Association Between Clinical Factors and Result of Immune Checkpoint Inhibitor Related Myasthenia Gravis: A Single Center Experience and Systematic Review. Front. Neurol. 2022, 13, 858628. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-T.; Chen, Y.-P.; Lin, W.-C.; Su, W.-C.; Sun, Y.-T. Immune Checkpoint Inhibitor-Induced Myasthenia Gravis. Front. Neurol. 2020, 11, 634. [Google Scholar] [CrossRef] [PubMed]
- Dresser, L.; Wlodarski, R.; Rezania, K.; Soliven, B. Myasthenia Gravis: Epidemiology, Pathophysiology and Clinical Manifestations. JCM 2021, 10, 2235. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.N.; Bai, X.; Quah, T.; Lo, S.N.; Allayous, C.; Callaghan, S.; Martínez-Vila, C.; Wallace, R.; Bhave, P.; Reijers, I.L.M.; et al. Delayed Immune-Related Adverse Events with Anti-PD-1-Based Immunotherapy in Melanoma. Ann. Oncol. 2021, 32, 917–925. [Google Scholar] [CrossRef]
- Ghisoni, E.; Wicky, A. Late-onset and long-lasting immune-related adverse events from immune checkpoint-inhibitors: An overlooked aspect in immunotherapy. Eur. J. Cancer 2021, 149, 153–164. [Google Scholar] [CrossRef]
- Johansen, A.; Christensen, S.J.; Scheie, D.; Højgaard, J.L.S.; Kondziella, D. Neuromuscular Adverse Events Associated with Anti-PD-1 Monoclonal Antibodies: Systematic Review. Neurology 2019, 92, 663–674. [Google Scholar] [CrossRef]
- Gilhus, N.E. Myasthenia Gravis. N. Engl. J. Med. 2016, 375, 2570–2581. [Google Scholar] [CrossRef]
- Kannaditharayil, D.; Napier, F.; Granit, V.; Bieri, P.; Herskovitz, S. Abnormal Spontaneous Activity on Needle Electromyography in Myasthenia Gravis: Abnormal Activity on Needle EMG in MG. Muscle Nerve 2017, 56, E11–E12. [Google Scholar] [CrossRef]
- Tsironis, T.; Catania, S. Reversible Spontaneous EMG Activity during Myasthenic Crisis: Two Case Reports. eNeurologicalSci 2019, 14, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K. Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. JCO 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Vogrig, A.; Muñiz-Castrillo, S.; Farina, A.; Honnorat, J.; Joubert, B. How to Diagnose and Manage Neurological Toxicities of Immune Checkpoint Inhibitors: An Update. J. Neurol. 2022, 269, 1701–1714. [Google Scholar] [CrossRef]
- Dubey, D.; David, W.S.; Amato, A.A.; Reynolds, K.L.; Clement, N.F.; Chute, D.F.; Cohen, J.V.; Lawrence, D.P.; Mooradian, M.J.; Sullivan, R.J.; et al. Varied Phenotypes and Management of Immune Checkpoint Inhibitor-Associated Neuropathies. Neurology 2019, 93, e1093–e1103. [Google Scholar] [CrossRef]
- Johnson, D.B.; Manouchehri, A.; Haugh, A.M.; Quach, H.T.; Balko, J.M.; Lebrun-Vignes, B.; Mammen, A.; Moslehi, J.J.; Salem, J.-E. Neurologic Toxicity Associated with Immune Checkpoint Inhibitors: A Pharmacovigilance Study. J. Immunother. Cancer 2019, 7, 134. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).