Target Low-Density Lipoprotein-Cholesterol and Secondary Prevention for Patients with Acute Myocardial Infarction: A Korean Nationwide Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of Data
2.2. Study Population
2.3. Study Variables and Endpoints
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Statin Exposure and LDL-C Reduction
3.3. Clinical Outcomes
3.4. Secondary Analyses
3.5. Predictors of the Primary Endpoint
4. Discussion
4.1. Achieving Target LDL-C Goal for Secondary Prevention following Acute MI
4.2. Degree of LDL-C Reduction and Clinical Events
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Szummer, K.; Wallentin, L.; Lindhagen, L.; Alfredsson, J.; Erlinge, D.; Held, C.; James, S.; Kellerth, T.; Lindahl, B.; Ravn-Fischer, A.; et al. Improved outcomes in patients with ST-elevation myocardial infarction during the last 20 years are related to implementation of evidence-based treatments: Experiences from the SWEDEHEART registry 1995–2014. Eur. Heart J. 2017, 38, 3056–3065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, G.G.; Olsson, A.G.; Ezekowitz, M.D.; Ganz, P.; Oliver, M.F.; Waters, D.; Zeiher, A.; Chaitman, B.R.; Leslie, S.; Stern, T. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: The MIRACL study: A randomized controlled trial. JAMA 2001, 285, 1711–1718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lemos, J.A.; Blazing, M.A.; Wiviott, S.D.; Lewis, E.F.; Fox, K.A.; White, H.D.; Rouleau, J.L.; Pedersen, T.R.; Gardner, L.H.; Mukherjee, R.; et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: Phase Z of the A to Z trial. JAMA 2004, 292, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Gitt, A.K.; Lautsch, D.; Ferrières, J.; De Ferrari, G.M.; Vyas, A.; Baxter, C.A.; Bash, L.D.; Ashton, V.; Horack, M.; Almahmeed, W.; et al. Cholesterol target value attainment and lipid-lowering therapy in patients with stable or acute coronary heart disease: Results from the Dyslipidemia International Study II. Atherosclerosis 2017, 266, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Lee, S.Y.; Kim, J.S.; Choi, K.M.; Lee, K.W.; Lee, S.C.; Cho, J.R.; Oh, S.J.; Kim, J.H.; Choi, S.H. Achievement of LDL-C Targets Defined by ESC/EAS (2011) Guidelines in Risk-Stratified Korean Patients with Dyslipidemia Receiving Lipid-Modifying Treatments. Endocrinol. Metab. 2020, 35, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chae, S.C.; Oh, D.J.; Kim, H.S.; Kim, Y.J.; Ahn, Y.; Cho, M.C.; Kim, C.J.; Yoon, J.H.; Park, H.Y.; et al. Multicenter Cohort Study of Acute Myocardial Infarction in Korea–Interim Analysis of the Korea Acute Myocardial Infarction Registry-National Institutes of Health Registry. Circ. J. 2016, 80, 1427–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnaveni, P.; Gowda, V.M. Assessing the Validity of Friedewald’s Formula and Anandraja’s Formula For Serum LDL-Cholesterol Calculation. J. Clin. Diagn. Res. 2015, 9, BC01–BC04. [Google Scholar] [CrossRef] [PubMed]
- Moussa, I.D.; Klein, L.W.; Shah, B.; Mehran, R.; Mack, M.J.; Brilakis, E.S.; Reilly, J.P.; Zoghbi, G.; Holper, E.; Stone, G.W. Consideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: An expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI). J. Am. Coll. Cardiol. 2013, 62, 1563–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinzl, H.; Kaider, A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput. Methods Programs Biomed. 1997, 54, 201–208. [Google Scholar] [CrossRef]
- Austin, P.C.; Lee, D.S.; Fine, J.P. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation 2016, 133, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef] [PubMed]
- Rhee, E.J.; Kim, H.C.; Kim, J.H.; Lee, E.Y.; Kim, B.J.; Kim, E.M.; Song, Y.; Lim, J.H.; Kim, H.J.; Choi, S.; et al. 2018 Guidelines for the management of dyslipidemia. Korean J. Intern. Med. 2019, 34, 723–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.H.; Jeong, M.H.; Park, K.W.; Kim, H.S.; Lee, S.R.; Chae, J.K.; Hong, Y.J.; Kim, J.H.; Ahn, Y.; Cho, J.G.; et al. Comparison of the effects of two low-density lipoprotein cholesterol goals for secondary prevention after acute myocardial infarction in real-world practice: ≥50% reduction from baseline versus <70 mg/dL. Int. J. Cardiol. 2015, 187, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.; Suh, S.Y.; Lee, K.; Kang, W.C.; Han, S.H.; Ahn, Y.; Jeong, M.H. Clinical Outcomes according to the Achievement of Target Low Density Lipoprotein-Cholesterol in Patients with Acute Myocardial Infarction. Korean Circ. J. 2017, 47, 31–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubert, J.; Lindahl, B.; Melhus, H.; Renlund, H.; Leosdottir, M.; Yari, A.; Ueda, P.; James, S.; Reading, S.R.; Dluzniewski, P.J.; et al. Low-density lipoprotein cholesterol reduction and statin intensity in myocardial infarction patients and major adverse outcomes: A Swedish nationwide cohort study. Eur. Heart J. 2021, 42, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists, C.; Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef] [Green Version]
- Cholesterol Treatment Trialists’ (CTT) Collaborators; Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; Voysey, M.; Gray, A.; Collins, R.; et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet 2012, 380, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists’ (CTT) Collaborators; Fulcher, J.; O’Connell, R.; Voysey, M.; Emberson, J.; Blackwell, L.; Mihaylova, B.; Simes, J.; Collins, R.; Kirby, A.; et al. Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015, 385, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Characteristics | Overall (N = 5049) | Non-Achiever (n = 3935) | Achiever (n = 1114) | p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, year | 60.0 [52.0; 70.0] | 61.0 [52.0; 70.0] | 60.0 [52.0; 70.0] | 0.204 |
| Female sex | 1060 (21.0%) | 872 (22.2%) | 188 (16.9%) | <0.001 |
| BMI > 23 kg/m2 | 3455 (68.4%) | 2657 (67.5%) | 798 (71.6%) | 0.010 |
| Medical history | ||||
| Current smoker | 2226 (44.1%) | 1710 (43.5%) | 516 (46.3%) | 0.096 |
| Hypertension | 2349 (46.5%) | 1854 (47.1%) | 495 (44.4%) | 0.121 |
| Diabetes | 1314 (26.0%) | 1031 (26.2%) | 283 (25.4%) | 0.620 |
| Dyslipidemia | 708 (14.0%) | 615 (15.6%) | 93 (8.3%) | <0.001 |
| History of MI | 257 (5.1%) | 240 (6.1%) | 17 (1.5%) | <0.001 |
| History of PCI/CABG | 203 (4.0%) | 184 (4.7%) | 19 (1.7%) | <0.001 |
| History of HF | 43 (0.9%) | 39 (1.0%) | 4 (0.4%) | 0.066 |
| History of CVA | 235 (4.7%) | 198 (5.0%) | 37 (3.3%) | 0.021 |
| Chronic kidney disease | 109 (2.2%) | 92 (2.3%) | 17 (1.5%) | 0.126 |
| Laboratory variables | ||||
| LDL-C (mg/dL) | 118.0 [92.6; 144.0] | 112.2 [87.0; 140.9] | 133.0 [116.0; 150.0] | <0.001 |
| HDL-C (mg/dL) | 42.0 [35.0; 49.0] | 41.0 [35.0; 49.0] | 43.0 [36.2; 50.0] | 0.002 |
| Triglyceride (mg/dL) | 112.0 [76.0; 172.0] | 111.0 [74.0; 172.0] | 116.0 [83.0; 172.0] | 0.025 |
| Total cholesterol (mg/dL) | 180.0 [154.0; 210.0] | 175.0 [148.0; 207.0] | 195.0 [175.5; 217.0] | <0.001 |
| hs-CRP (mg/L) | 0.8 [0.0; 3.1] | 0.7 [0.0; 3.0] | 1.0 [0.0; 3.6] | 0.022 |
| HbA1c (%) | 5.9 [5.6; 6.8] | 5.9 [5.5; 6.8] | 5.9 [5.6; 6.9] | 0.213 |
| LVEF < 40% | 560 (11.1%) | 454 (11.5%) | 106 (9.5%) | 0.065 |
| Clinical presentation | ||||
| STEMI | 2632 (52.1%) | 2039 (51.8%) | 593 (53.2%) | 0.423 |
| Multivessel disease | 2251 (44.6%) | 1757 (44.7%) | 494 (44.3%) | 0.883 |
| LM disease | 200 (4.0%) | 166 (4.2%) | 34 (3.1%) | 0.094 |
| Cardiogenic shock | 302 (6.0%) | 255 (6.5%) | 47 (4.2%) | 0.006 |
| Newly developed HF | 141 (2.8%) | 114 (2.9%) | 27 (2.4%) | 0.457 |
| Infarct-related artery | ||||
| Left main | 86 (1.7%) | 74 (1.9%) | 12 (1.1%) | |
| Left anterior descending | 2263 (44.8%) | 1720 (43.7%) | 543 (48.7%) | |
| Left circumflex | 862 (17.1%) | 662 (16.8%) | 200 (18.0%) | |
| Right | 1605 (31.8%) | 1277 (32.5%) | 328 (29.4%) | |
| PCI results | ||||
| Culprit-only | 1423 (28.2%) | 1098 (27.9%) | 325 (29.2%) | 0.427 |
| Complete revascularization | 3381 (67.0%) | 2625 (66.7%) | 756 (67.9%) | 0.492 |
| Discharge medication | ||||
| Statin | 4824 (95.5%) | 3729 (94.8%) | 1095 (98.3%) | <0.001 |
| No therapy | 225 (4.5%) | 206 (5.2%) | 19 (1.7%) | |
| Low-intensity | 79 (1.6%) | 64 (1.6%) | 15 (1.3%) | |
| Medium-intensity | 2988 (59.2%) | 2480 (63.0%) | 508 (45.6%) | |
| High-intensity | 1757 (34.8%) | 1185 (30.1%) | 572 (51.3%) | |
| Aspirin | 5042 (99.9%) | 3930 (99.9%) | 1112 (99.8%) | 1.000 |
| Clopidogrel | 3694 (73.2%) | 2935 (74.6%) | 759 (68.1%) | <0.001 |
| Prasugrel | 647 (12.8%) | 518 (13.2%) | 129 (11.6%) | 0.178 |
| Ticagrelor | 1362 (36.3%) | 978 (34.1%) | 384 (43.3%) | <0.001 |
| Beta-blocker | 4388 (86.9%) | 3415 (86.8%) | 973 (87.3%) | 0.662 |
| ACEi/ARB | 4097 (81.1%) | 3183 (80.9%) | 914 (82.0%) | 0.407 |
| CCB | 355 (7.0%) | 291 (7.4%) | 64 (5.7%) | 0.066 |
| Characteristics | Overall (N = 5049) | Non-Achiever (n = 3935) | Achiever (n = 1114) | p-Value |
|---|---|---|---|---|
| Laboratory variables | ||||
| LDL-C (mg/dL) | 73.0 [59.0; 90.0] | 80.0 [67.0; 95.0] | 53.4 [45.2; 61.0] | <0.001 |
| LDL-C reduction (mg/dL) | 44.2 [17.0; 71.0] | 33.0 [8.0; 55.0] | 77.0 [65.0; 93.0] | <0.001 |
| LDL-C ≤ 70 mg/dL | 2303 (45.6%) | 1189 (30.2%) | 1114 (100.0%) | <0.001 |
| ≥50% LDL-C reduction | 1438 (28.5%) | 324 (8.2%) | 1114 (100.0%) | <0.001 |
| HDL-C (mg/dL) | 43.0 [37.0; 51.0] | 43.0 [37.0; 51.0] | 42.0 [35.0; 50.0] | <0.001 |
| Triglyceride (mg/dL) | 116.0 [83.0; 166.0] | 118.0 [85.0; 171.0] | 104.5 [76.0; 148.0] | <0.001 |
| Total cholesterol (mg/dL) | 136.0 [118.0; 157.0] | 144.0 [127.0; 163.0] | 114.0 [103.0; 125.0] | <0.001 |
| hs-CRP (mg/L) | 0.8 [0.3; 2.1] | 0.8 [0.3; 2.1] | 0.8 [0.3; 2.0] | 0.820 |
| HbA1c (%) | 6.2 [5.7; 7.0] | 6.2 [5.7; 7.0] | 6.2 [5.8; 7.1] | 0.198 |
| LVEF < 40% | 191 (6.5%) | 159 (7.1%) | 32 (4.5%) | 0.017 |
| On-going medications | ||||
| Statin | 4580 (90.7%) | 3541 (90.0%) | 1039 (93.3%) | <0.001 |
| Aspirin | 4380 (86.7%) | 3427 (87.1%) | 953 (85.5%) | 0.314 |
| Clopidogrel | 2009 (39.8%) | 1563 (39.7%) | 446 (40.0%) | 0.140 |
| Prasugrel | 162 (3.2%) | 141 (3.6%) | 21 (1.9%) | 0.067 |
| Ticagrelor | 232 (4.6%) | 157 (4.0%) | 75 (6.7%) | <0.001 |
| Beta-blocker | 3774 (74.7%) | 2942 (74.8%) | 832 (74.7%) | 0.584 |
| ACEi/ARB | 2660 (52.7%) | 2066 (52.5%) | 594 (53.3%) | 0.654 |
| CCB | 382 (7.6%) | 310 (7.9%) | 72 (6.5%) | 0.386 |
| Non-Achiever (n = 3935) | Achiever (n = 1114) | Log-Rank p-Value | Adjusted HR * [95% CI] | p-Value | |
|---|---|---|---|---|---|
| Primary Endpoint | |||||
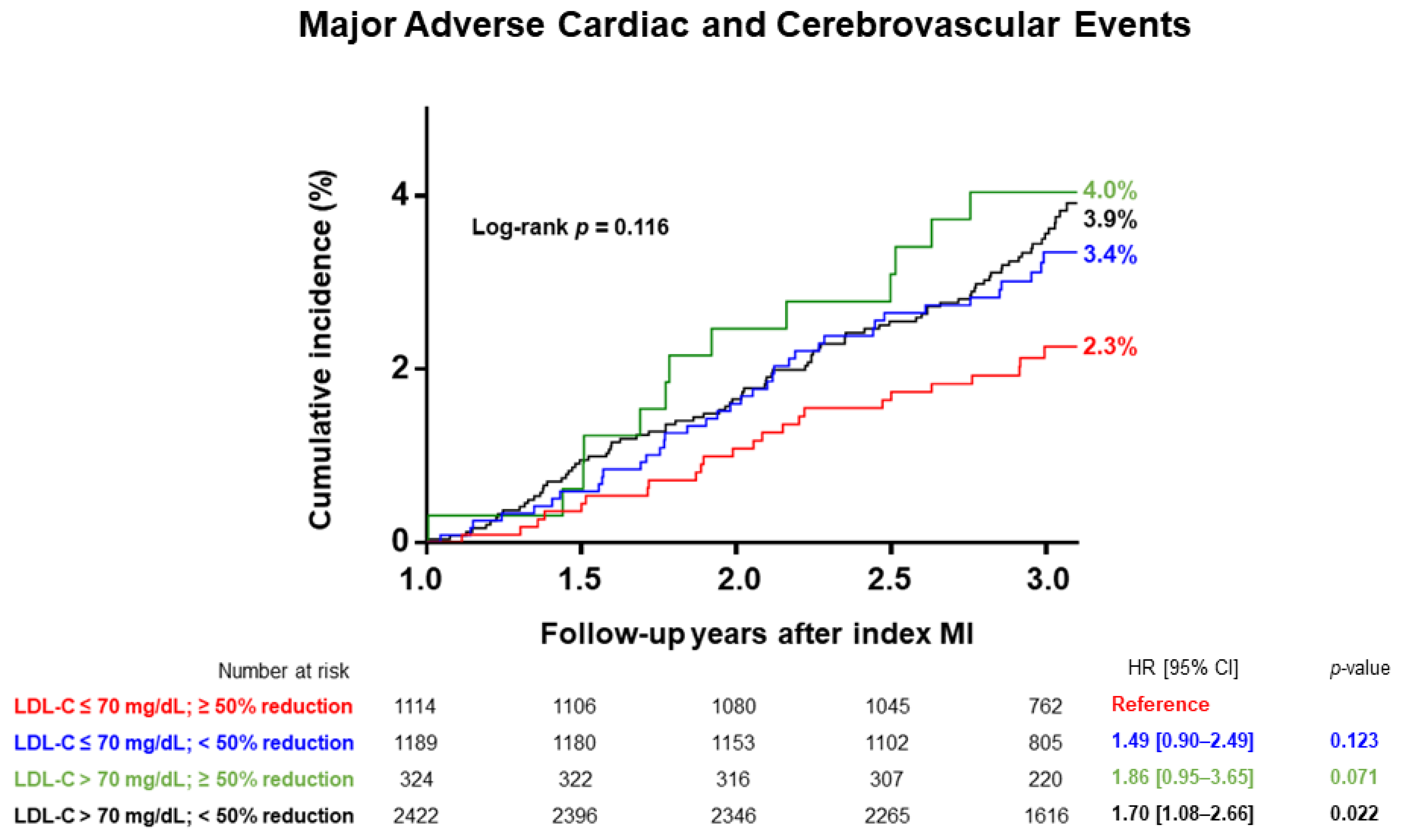
| MACCE | 139 (3.5%) | 24 (2.2%) | 0.022 | 0.63 [0.40–0.98] | 0.041 |
| Secondary Endpoints | |||||
| All-cause mortality | 93 (2.4%) | 18 (1.6%) | 0.140 | 0.77 [0.46–1.31] | 0.339 |
| Cardiovascular mortality | 52 (1.3%) | 9 (0.8%) | 0.169 | 0.70 [0.33–1.45] | 0.334 |
| Recurrent MI | 62 (1.6%) | 9 (0.8%) | 0.055 | 0.48 [0.24–0.98] | 0.044 |
| Ischaemic stroke | 33 (0.8%) | 6 (0.5%) | 0.312 | 0.67 [0.28–1.64] | 0.384 |
| Repeat revascularization | 145 (3.7%) | 34 (3.1%) | 0.311 | 0.81 [0.56–1.19] | 0.290 |
| Hospitalization for HF | 56 (1.4%) | 9 (0.8%) | 0.109 | 0.82 [0.39–1.73] | 0.606 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Cha, J.-J.; Lim, S.; An, J.; Kim, M.-N.; Hong, S.J.; Joo, H.J.; Park, J.H.; Yu, C.W.; Lim, D.-S.; et al. Target Low-Density Lipoprotein-Cholesterol and Secondary Prevention for Patients with Acute Myocardial Infarction: A Korean Nationwide Cohort Study. J. Clin. Med. 2022, 11, 2650. https://doi.org/10.3390/jcm11092650
Kim JH, Cha J-J, Lim S, An J, Kim M-N, Hong SJ, Joo HJ, Park JH, Yu CW, Lim D-S, et al. Target Low-Density Lipoprotein-Cholesterol and Secondary Prevention for Patients with Acute Myocardial Infarction: A Korean Nationwide Cohort Study. Journal of Clinical Medicine. 2022; 11(9):2650. https://doi.org/10.3390/jcm11092650
Chicago/Turabian StyleKim, Ju Hyeon, Jung-Joon Cha, Subin Lim, Jungseok An, Mi-Na Kim, Soon Jun Hong, Hyung Joon Joo, Jae Hyoung Park, Cheol Woong Yu, Do-Sun Lim, and et al. 2022. "Target Low-Density Lipoprotein-Cholesterol and Secondary Prevention for Patients with Acute Myocardial Infarction: A Korean Nationwide Cohort Study" Journal of Clinical Medicine 11, no. 9: 2650. https://doi.org/10.3390/jcm11092650
APA StyleKim, J. H., Cha, J.-J., Lim, S., An, J., Kim, M.-N., Hong, S. J., Joo, H. J., Park, J. H., Yu, C. W., Lim, D.-S., Byeon, K., Kim, S.-W., Shin, E.-S., Cha, K. S., Chae, J. K., Ahn, Y., Jeong, M. H., & Ahn, T. H. (2022). Target Low-Density Lipoprotein-Cholesterol and Secondary Prevention for Patients with Acute Myocardial Infarction: A Korean Nationwide Cohort Study. Journal of Clinical Medicine, 11(9), 2650. https://doi.org/10.3390/jcm11092650







