Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) in Patients after Acute Stroke: Relation to Stroke Severity, Myocardial Injury, and Impact on Prognosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Laboratory Analysis
2.3. Electrocardiogram (ECG) and Echocardiographic Analysis
2.4. Statistical Analysis
3. Results
3.1. Patients’ Population
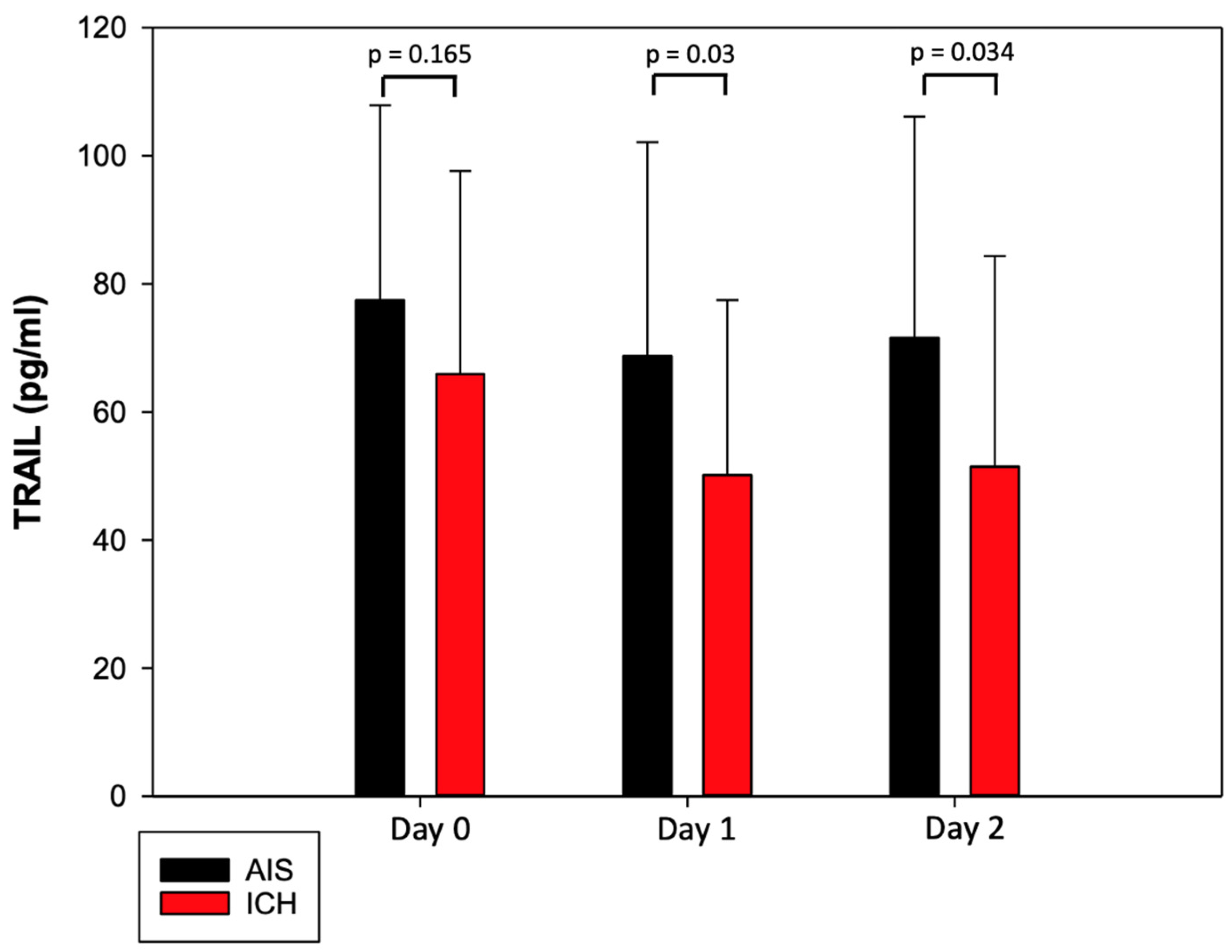
3.2. Characteristics of TRAIL and Its Dynamic Changes after Acute Stroke
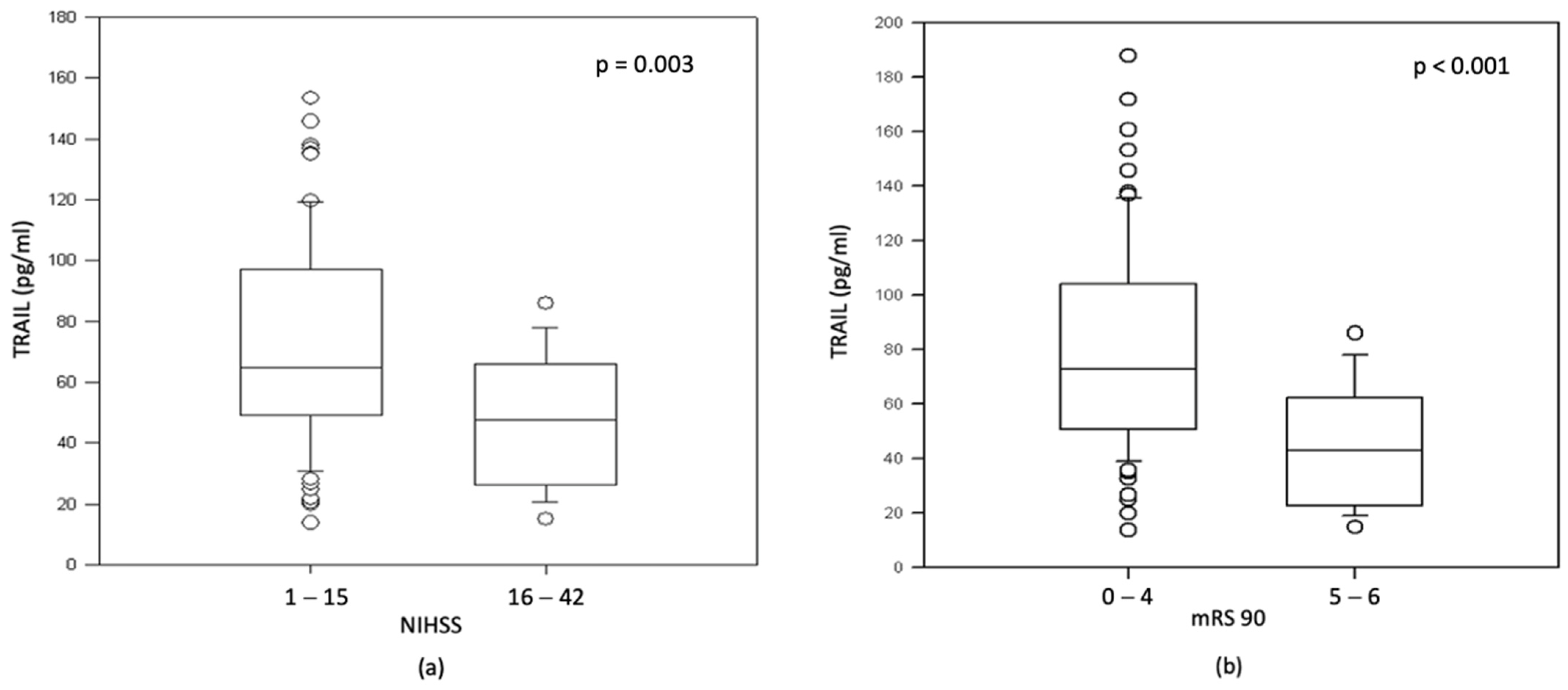
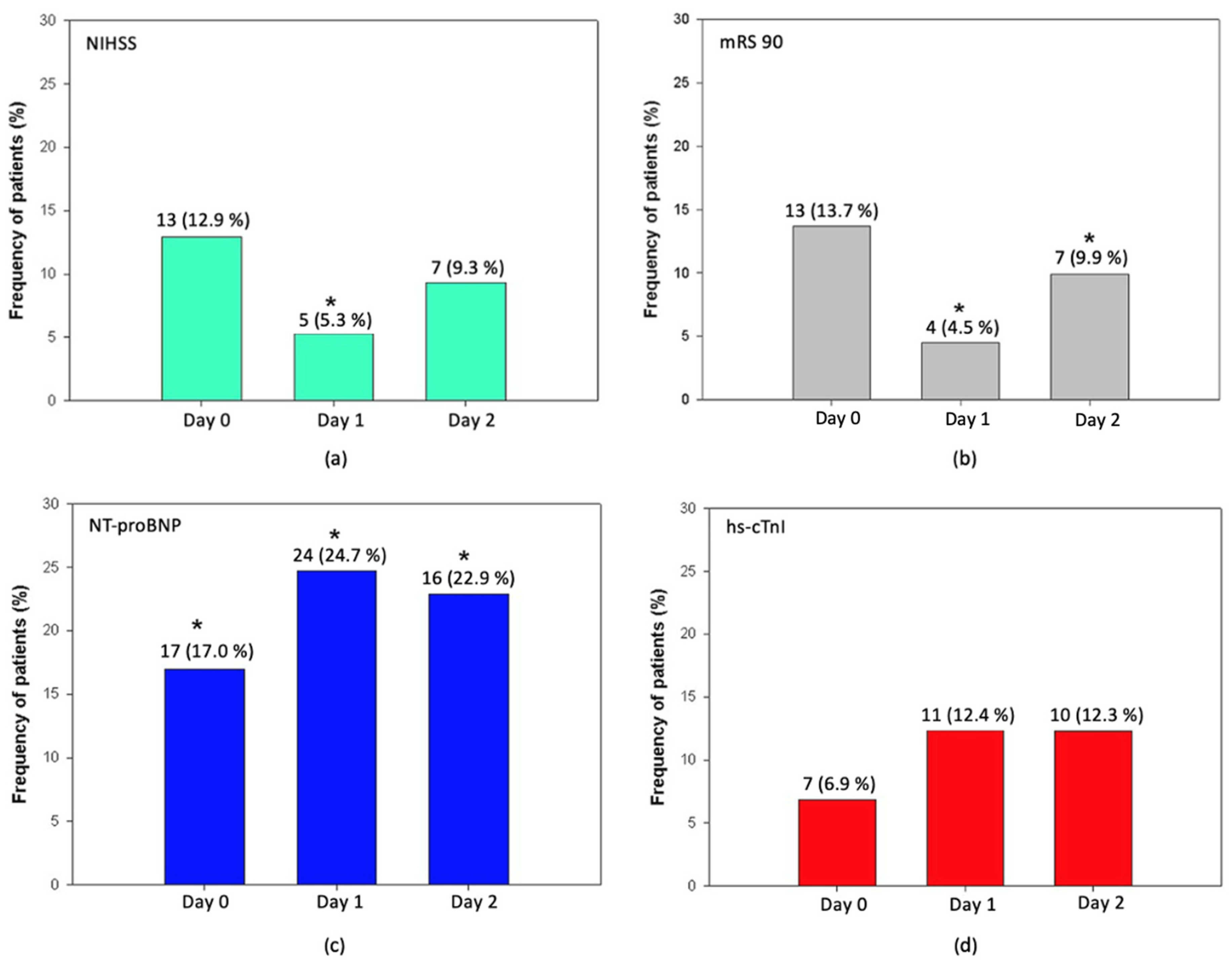
3.3. TRAIL and NT-proBNP Are Associated with Stroke Severity and Worse Neurological Outcome in AIS
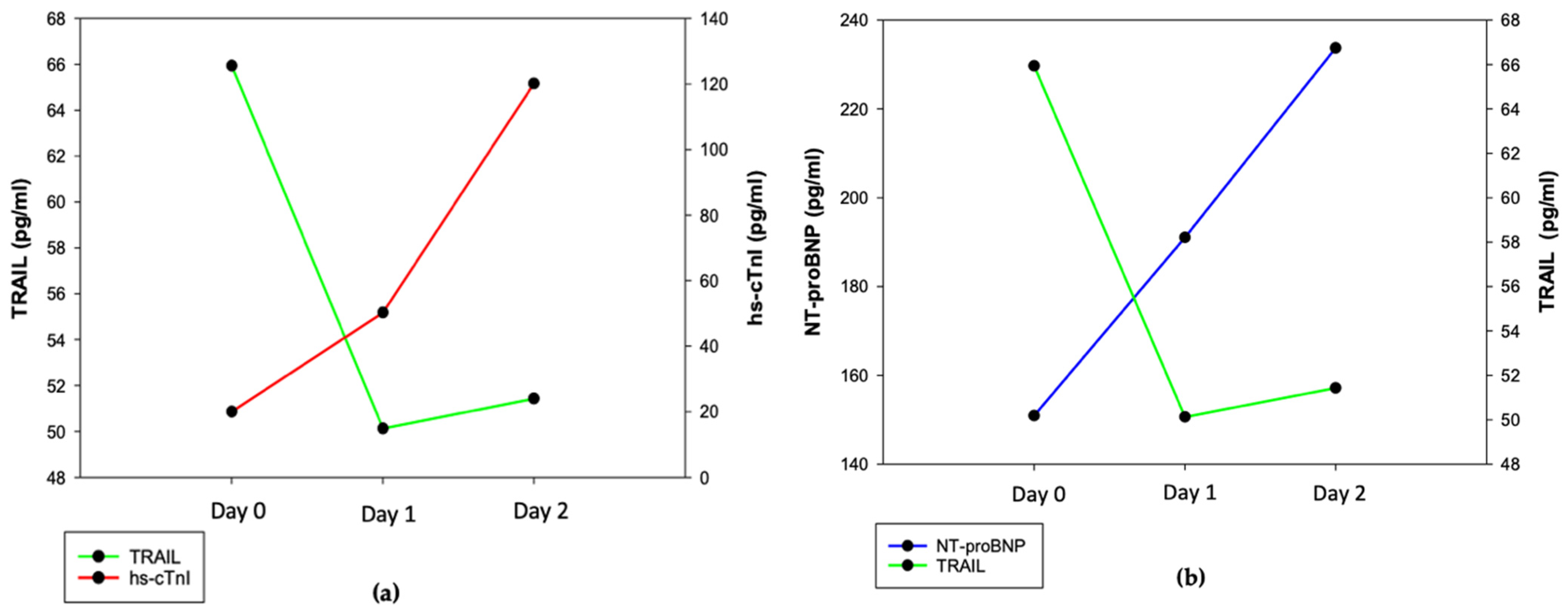
3.4. TRAIL and Markers of Myocardial Injury in Patients after Acute Stroke
3.5. TRAIL and ECG Changes in AIS
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wiley, S.R.; Schooley, K.; Smolak, P.J.; Din, W.S.; Huang, C.P.; Nicholl, J.K.; Sutherland, G.R.; Smith, T.D.; Rauch, C.; Smith, C.A.; et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995, 3, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Stuckey, D.W.; Shah, K. TRAIL on trial: Preclinical advances in cancer therapy. Trends Mol. Med. 2013, 19, 685–694. [Google Scholar] [CrossRef] [Green Version]
- Gasparini, C.; Vecchi Brumatti, L.; Monasta, L.; Zauli, G. TRAIL-based therapeutic approaches for the treatment of pediatric malignancies. Curr. Med. Chem. 2013, 20, 2254–2271. [Google Scholar] [CrossRef] [PubMed]
- Corallini, F.; Rimondi, E.; Secchiero, P. TRAIL and osteoprotegerin: A role in endothelial physiopathology? Front. Biosci. 2008, 13, 135–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavurma, M.M.; Bennett, M.R. Expression, regulation and function of trail in atherosclerosis. Biochem. Pharmacol. 2008, 75, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Kakareko, K.; Rydzewska-Rosolowska, A.; Zbroch, E.; Hryszko, T. TRAIL and Cardiovascular Disease-A Risk Factor or Risk Marker: A Systematic Review. J. Clin. Med. 2021, 10, 1252. [Google Scholar] [CrossRef]
- Teringova, E.; Kozel, M.; Knot, J.; Kocka, V.; Benesova, K.; Tousek, P. Relationship between TRAIL and Left Ventricular Ejection Fraction in Patients with ST-Elevation Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention. BioMed Res. Int. 2018, 2018, 3709084. [Google Scholar] [CrossRef] [Green Version]
- Volpato, S.; Ferrucci, L.; Secchiero, P.; Corallini, F.; Zuliani, G.; Fellin, R.; Guralnik, J.M.; Bandinelli, S.; Zauli, G. Association of tumor necrosis factor-related apoptosis-inducing ligand with total and cardiovascular mortality in older adults. Atherosclerosis 2011, 215, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Mori, K.; Ikari, Y.; Jono, S.; Shioi, A.; Ishimura, E.; Emoto, M.; Inaba, M.; Hara, K.; Nishizawa, Y. Association of serum TRAIL level with coronary artery disease. Thromb. Res. 2010, 125, 322–325. [Google Scholar] [CrossRef]
- Secchiero, P.; Corallini, F.; Ceconi, C.; Parrinello, G.; Volpato, S.; Ferrari, R.; Zauli, G. Potential prognostic significance of decreased serum levels of TRAIL after acute myocardial infarction. PLoS ONE 2009, 4, e4442. [Google Scholar] [CrossRef]
- Niessner, A.; Hohensinner, P.J.; Rychli, K.; Neuhold, S.; Zorn, G.; Richter, B.; Hulsmann, M.; Berger, R.; Mortl, D.; Huber, K.; et al. Prognostic value of apoptosis markers in advanced heart failure patients. Eur. Heart J. 2009, 30, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Skau, E.; Henriksen, E.; Wagner, P.; Hedberg, P.; Siegbahn, A.; Leppert, J. GDF-15 and TRAIL-R2 are powerful predictors of long-term mortality in patients with acute myocardial infarction. Eur. J. Prev. Cardiol. 2017, 24, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.P.; Sharma, A.; Mehta, C.; Bakris, G.; Rossignol, P.; White, W.B.; Zannad, F. Multi-proteomic approach to predict specific cardiovascular events in patients with diabetes and myocardial infarction: Findings from the EXAMINE trial. Clin. Res. Cardiol. 2021, 110, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Arcidiacono, M.V.; Rimondi, E.; Maietti, E.; Melloni, E.; Tisato, V.; Gallo, S.; Valdivielso, J.M.; Fernandez, E.; Betriu, A.; Voltan, R.; et al. Relationship between low levels of circulating TRAIL and atheromatosis progression in patients with chronic kidney disease. PLoS ONE 2018, 13, e0203716. [Google Scholar] [CrossRef] [PubMed]
- Voltan, R.; Secchiero, P.; Casciano, F.; Milani, D.; Zauli, G.; Tisato, V. Redox signaling and oxidative stress: Cross talk with TNF-related apoptosis inducing ligand activity. Int. J. Biochem. Cell Biol. 2016, 81, 364–374. [Google Scholar] [CrossRef] [Green Version]
- Xia, P.; Liu, Y.; Cheng, Z. Signaling Pathways in Cardiac Myocyte Apoptosis. BioMed Res. Int. 2016, 2016, 9583268. [Google Scholar] [CrossRef] [PubMed]
- Tisato, V.; Gonelli, A.; Voltan, R.; Secchiero, P.; Zauli, G. Clinical perspectives of TRAIL: Insights into central nervous system disorders. Cell. Mol. Life Sci. 2016, 73, 2017–2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tufekci, K.U.; Vurgun, U.; Yigitaslan, O.; Keskinoglu, P.; Yaka, E.; Kutluk, K.; Genc, S. Follow-up Analysis of Serum TNF-Related Apoptosis-Inducing Ligand Protein and mRNA Expression in Peripheral Blood Mononuclear Cells from Patients with Ischemic Stroke. Front. Neurol. 2018, 9, 102. [Google Scholar] [CrossRef] [Green Version]
- Pan, X.; Pang, M.; Ma, A.; Wang, K.; Zhang, Z.; Zhong, Q.; Yang, S. Association of TRAIL and Its Receptors with Large-Artery Atherosclerotic Stroke. PLoS ONE 2015, 10, e0136414. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.H.; Park, M.G.; Noh, K.H.; Park, H.R.; Lee, H.W.; Son, S.M.; Park, K.P. Low serum TNF-related apoptosis-inducing ligand (TRAIL) levels are associated with acute ischemic stroke severity. Atherosclerosis 2015, 240, 228–233. [Google Scholar] [CrossRef]
- Soto-Camara, R.; Gonzalez-Santos, J.; Gonzalez-Berna, J.; Trejo-Gabriel-Galan, J.M. Factors associated with a rapid call for assistance for patients with ischemic stroke. Emergencias 2020, 32, 33–39. [Google Scholar]
- Scheitz, J.F.; Nolte, C.H.; Laufs, U.; Endres, M. Application and interpretation of high-sensitivity cardiac troponin assays in patients with acute ischemic stroke. Stroke 2015, 46, 1132–1140. [Google Scholar] [CrossRef] [Green Version]
- Kerr, G.; Ray, G.; Wu, O.; Stott, D.J.; Langhorne, P. Elevated troponin after stroke: A systematic review. Cerebrovasc. Dis. 2009, 28, 220–226. [Google Scholar] [CrossRef]
- Faiz, K.W.; Thommessen, B.; Einvik, G.; Omland, T.; Ronning, O.M. Prognostic value of high-sensitivity cardiac troponin T in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 2014, 23, 241–248. [Google Scholar] [CrossRef] [PubMed]
- de Lemos, J.A.; Morrow, D.A.; Bentley, J.H.; Omland, T.; Sabatine, M.S.; McCabe, C.H.; Hall, C.; Cannon, C.P.; Braunwald, E. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N. Engl. J. Med. 2001, 345, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Levy, D.; Benjamin, E.J.; Leip, E.P.; Omland, T.; Wolf, P.A.; Vasan, R.S. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N. Engl. J. Med. 2004, 350, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Fonarow, G.C.; Peacock, W.F.; Phillips, C.O.; Givertz, M.M.; Lopatin, M.; ADHERE Scientific Advisory Committee and Investigators. Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J. Am. Coll. Cardiol. 2007, 49, 1943–1950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cushman, M.; Judd, S.E.; Howard, V.J.; Kissela, B.; Gutierrez, O.M.; Jenny, N.S.; Ahmed, A.; Thacker, E.L.; Zakai, N.A. N-terminal pro-B-type natriuretic peptide and stroke risk: The reasons for geographic and racial differences in stroke cohort. Stroke 2014, 45, 1646–1650. [Google Scholar] [CrossRef] [Green Version]
- Di Castelnuovo, A.; Veronesi, G.; Costanzo, S.; Zeller, T.; Schnabel, R.B.; de Curtis, A.; Salomaa, V.; Borchini, R.; Ferrario, M.; Giampaoli, S.; et al. NT-proBNP (N-Terminal Pro-B-Type Natriuretic Peptide) and the Risk of Stroke. Stroke 2019, 50, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Osmancik, P.; Teringova, E.; Tousek, P.; Paulu, P.; Widimsky, P. Prognostic value of TNF-related apoptosis inducing ligand (TRAIL) in acute coronary syndrome patients. PLoS ONE 2013, 8, e53860. [Google Scholar] [CrossRef] [Green Version]
- Shichita, T.; Ito, M.; Yoshimura, A. Post-ischemic inflammation regulates neural damage and protection. Front. Cell. Neurosci. 2014, 8, 319. [Google Scholar] [CrossRef]
- Mabuchi, T.; Kitagawa, K.; Ohtsuki, T.; Kuwabara, K.; Yagita, Y.; Yanagihara, T.; Hori, M.; Matsumoto, M. Contribution of microglia/macrophages to expansion of infarction and response of oligodendrocytes after focal cerebral ischemia in rats. Stroke 2000, 31, 1735–1743. [Google Scholar] [CrossRef]
- Martin-Villalba, A.; Herr, I.; Jeremias, I.; Hahne, M.; Brandt, R.; Vogel, J.; Schenkel, J.; Herdegen, T.; Debatin, K.M. CD95 ligand (Fas-L/APO-1L) and tumor necrosis factor-related apoptosis-inducing ligand mediate ischemia-induced apoptosis in neurons. J. Neurosci. 1999, 19, 3809–3817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannakoulas, G.; Hatzitolios, A.; Karvounis, H.; Koliakos, G.; Charitandi, A.; Dimitroulas, T.; Savopoulos, C.; Tsirogianni, E.; Louridas, G. N-terminal pro-brain natriuretic peptide levels are elevated in patients with acute ischemic stroke. Angiology 2005, 56, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Di Angelantonio, E.; Fiorelli, M.; Toni, D.; Sacchetti, M.L.; Lorenzano, S.; Falcou, A.; Ciarla, M.V.; Suppa, M.; Bonanni, L.; Bertazzoni, G.; et al. Prognostic significance of admission levels of troponin I in patients with acute ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 2005, 76, 76–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, H.; Fogh Christensen, A.; Boysen, G. Abnormalities on ECG and telemetry predict stroke outcome at 3 months. J. Neurol. Sci. 2005, 234, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.; Boysen, G.; Christensen, A.F.; Johannesen, H.H. Insular lesions, ECG abnormalities, and outcome in acute stroke. J. Neurol. Neurosurg. Psychiatry 2005, 76, 269–271. [Google Scholar] [CrossRef] [Green Version]
- Eom, Y.W.; Jung, H.Y.; Oh, J.E.; Lee, J.W.; Ahn, M.S.; Youn, Y.J.; Ahn, S.G.; Kim, J.Y.; Lee, S.H.; Yoon, J.; et al. Isoproterenol Enhances Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand-Induced Apoptosis in Human Embryonic Kidney Cells through Death Receptor 5 up-Regulation. Korean Circ. J. 2016, 46, 93–98. [Google Scholar] [CrossRef]
- Secchiero, P.; Gonelli, A.; Corallini, F.; Ceconi, C.; Ferrari, R.; Zauli, G. Metalloproteinase 2 cleaves in vitro recombinant TRAIL: Potential implications for the decreased serum levels of TRAIL after acute myocardial infarction. Atherosclerosis 2010, 211, 333–336. [Google Scholar] [CrossRef]
- Secchiero, P.; Gonelli, A.; Carnevale, E.; Milani, D.; Pandolfi, A.; Zella, D.; Zauli, G. TRAIL promotes the survival and proliferation of primary human vascular endothelial cells by activating the Akt and ERK pathways. Circulation 2003, 107, 2250–2256. [Google Scholar] [CrossRef] [Green Version]
- Zauli, G.; Pandolfi, A.; Gonelli, A.; Di Pietro, R.; Guarnieri, S.; Ciabattoni, G.; Rana, R.; Vitale, M.; Secchiero, P. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) sequentially upregulates nitric oxide and prostanoid production in primary human endothelial cells. Circ. Res. 2003, 92, 732–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secchiero, P.; Candido, R.; Corallini, F.; Zacchigna, S.; Toffoli, B.; Rimondi, E.; Fabris, B.; Giacca, M.; Zauli, G. Systemic tumor necrosis factor-related apoptosis-inducing ligand delivery shows antiatherosclerotic activity in apolipoprotein E-null diabetic mice. Circulation 2006, 114, 1522–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secchiero, P.; Corallini, F.; di Iasio, M.G.; Gonelli, A.; Barbarotto, E.; Zauli, G. TRAIL counteracts the proadhesive activity of inflammatory cytokines in endothelial cells by down-modulating CCL8 and CXCL10 chemokine expression and release. Blood 2005, 105, 3413–3419. [Google Scholar] [CrossRef] [PubMed]
| Overall, n = 120 | AIS, n = 104 | ICH, n = 16 | p Value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, Mean year (SD) | 70.9 (12) | 70.8 (11.8) | 71.8 (13.5) | 0.84 |
| Male, n (%) | 63 (52.5) | 53 (51) | 10 (62) | 0.43 |
| Arterial hypertension, n (%) | 93 (77.5) | 78 (75) | 15 (93.7) | 0.12 |
| Smoking, n (%) | 51 (42.5) | 43 (41.3) | 8 (50) | 0.81 |
| Dyslipidemia, n (%) | 60 (50) | 53 (51) | 7 (44) | 0.79 |
| Diabetes mellitus, n (%) | 29 (24.1) | 24 (23.1) | 5 (31.3) | 0.53 |
| Ischemic heart disease, n (%) | 8 (6.7) | 6 (5.8) | 2 (12.5) | 0.29 |
| History of stroke/TIA, n (%) | 13 (10.8) | 10 (9.6) | 3 (18.8) | 0.38 |
| Atrial fibrillation, n (%) | 26 (21.7) | 23 (22.1) | 3 (18.8) | 0.21 |
| Renal insufficiency, n (%) | 9 (7.5) | 6 (5.8) | 3 (18.8) | 0.09 |
| History of myocardial infarction, n (%) | 4 (3.3) | 3 (2.9) | 1 (6.3) | 0.44 |
| Assessments | ||||
| Symptom duration | ||||
| <4.5 h, n (%) | 93 (77.5) | 81 (77.9) | 12 (75) | 0.75 |
| <12 h, n (%) | 115 (95.8) | 101 (97.1) | 14 (87.5) | 0.13 |
| NIHSS | ||||
| 0 (No stroke symptoms) | 0 | |||
| 1–4 (Minor) | 30 (28.8) | |||
| 5–15 (Moderate) | 52 (50) | |||
| 16–20 (Moderate to severe) | 16 (15.4) | |||
| 21–42 (Severe) | 6 (5.8) | |||
| mRS 90 days | 0.02 | |||
| 0 (No symptoms) (%) | 34 (34.7) | 1 (6.3) | ||
| 1 (No disability despite symptoms) (%) | 22 (22.5) | 1 (6.3) | ||
| 2 (Slight disability) (%) | 12 (12.2) | 1 (6.3) | ||
| 3 (Moderate disability) (%) | 6 (6.1) | 1 (6.3) | ||
| 4 (Moderate severe disability) (%) | 6 (6.1) | 1 (6.3) | ||
| 5 (Severe disability) (%) | 5 (5.1) | 1 (6.3) | ||
| 6 (Dead) (%) | 13 (13.3) | 8 (57.1) |
| N (%) | p Value | |
|---|---|---|
| Arrythmias | ||
| Atrial fibrillation | 20 (21.3%) | 0.13 |
| AV block I.degree | 8 (8.5%) | 0.78 |
| PVC | 7 (7.4%) | 0.04 |
| LAH | 4 (4.3%) | 0.49 |
| RBBB | 4 (4.3%) | 0.37 |
| Sinus tachycardia | 3 (3.2%) | 0.75 |
| Morphological changes | ||
| QTc prolongation | 16 (17.0%) | 0.052 |
| ST segment depression | 11 (11.7%) | 0.29 |
| T wave inversion | 10 (10.6%) | 0.14 |
| Flat T wave | 6 (6.4%) | 0.92 |
| U wave | 2 (2.1%) | 0.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihalovic, M.; Mikulenka, P.; Línková, H.; Neuberg, M.; Štětkářová, I.; Peisker, T.; Lauer, D.; Tousek, P. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) in Patients after Acute Stroke: Relation to Stroke Severity, Myocardial Injury, and Impact on Prognosis. J. Clin. Med. 2022, 11, 2552. https://doi.org/10.3390/jcm11092552
Mihalovic M, Mikulenka P, Línková H, Neuberg M, Štětkářová I, Peisker T, Lauer D, Tousek P. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) in Patients after Acute Stroke: Relation to Stroke Severity, Myocardial Injury, and Impact on Prognosis. Journal of Clinical Medicine. 2022; 11(9):2552. https://doi.org/10.3390/jcm11092552
Chicago/Turabian StyleMihalovic, Michal, Petr Mikulenka, Hana Línková, Marek Neuberg, Ivana Štětkářová, Tomáš Peisker, David Lauer, and Petr Tousek. 2022. "Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) in Patients after Acute Stroke: Relation to Stroke Severity, Myocardial Injury, and Impact on Prognosis" Journal of Clinical Medicine 11, no. 9: 2552. https://doi.org/10.3390/jcm11092552
APA StyleMihalovic, M., Mikulenka, P., Línková, H., Neuberg, M., Štětkářová, I., Peisker, T., Lauer, D., & Tousek, P. (2022). Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL) in Patients after Acute Stroke: Relation to Stroke Severity, Myocardial Injury, and Impact on Prognosis. Journal of Clinical Medicine, 11(9), 2552. https://doi.org/10.3390/jcm11092552





