Inhaled Sedation for Invasively Ventilated COVID-19 Patients: A Systematic Review
Abstract
:1. Introduction
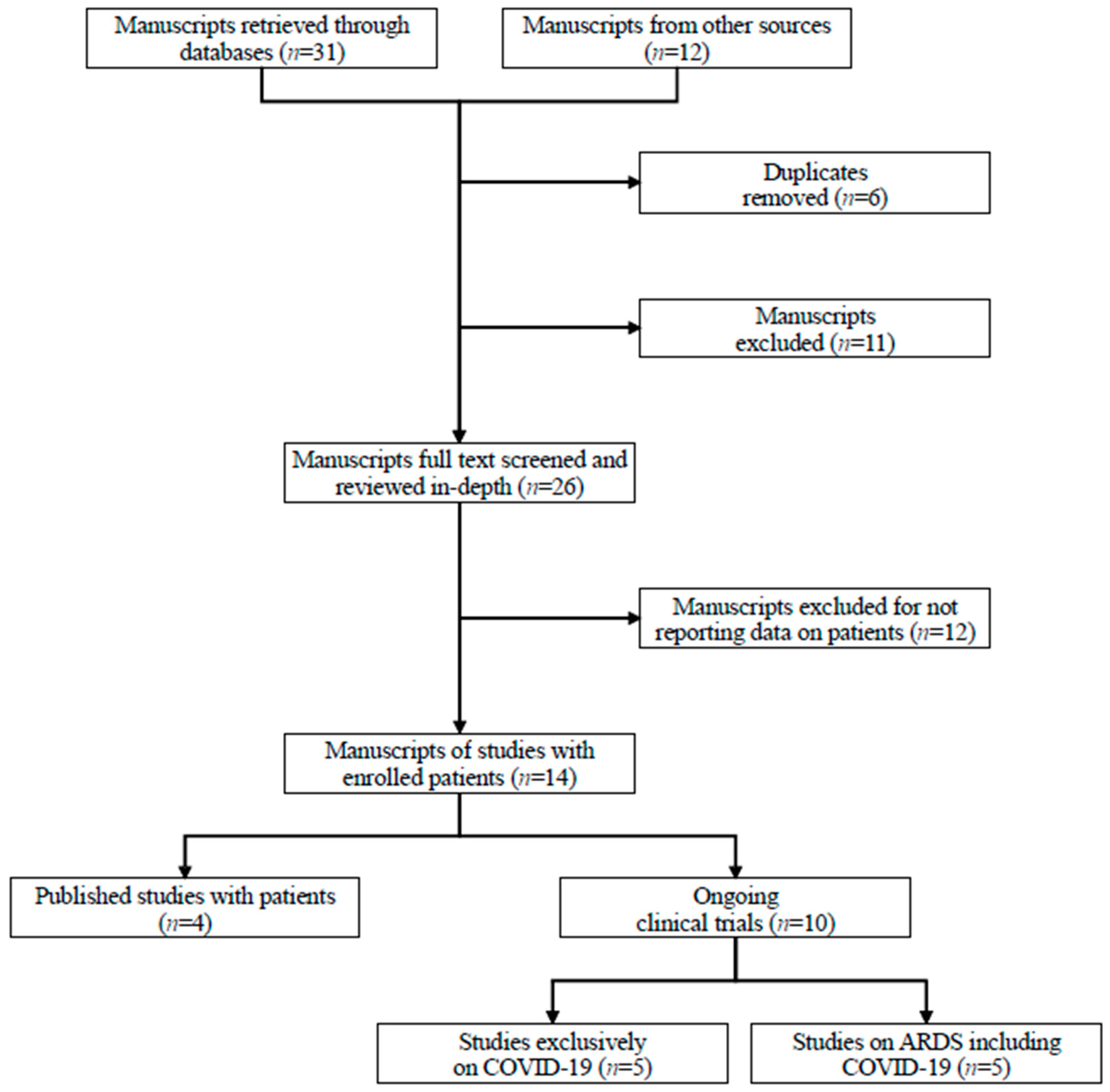
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hopkins, J. Coronavirus Resource Center. November 2021. Available online: https://coronavirus.jhu.edu/ (accessed on 25 April 2022).
- Welker, C.; Huang, J.; Gil, I.; Ramakrishna, H. 2021 Acute Respiratory Distress Syndrome Update, with Coronavirus Disease 2019 Focus. J. Cardiothorac. Vasc. Anesth. 2022, 36, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Stollings, L.M.; Jia, L.J.; Tang, P.; Dou, H.; Lu, B.; Xu, Y. Immune Modulation by Volatile Anesthetics. Anesthesiology 2016, 125, 399–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suter, D.; Spahn, D.R.; Blumenthal, S.; Reyes, L.; Booy, C.; Z’graggen, B.R.; Beck-Schimmer, B. The immunomodulatory effect of sevoflurane in endotoxin-injured alveolar epithelial cells. Anesth. Analg. 2007, 104, 638–645. [Google Scholar] [CrossRef] [Green Version]
- Steurer, M.; Schläpfer, M.; Steurer, M.; Roth Z’graggen, B.; Booy, C.; Reyes, L.; Spahn, D.R.; Beck-Schimmer, B. The volatile anaesthetic sevoflurane attenuates lipopolysaccharide-induced injury in alveolar macrophages. Clin. Exp. Immunol. 2009, 155, 224–230. [Google Scholar] [CrossRef]
- Voigtsberger, S.; Lachmann, R.A.; Leutert, A.C.; Schläpfer, M.; Booy, C.; Reyes, L.; Urner, M.; Schild, J.; Schimmer, R.C.; Beck-Schimmer, B. Sevoflurane Ameliorates Gas Exchange and Attenuates Lung Damage in Experimental Lipopolysaccharide-induced Lung Injury. Anesthesiology 2009, 111, 1238–1248. [Google Scholar] [CrossRef] [Green Version]
- Harr, J.N.; Moore, E.E.; Stringham, J.; Wohlauer, M.V.; Fragoso, M.; Jones, W.L.; Gamboni, F.; Silliman, C.C.; Banerjee, A. Isoflurane prevents acute lung injury through ADP-mediated platelet inhibition. Surgery 2012, 152, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Serrano, M.; Gerónimo-Pardo, M.; Martínez-Monsalve, A.; Crespo-Sánchez, M.D. Antibacterial effect of sevoflurane and isoflurane. Rev. Esp. Quimioter. 2017, 30, 84–89. [Google Scholar]
- Landoni, G.; Pasin, L.; Cabrini, L.; Scandroglio, A.M.; Baiardo Redaelli, M.; Votta, C.D.; Bellandi, M.; Borghi, G.; Zangrillo, A. Volatile Agents in Medical and Surgical Intensive Care Units: A Meta-Analysis of Randomized Clinical Trials. J. Cardiothorac. Vasc. Anesth. 2016, 30, 1005–1014. [Google Scholar] [CrossRef]
- Faller, S.; Strosing, K.M.; Ryter, S.W.; Buerkle, H.; Loop, T.; Schmidt, R.; Hoetzel, A. The volatile anesthetic isoflurane prevents ventilator-induced lung injury via phosphoinositide 3-kinase/Akt signaling in mice. Anesth. Analg. 2012, 114, 747–756. [Google Scholar] [CrossRef]
- Fortis, S.; Spieth, P.M.; Lu, W.Y.; Parotto, M.; Haitsma, J.J.; Slutsky, A.S.; Zhong, N.; Mazer, C.D.; Zhang, H. Effects of anesthetic regimes on inflammatory responses in a rat model of acute lung injury. Intensive Care Med. 2012, 38, 1548–1555. [Google Scholar] [CrossRef]
- Jabaudon, M.; Boucher, P.; Imhoff, E.; Chabanne, R.; Faure, J.S.; Roszyk, L.; Thibault, S.; Blondonnet, R.; Clairefond, G.; Guérin, R.; et al. Sevoflurane for Sedation in Acute Respiratory Distress Syndrome. A Randomized Controlled Pilot Study. Am. J. Respir. Crit. Care Med. 2017, 195, 792–800. [Google Scholar] [CrossRef]
- Kermad, A.; Speltz, J.; Danziger, G.; Mertke, T.; Bals, R.; Volk, T.; Lepper, P.M.; Meiser, A. Comparison of isoflurane and propofol sedation in critically ill COVID-19 patients-a retrospective chart review. J. Anesth. 2021, 35, 625–632. [Google Scholar] [CrossRef]
- Ferrière, N.; Bodenes, L.; Bailly, P.; L’Her, E. Shortage of anesthetics: Think of inhaled sedation! J. Crit. Care 2021, 63, 104–105. [Google Scholar] [CrossRef]
- Flinspach, A.N.; Zacharowski, K.; Ioanna, D.; Adam, E.H. Volatile Isoflurane in Critically Ill Coronavirus Disease 2019 Patients-A Case Series and Systematic Review. Crit. Care Explor. 2020, 2, e0256. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Coppola, S.; Cenci, S.; Cozzolino, M.; Chiumello, D. Sevoflurane sedation and nephrogenic diabetes insipidus in patients affected with severe acute respiratory syndrome coronavirus 2. Eur. J. Anaesthesiol. 2021, 38, 438–441. [Google Scholar] [CrossRef]
- Hanidziar, D.; Baldyga, K.; Ji, C.S. Standard sedation and sedation with isoflurane in mechanically ventilated patients with coronavirus disease 2019. Crit. Care Explor. 2021, 3, e0370. [Google Scholar] [CrossRef]
- Cabibel, R.; Gerard, L.; Maiter, D.; Collin, V.; Hantson, P. Complete Nephrogenic Diabetes Insipidus After Prolonged Sevoflurane Sedation: A Case Report of 3 Cases. A&A Pract. 2019, 12, 155–159. [Google Scholar] [CrossRef]
- Hanidziar, D.; Bittner, E.A. Sedation of Mechanically Ventilated COVID-19 Patients: Challenges and Special Considerations. Anesth. Analg. 2020, 131, e40–e41. [Google Scholar] [CrossRef]
- Marini, J.J.; Gattinoni, L. Management of COVID-19 Respiratory Distress. JAMA 2020, 323, 2329–2330. [Google Scholar] [CrossRef]
- Meng, L.; Qiu, H.; Wan, L.; Ai, Y.; Xue, Z.; Guo, Q.; Deshpande, R.; Zhang, L.; Meng, J.; Tong, C.; et al. Intubation and Ventilation amid the COVID-19 Outbreak: Wuhan’s Experience. Anesthesiology 2020, 132, 1317–1332. [Google Scholar] [CrossRef] [Green Version]
- Sorbello, M.; El-Boghdadly, K.; Di Giacinto, I.; Cataldo, R.; Esposito, C.; Falcetta, S.; Merli, G.; Cortese, G.; Corso, R.M.; Bressan, F.; et al. The Italian coronavirus disease 2019 outbreak: Recommendations from clinical practice. Anaesthesia 2020, 75, 724–732. [Google Scholar] [CrossRef]
- Wolf, A.; Mörgeli, R.; Müller, A.; Weiss, B.; Spies, C. Delir, Analgesie und Sedierung in der Intensivmedizin: Entwicklung eines protokollbasierten Managements [Delirium, analgesia, and sedation in intensive care medicine: Development of a protocol-based management approach]. Med. Klin. Intensivmed. Notfmed. 2017, 112, 65–74. [Google Scholar] [CrossRef]
- ASA/APSF Guidance for Use of Volatile Anesthesic for Sedation of ICU Patients Emergency Use for the COVID-19 Pandemic. (Modified from a protocol produced by Brian O’Gara MD MPH, Assistant Professor of Anesthesia, Beth Israel Deaconess Medical Center, Department of Anesthesia, Critical Care, and Pain Medicine, Harvard Medical School). Am. Soc. Anesthesiol. Available online: https://www.asahq.org/in-the-spotlight/coronavirus-covid-19-information/purposing-anesthesia-machines-for-ventilators (accessed on 25 April 2022).
- Meiser, A.; Volk, T.; Wallenborn, J.; Guenther, U.; Becher, T.; Bracht, H.; Schwarzkopf, K.; Knafelj, R.; Faltlhauser, A.; Thal, S.C.; et al. Inhaled isoflurane via the anaesthetic conserving device versus propofol for sedation of invasively ventilated patients in intensive care units in Germany and Slovenia: An open-label, phase 3, randomised controlled, non-inferiority trial. Lancet Respir. Med. 2021, 9, 1231–1240. [Google Scholar] [CrossRef]
- French Agency for the Safety of Health Products. November 2021. Available online: https://www.emergobyul.com/resources/asnm-french-agency-safety-health-products (accessed on 25 April 2022).
- Kim, H.Y.; Lee, J.E.; Kim, H.Y.; Kim, J. Volatile sedation in the intensive care unit: A systematic review and meta-analysis. Medicine 2017, 96, e8976. [Google Scholar] [CrossRef]
- Jerath, A.; Ferguson, N.D.; Cuthbertson, B. Inhalational volatile-based sedation for COVID-19 pneumonia and ARDS. Intensive Care Med. 2020, 46, 1563–1566. [Google Scholar] [CrossRef]
- Jerath, A.; Panckhurst, J.; Parotto, M.; Lightfoot, N.; Wasowicz, M.; Ferguson, N.D.; Steel, A.; Beattie, W.S. Safety and Efficacy of Volatile Anesthetic Agents Compared with Standard Intravenous Midazolam/Propofol Sedation in Ventilated Critical Care Patients: A Meta-analysis and Systematic Review of Prospective Trials. Anesth. Analg. 2017, 124, 1190–1199. [Google Scholar] [CrossRef]
- Bellgardt, M.; Bomberg, H.; Herzog-Niescery, J.; Dasch, B.; Vogelsang, H.; Weber, T.P.; Steinfort, C.; Uhl, W.; Wagenpfeil, S.; Volk, T.; et al. Survival after long-term isoflurane sedation as opposed to intravenous sedation in critically ill surgical patients: Retrospective analysis. Eur. J. Anaesthesiol. 2016, 33, 6–13. [Google Scholar] [CrossRef]
- Orser, B.A.; Wang, D.S.; Lu, W.Y. Sedating ventilated COVID-19 patients with inhalational anesthetic drugs. EBioMedicine 2020, 55, 102770. [Google Scholar] [CrossRef]
- Nieuwenhuijs-Moeke, G.J.; Jainandunsing, J.S.; Struys, M.M.R.F. Sevoflurane, a sigh of relief in COVID-19? Br. J. Anaesth. 2020, 125, 118–121. [Google Scholar] [CrossRef]
- Suleiman, A.; Qaswal, A.B.; Alnouti, M.; Yousef, M.; Suleiman, B.; Jarbeh, M.E.; Alshawabkeh, G.; Bsisu, I.; Santarisi, A.; Ababneh, M. Sedating Mechanically Ventilated COVID-19 Patients with Volatile Anesthetics: Insights on the Last-Minute Potential Weapons. Sci. Pharm. 2021, 89, 6. [Google Scholar] [CrossRef]
- Rand, A.; Zahn, P.K.; Schildhauer, T.A.; Waydhas, C.; Hamsen, U. Inhalative sedation with small tidal volumes under venovenous ECMO. J. Artif. Organs 2018, 21, 201–205. [Google Scholar] [CrossRef]
- Meiser, A.; Groesdonk, H.V.; Bonnekessel, S.; Volk, T.; Bomberg, H. Inhalation Sedation in Subjects with ARDS Undergoing Continuous Lateral Rotational Therapy. Respir. Care 2018, 63, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Sherren, P.B.; Ostermann, M.; Agarwal, S.; Meadows, C.I.S.; Ioannou, N.; Camporota, L. COVID-19-related organ dysfunction and management strategies on the intensive care unit: A narrative review. Br. J. Anaesth. 2020, 125, 912–925. [Google Scholar] [CrossRef]
- Son, K.H.; Lee, S.I.; Choi, C.H.; Park, C.H. Mechanical Failure of Extracorporeal Membrane Oxygenation Induced by Hypertriglyceridemia. Ann. Thorac. Surg. 2017, 104, e85. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Xu, L.; Huang, B.; Wu, L. Propofol increases angiotensin-converting enzyme 2 expression in human pulmonary artery endothelial cells. Pharmacology 2012, 90, 342–347. [Google Scholar] [CrossRef]
- Hirota, K.; Lambert, D.G. Propofol and SARS-CoV-2 infection. Br. J. Anaesth. 2020, 125, e475–e476. [Google Scholar] [CrossRef]
- Sohn, J.T. Propofol and sedation in patients with coronavirus disease. Am. J. Emerg. Med. 2021, 42, 250. [Google Scholar] [CrossRef]
- Zangrillo, A.; Lomivorotov, V.V.; Pasyuga, V.V.; Belletti, A.; Gazivoda, G.; Monaco, F.; Neto, C.N.; Likhvantsev, V.V.; Bradic, N.; Lozovskiy, A.; et al. Effect of Volatile Anesthetics on Myocardial Infarction After Coronary Artery Surgery: A Post Hoc Analysis of a Randomized Trial. J. Cardiothorac. Vasc. Anesth. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Kaura, V.; Hopkins, P.M. Sevoflurane may not be a complete sigh of relief in COVID-19. Br. J. Anaesth. 2020, 125, e487–e488. [Google Scholar] [CrossRef]
- Sackey, P.V.; Martling, C.R.; Nise, G.; Radell, P.J. Ambient isoflurane pollution and isoflurane consumption during intensive care unit sedation with the Anesthetic Conserving Device. Crit. Care Med. 2005, 33, 585–590. [Google Scholar] [CrossRef]
- González-Rodríguez, R.; Muñoz Martínez, A.; Galan Serrano, J.; Moral García, M.V. Health worker exposure risk during inhalation sedation with sevoflurane using the (AnaConDa®) anaesthetic conserving device. Rev. Esp. Anestesiol. Reanim. 2014, 61, 133–139. [Google Scholar] [CrossRef]
- Zhang, Y.; Shan, G.J.; Zhang, Y.X.; Cao, S.-J.; Zhu, S.-N.; Li, H.-J.; Ma, D.; Wang, D.-X. Propofol compared with sevoflurane general anaesthesia is associated with decreased delayed neurocognitive recovery in older adults. Br. J. Anaesth. 2018, 121, 595–604. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, D.; Wang, H.; Wang, Z.; Song, F.; Li, H.; Ling, L.; Shen, Z.; Hu, C.; Peng, J.; et al. Intravenous versus Volatile Anesthetic Effects on Postoperative Cognition in Elderly Patients Undergoing Laparoscopic Abdominal Surgery. Anesthesiology 2021, 134, 381–394. [Google Scholar] [CrossRef]
- Charlesworth, M.; Swinton, F. Anaesthetic gases, climate change, and sustainable practice. Lancet Planet. Health 2017, 1, e216–e217. [Google Scholar] [CrossRef]
- Boldt, J.; Jaun, N.; Kumle, B.; Heck, M.; Mund, K. Economic considerations of the use of new anesthetics: A comparison of propofol, sevoflurane, desflurane, and isoflurane. Anesth. Analg. 1998, 86, 504–509, Erratum in Anesth. Analg. 2020, 131, e240. [Google Scholar] [CrossRef]
- Chen, C.; Ji, M.; Xu, Q.; Zhang, Y.; Sun, Q.; Liu, J.; Zhu, S.; Li, W. Sevoflurane attenuates stress-enhanced fear learning by regulating hippocampal BDNF expression and Akt/GSK-3β signaling pathway in a rat model of post-traumatic stress disorder. J. Anesth. 2015, 29, 600–608. [Google Scholar] [CrossRef]
| Authors | Year | Month | Setting | Patients Enrolled (n) | Agent | Comparison Group (n) | Delivery System | Reason | Duration of Volatile Sedation (Mean ± SD, Days) | ECMO | Study Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coppola S. [17] | 2021 | April | ICU | 2 | Sevoflurane | ND | ND | Suboptimal sedation | 9.5 ± 0.71 | ND | The prolonged use of sevoflurane together with the ARDS related inflammatory and hemodynamic mechanisms on renal function could be nephrotoxic |
| Flinspach A. [15] | 2020 | October | ICU | 5 | Isoflurane | ND | Mirus | 3/5: suboptimal sedation 2/5: suboptimal sedation + bronchoconstriction | 4.3 ± 3.08 | 3 | Isoflurane achieved the required deep sedation and reduced the need for IV sedation |
| Hanidziar D. [18] | 2021 | March | Operating theatre used as ICU | 18 | Isoflurane + IV sedation | 17 | Apollo | 6/18: as primary sedative during neuromuscular blockade 12/18: as an adjunct to multiple IV agents | 5.6 ± 2.99 | ND | Isoflurane was associated with a significant decrease in propofol and hydromorphone infusions |
| Kermad A. [13] | 2021 | June | ICU | 18 | Isoflurane ± IV sedation | 12 | AnaConDa | ND | ND | 9 | Isoflurane provided sufficient sedation with less NMBAs, less polypharmacy, and lower opioid doses compared to propofol |
| Authors | Year | NCT | Patients Enrolled (n) | Agent | Comparison Group (n) | COVID-19 Patients | RCTs | Recruitment Status | Objective |
|---|---|---|---|---|---|---|---|---|---|
| Blondonnet R. | 2020 | 04023305 | 43 | Sevoflurane | ND | ND | YES | Recruiting | Pharmacokinetic models of sevoflurane-induced sedation during ARDS depending on the lung imaging phenotype. |
| Fundación para la Investigación del Hospital Clínico de Valencia | 2020 | 04359862 | 19 | Sevoflurane | Propofol | YES | YES | Terminated (low recruitment ratio) | PaO2/FiO2 on day two in patients with COVID-19 ARDS. |
| Jabaudon M. | 2020 | 04383730 | 203 | Isoflurane or sevoflurane | Intravenous sedation | YES | NO | Completed | Number of days off the ventilator on day 28. |
| Jabaudon M. | 2020 | 04235608 | 700 | Sevoflurane | Propofol | ND | YES | Recruiting | Mortality and morbidity. |
| Jerath A. | 2020 | 04415060 | 752 | Isoflurane or sevoflurane | Propofol | ND | YES | Recruiting | Hospital mortality, ventilator-free days, ICU free days and participant quality of life at 3- and 12-months post discharge. |
| Lai C. | 2020 | 04530188 | 68 | Sevoflurane | Propofol | ND | YES | Not yet recruiting | PVPI and the amount of EVLW in patients with moderate-to-severe ARDS. |
| Likhvantsev V. | 2019 | 04014218 | 80 | Sevoflurane | Propofol | ND | YES | ND | 28-day mortality. |
| Palacios Chavarria A. | 2020 | 04998253 | 24 | Sevoflurane | Propofol | YES | YES | Completed | Difference in the oxygenation hypoxic pulmonary vasoconstriction. |
| Schimmer B. | 2020 | 04355962 | 68 | Sevoflurane | Intravenous sedation | YES | YES | Completed | Mortality and organ dysfunction at day 28. |
| Xie Z | 2020 | 04492943 | 35 | Isoflurane or sevoflurane | ND | ND | NO | Completed | Survival. |
| Authors | Year | Month | Agent | Target Cells | Effect | Results |
|---|---|---|---|---|---|---|
| L.M. Stolling [3] | 2016 | Aug | Sevoflurane, Desflurane, Isoflurane, Halotane | Neutrophil, Macrophage, NK cell, T lymphocyte, B lympohocyte | Decreased cell numbers and cytokine release, Promotion of cell-mediated immunity | The majority of studies reported thus far show that volatile anesthetics have immunosuppressive effects. |
| D. Suter [4] | 2007 | Mar | Sevoflurane | Alveolar epithelial cells | Suppression of the expression of inflammatory mediators. These results suggest that sevoflurane reduces AEC-induced accumulation of neutrophils in LPS injury. | This study shows that sevoflurane alters the LPS-induced inflammatory response, not only with respect to the expression pattern of inflammatory mediators, but also regarding the biological consequences with less accumulation of effector cells such as neutrophils. |
| M. Steurer [5] | 2009 | Feb | Sevoflurane | Alveolar macrophages | Protective effect of post-conditioning with the volatile anaesthetic sevoflurane on endotoxin-induced injury in AM attenuating cytokine and chemokine production. | Pharmacological post-conditioning prevents enhanced expression of inflammatory mediators and attenuates increased chemotaxis. |
| S. Voigtsberger [6] | 2009 | Dec | Sevoflurane | ND | Expression of the cytokine’s protein in bronchoalveolar lavage fluid as well as messenger RNA in lung tissue was significantly lower in the sevoflurane-lipopolysaccharide group compared with the propofol-lipopolysaccharide group. | Significant improvement of the ratio of oxygen tension to inspired oxygen fraction was shown with sevoflurane compared with propofol. |
| J.N. Harr [7] | 2012 | Aug | Isoflurane | Platelet | Inhibition of the platelet ADP-pathway | Isoflurane attenuates ALI through an antiplatelet mechanism. |
| M. Martínez-Serrano [8] | 2017 | Apr | Sevoflurane, Isoflurane | Against pathogens resistant | Halogenated anaesthetics have shown antibacterial activity. | Both halogenated agents, but particularly isoflurane, showed in vitro antibacterial activity against pathogens resistant to conventional antibiotics. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landoni, G.; Belloni, O.; Russo, G.; Bonaccorso, A.; Carà, G.; Jabaudon, M. Inhaled Sedation for Invasively Ventilated COVID-19 Patients: A Systematic Review. J. Clin. Med. 2022, 11, 2500. https://doi.org/10.3390/jcm11092500
Landoni G, Belloni O, Russo G, Bonaccorso A, Carà G, Jabaudon M. Inhaled Sedation for Invasively Ventilated COVID-19 Patients: A Systematic Review. Journal of Clinical Medicine. 2022; 11(9):2500. https://doi.org/10.3390/jcm11092500
Chicago/Turabian StyleLandoni, Giovanni, Olivia Belloni, Giada Russo, Alessandra Bonaccorso, Gianmarco Carà, and Matthieu Jabaudon. 2022. "Inhaled Sedation for Invasively Ventilated COVID-19 Patients: A Systematic Review" Journal of Clinical Medicine 11, no. 9: 2500. https://doi.org/10.3390/jcm11092500
APA StyleLandoni, G., Belloni, O., Russo, G., Bonaccorso, A., Carà, G., & Jabaudon, M. (2022). Inhaled Sedation for Invasively Ventilated COVID-19 Patients: A Systematic Review. Journal of Clinical Medicine, 11(9), 2500. https://doi.org/10.3390/jcm11092500