Pandemic Preparedness: The Importance of Adequate Immune Fitness
Abstract
:1. Introduction
2. Methods
3. Results
3.1. CLOFIT Study
3.2. COTEST Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prati, G.; Mancini, A.D. The psychological impact of COVID-19 pandemic lockdowns: A review and meta-analysis of longitudinal studies and natural experiments. Psychol. Med. 2021, 51, 201–211. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Public Health and the Environment (RIVM). Risicogroepen en COVID-19. Available online: https://www.rivm.nl/coronavirus-covid-19/risicogroepen (accessed on 9 March 2022).
- Lee, K.; Jeong, G.-C.; Yim, J. Consideration of the psychological and mental health of the elderly during COVID-19: A theoretical review. Int. J. Environ. Res. Public Health 2020, 17, 8098. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Aziz, R.; Al Mahri, S.; Malik, S.S.; Haji, E.; Husain Khan, A.; Saleem Khatlani, T.; Bouchama, A. Obesity and COVID-19: What makes obese host so vulnerable? Immun. Ageing 2021, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Almeida Pititto, B.; Dualib, P.M.; Zajdenverg, L.; Rodrigues Dantas, J.; Dias de Souza, F.; Rodacki, M.; Casaccia Bertoluci, M. Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: A meta-analysis. Diabetol. Metab. Syndr. 2020, 12, 75. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Weyand, C.M.; Goronzy, J.J. Aging of the immune system. Mechanisms and therapeutic targets. Ann. Am. Thorac. Soc. 2016, 13 (Suppl. S5), S422–S428. [Google Scholar] [CrossRef]
- Wilod Versprille, L.J.F.; van de Loo, A.J.A.E.; Mackus, M.; Arnoldy, L.; Sulzer, T.A.L.; Vermeulen, S.A.; Abdulahad, S.; Huls, H.; Baars, T.; Kraneveld, A.D.; et al. Development and validation of the Immune Status Questionnaire (ISQ). Int. J. Environ. Res. Public Health 2019, 16, E4743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Schrojenstein Lantman, M.; Otten, L.S.; Mackus, M.; de Kruijff, D.; van de Loo, A.J.A.E.; Kraneveld, A.D.; Garssen, J.; Verster, J.C. Mental resilience, perceived immune functioning, and health. J. Multidiscip. Healthc. 2017, 10, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Vrousgos, G. Lifestyle Factors that can induce an independent and persistent low-grade systemic inflammatory response: A wholistic approach. Open Med. J. 2016, 3, 34–48. [Google Scholar] [CrossRef]
- Taghizadeh-Hesary, F.; Akbari, H. The powerful immune system against powerful COVID-19: A hypothesis. Med. Hypotheses 2020, 140, 109762. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Kiani, P.; Merlo, A.; Saeed, H.M.; Benson, S.; Bruce, G.; Hoorn, R.; Kraneveld, A.D.; Severeijns, N.R.; Sips, A.S.M.; Scholey, A.; et al. Immune fitness, and the psychosocial and health consequences of the COVID-19 pandemic lockdown in The Netherlands: Methodology and design of the CLOFIT study. Eur. J. Investig. Health Psychol. Educ. 2021, 11, 199–218. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Public Health and the Environment (RIVM). Aandoeningen. Welke Aandoeningen Hebben We in De Toekomst? Available online: https://www.vtv2018.nl/aandoeningen (accessed on 5 October 2020).
- RIVM Symptoms. Available online: https://www.rivm.nl/coronavirus-covid-19/ziekte (accessed on 1 May 2020).
- Centers for Disease Control and Prevention. Symptoms of COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html (accessed on 1 November 2020).
- Hulme, K.D.; Noye, E.C.; Short, K.R.; Labzin, L.I. Dysregulated inflammation during obesity: Driving disease severity in influenza virus and SARS-CoV-2 infections. Front. Immunol. 2021, 12, 770066. [Google Scholar] [CrossRef]
- Kompaniyets, L.; Pennington, A.F.; Goodman, A.B.; Rosenblum, H.G.; Belay, B.; Ko, J.Y.; Chevinsky, J.R.; Schieber, L.Z.; Summers, A.D.; Lavery, A.M.; et al. Underlying medical conditions and severe illness among 540,667 adults hospitalized with COVID-19, March 2020–March 2021. Prev. Chronic. Dis. 2021, 18, 210123. [Google Scholar] [CrossRef]
- Liu, L.; Ni, S.Y.; Yan, W.; Lu, Q.D.; Zhao, Y.M.; Xu, Y.Y.; Mei, H.; Shi, L.; Yuan, K.; Han, Y.; et al. Mental and neurological disorders and risk of COVID-19 susceptibility, illness severity and mortality: A systematic review, meta-analysis and call for action. EClinical Med. 2021, 40, 101111. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, S.; Panigrahi, C.; Barua, S.; Sahoo, M.; Mandliya, S. Food nutrients as inherent sources of immunomodulation during COVID-19 pandemic. Lebensm. Wiss. Technol. 2022, 158, 113154. [Google Scholar] [CrossRef] [PubMed]
- Filgueira, T.O.; Castoldi, A.; Santos, L.; de Amorim, G.J.; de Sousa Fernandes, M.S.; Anastácio, W.; Campos, E.Z.; Santos, T.M.; Souto, F.O. The relevance of a physical active lifestyle and physical fitness on immune defense: Mitigating disease burden, with focus on COVID-19 consequences. Front. Immunol. 2021, 12, 587146. [Google Scholar] [CrossRef]
- Schmitz, N.; van der Werf, Y.D.; Lammers-van der Holst, H.M. The importance of sleep and circadian rhythms for vaccination success and susceptibility to viral infections. Clocks Sleep 2022, 4, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Hendriksen, P.A.; Kiani, P.; Garssen, J.; Bruce, G.; Verster, J.C. Living alone or together during lockdown: Association with mood, immune fitness and experiencing COVID-19 symptoms. Psychol. Res. Behav. Manag. 2021, 14, 1947–1957. [Google Scholar] [CrossRef]
- Merlo, A.; Severeijns, N.R.; Benson, S.; Scholey, A.; Garssen, J.; Bruce, G.; Verster, J.C. Mood and changes in alcohol consumption in young adults during COVID-19 lockdown: A model explaining associations with perceived immune fitness and experiencing COVID-19 symptoms. Int. J. Environ. Res. Public Health 2021, 18, 10028. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Q.; Kaelber, D.C.; Xu, R.; Volkow, N.D. COVID-19 risk and outcomes in patients with substance use disorders: Analyses from electronic health records in the United States. Mol. Psychiat. 2021, 26, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, S.T.; Singh-Manoux, A.; Pentti, J.; Madsen, I.; Sabia, S.; Alfredsson, L.; Bjorner, J.B.; Borritz, M.; Burr, H.; Goldberg, M.; et al. Association of healthy lifestyle with years lived without major chronic diseases. JAMA Int. Med. 2020, 180, 760–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute for Public Health and the Environment (RIVM). Weekcijfers Coronavirus SARS-CoV-2. Available online: https://www.rivm.nl/coronavirus-covid-19/weekcijfers (accessed on 9 March 2022).
- Gans, J.S.; Goldfarb, A.; Agrawal, A.K.; Sennik, S.; Stein, J.; Rosella, L. False-Positive Results in Rapid Antigen Tests for SARS-CoV-2. JAMA 2022, 327, 485–486. [Google Scholar] [CrossRef] [PubMed]
| Variable | Overall | Men | Women | p-Value |
|---|---|---|---|---|
| n (%) | 1415 (100%) | 503 (35.5%) | 912 (64.5%) | <0.001 * |
| Age (year) | 45.0 (18.5) | 49.7 (18.4) | 42.4 (18.0) | <0.001 * |
| Height (m) | 1.73 (0.09) | 1.80 (0.08) | 1.69 (0.07) | <0.001 * |
| Weight (kg) | 79.3 (18.8) | 87.6 (17.4) | 74.8 (17.9) | <0.001 * |
| BMI (kg/m2) | 26.4 (5.8) | 26.9 (5.3) | 26.2 (6.1) | <0.001 * |
| Underlying disease (% yes) | 65.5 | 60.4 | 68.3 | 0.003 * |
| Immune fitness (2019) | 7.1 (2.4) | 7.8 (2.3) | 6.8 (2.5) | <0.001 * |
| Immune fitness (DL) | 7.1 (2.0) | 7.4 (1.8) | 6.9 (2.1) | <0.001 * |
| Number of COVID-19 Symptoms | 2.7 (2.2) | 2.4 (2.1) | 2.8 (2.3) | 0.010 * |
| Severity of COVID-19 Symptoms | 0.44 (0.5) | 0.38 (0.4) | 0.48 (0.5) | 0.001 * |
| Variable | Tested Positive | Tested Negative | p-Value |
|---|---|---|---|
| n | 88 | 837 | <0.001 * |
| Male/female (%) | 60.2/39.8 | 54.5/45.5 | 0.313 |
| Age (year) | 46.3 (13.3) | 47.0 (14.5) | 0.697 |
| BMI (kg/m2) | 26.1 (4.4) | 26.0 (4.3) | 0.848 |
| Underlying diseases (% yes) | 58.0 | 54.1 | 0.502 |
| Immune fitness (2019) | 8.1 (1.7) | 7.8 (2.0) | 0.466 |
| Immune fitness (T) | 7.3 (1.7) | 7.5 (1.6) | 0.714 |
| Number of COVID-19 symptoms | 5.2 (3.2) | 3.4 (3.0) | <0.001 * |
| Severity of COVID-19 symptoms | 0.46 (0.4) | 0.29 (0.3) | <0.001 * |
| Correlations with Immune Fitness (2019) | |||||||
|---|---|---|---|---|---|---|---|
| Correlation with COVID-19 Symptoms | Overall | Tested Positive | Tested Negative | Comparison | |||
| r | p-Value | r | p-Value | r | p-Value | p-Value | |
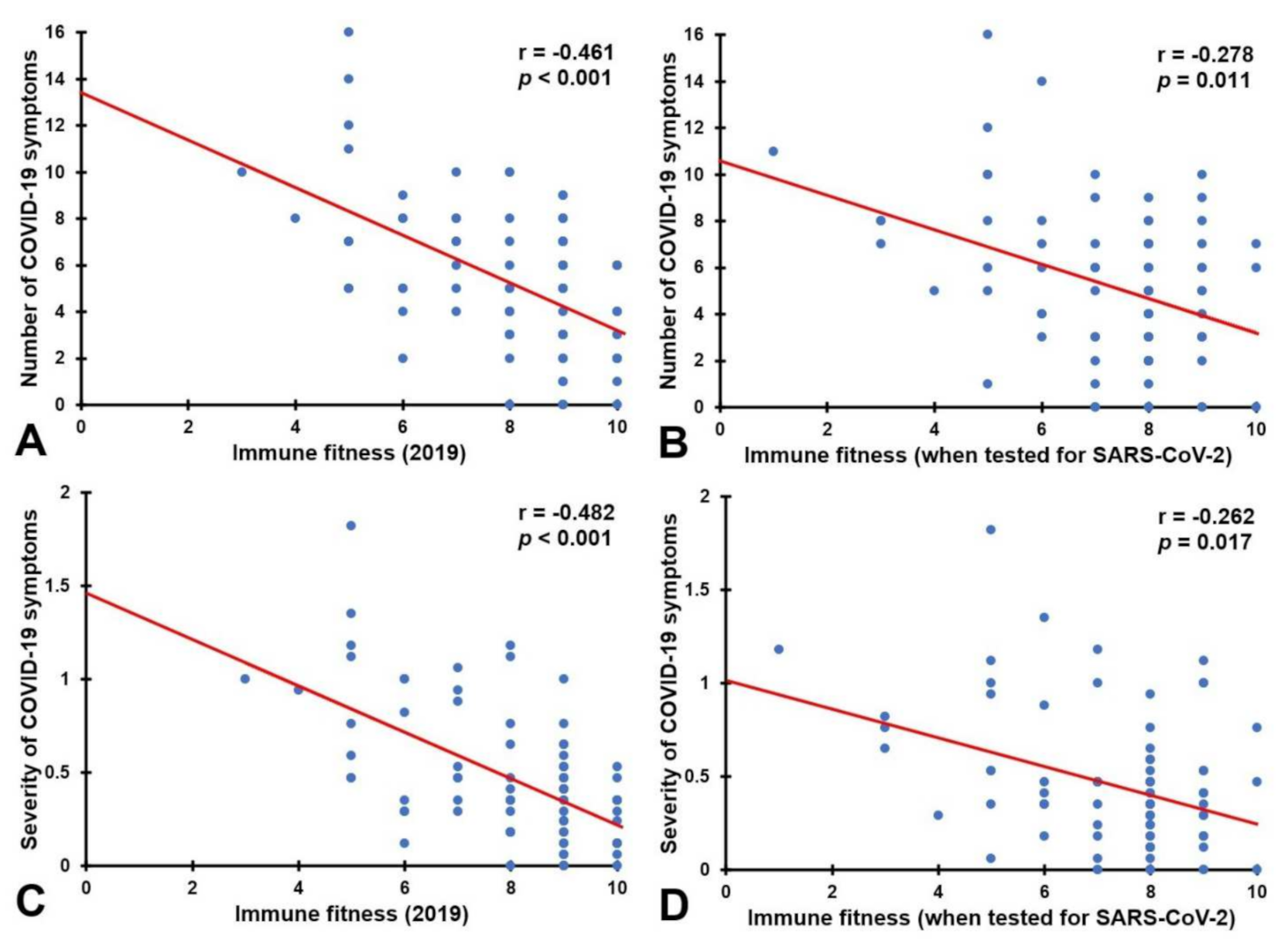
| Number of symptoms | −0.431 | <0.001 * | −0.461 | <0.001 * | −0.442 | <0.001 * | 0.834 |
| Severity of symptoms | −0.432 | <0.001 * | −0.482 | <0.001 * | −0.440 | <0.001 * | 0.638 |
| Correlations with immune fitness (T) | |||||||
| Number of symptoms | −0.451 | <0.001 * | −0.278 | 0.011 * | −0.473 | <0.001 * | 0.044 * |
| Severity of symptoms | −0.459 | <0.001 * | −0.262 | 0.017 * | −0.481 | <0.001 * | 0.024 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiani, P.; Balikji, J.; Kraneveld, A.D.; Garssen, J.; Bruce, G.; Verster, J.C. Pandemic Preparedness: The Importance of Adequate Immune Fitness. J. Clin. Med. 2022, 11, 2442. https://doi.org/10.3390/jcm11092442
Kiani P, Balikji J, Kraneveld AD, Garssen J, Bruce G, Verster JC. Pandemic Preparedness: The Importance of Adequate Immune Fitness. Journal of Clinical Medicine. 2022; 11(9):2442. https://doi.org/10.3390/jcm11092442
Chicago/Turabian StyleKiani, Pantea, Jessica Balikji, Aletta D. Kraneveld, Johan Garssen, Gillian Bruce, and Joris C. Verster. 2022. "Pandemic Preparedness: The Importance of Adequate Immune Fitness" Journal of Clinical Medicine 11, no. 9: 2442. https://doi.org/10.3390/jcm11092442
APA StyleKiani, P., Balikji, J., Kraneveld, A. D., Garssen, J., Bruce, G., & Verster, J. C. (2022). Pandemic Preparedness: The Importance of Adequate Immune Fitness. Journal of Clinical Medicine, 11(9), 2442. https://doi.org/10.3390/jcm11092442







