Chemotherapy-Related Cognitive Impairment in Patients with Breast Cancer Based on Functional Assessment and NIRS Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Setting
2.2. Procedure
2.3. Instruments and Measures
2.3.1. Interview and Clinical History
2.3.2. Neuropsychological Assessment
Subjective Neuropsychological Assessment: Functional Assessment of Cancer Therapy, Cognitive Scale (FACT-Cog), Version 3
Neuropsychological Objective Assessment: VFT and Procedure
Neuropsychological Objective Assessment: NIRS Measurements and Procedure
2.3.3. Statistical Analysis
3. Results
3.1. Comorbidity and Obstetric-Gynecologic Antecedents
3.2. Location of the Tumor, Anatomopathological Characteristics, and Family Antecedents
3.3. Therapeutic Management
3.4. Cognitive Impairment, NIRS Measures, and VFT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ahles, T.A.; Saykin, A.J.; McDonald, B.C.; Li, Y.; Furstenberg, C.T.; Hanscom, B.S.; Mulrooney, T.J.; Schwartz, G.N.; Kaufman, P.A. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. J. Clin. Oncol. 2010, 28, 4434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez Martín, B.; Fernández Rodríguez, E.J.; Rihuete Galve, M.I.; Cruz Hernández, J.J. Study of chemotherapy-induced cognitive impairment in women with breast cancer. Int. J. Environ. Res. Public Health 2020, 17, 8896. [Google Scholar] [CrossRef] [PubMed]
- Wefel, J.S.; Kesler, S.R.; Noll, K.R.; Schagen, S.B. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J. Clin. 2015, 65, 123–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myers, J.S. Chemotherapy-Related Cognitive Impairment: The Breast Cancer Experience. Oncol. Nurs. Forum 2012, 39, E31–E40. [Google Scholar] [CrossRef] [Green Version]
- Lindner, O.C.; Phillips, B.; McCabe, M.G.; Mayes, A.; Wearden, A.; Varese, F.; Talmi, D. A meta-analysis of cognitive impairment following adult cancer chemotherapy. Neuropsychology 2014, 28, 726. [Google Scholar] [CrossRef] [Green Version]
- Horowitz, T.S.; Suls, J.; Treviño, M. A call for a neuroscience approach to cancer-related cognitive impairment. Trends Neurosci. 2018, 41, 493–496. [Google Scholar] [CrossRef]
- Wefel, J.S.; Saleeba, A.K.; Buzdar, A.U.; Meyers, C.A. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer 2010, 116, 3348–3356. [Google Scholar] [CrossRef] [Green Version]
- Miao, H.; Li, J.; Hu, S.; He, X.; Partridge, S.C.; Ren, J.; Bian, Y.; Yu, Y.; Qiu, B. Long-term cognitive impairment of breast cancer patients after chemotherapy: A functional MRI study. Eur. J. Radiol. 2016, 85, 1053–1057. [Google Scholar] [CrossRef]
- Schmidt, J.E.; Beckjord, E.; Bovbjerg, D.H.; Low, C.A.; Posluszny, N.M.; Lowery, A.E.; Dew, M.A.; Nutt, S.; Arvey, S.R.; Rechis, R. Prevalence of perceived cognitive dysfunction in survivors of a wide range of cancers: Results from the 2010 LIVESTRONG survey. J. Cancer Surviv. 2016, 10, 302–311. [Google Scholar] [CrossRef] [Green Version]
- Tong, T.; Lu, H.; Zong, J.; Lv, Q.; Chu, X. Chemotherapy-related cognitive impairment in patients with breast cancer based on MRS and DTI analysis. Breast Cancer 2020, 27, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Ahles, T.A.; Saykin, A.J. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat. Rev. Cancer 2007, 7, 192–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin, M.-F.; Lord, C.; Andrews, J.; Juster, R.-P.; Sindi, S.; Arsenault-Lapierre, G.; Fiocco, A.J.; Lupien, S.J. Chronic stress, cognitive functioning and mental health. Neurobiol. Learn. Mem. 2011, 96, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Jansen, C.E.; Cooper, B.A.; Dodd, M.J.; Miaskowski, C.A. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Supportive Care Cancer 2011, 19, 1647–1656. [Google Scholar] [CrossRef]
- Pullens, M.J.; De Vries, J.; Roukema, J.A. Subjective cognitive dysfunction in breast cancer patients: A systematic review. Psychooncology 2010, 19, 1127–1138. [Google Scholar] [CrossRef]
- Cella, D.; Hernandez, L.; Bonomi, A.E.; Corona, M.; Vaquero, M.; Shiomoto, G.; Baez, L. Spanish language translation and initial validation of the functional assessment of cancer therapy quality-of-life instrument. Med. Care 1998, 36, 1407–1418. [Google Scholar] [CrossRef]
- Sousa, H.; Almeida, S.; Bessa, J.; Pereira, M.G. The developmental trajectory of cancer-related cognitive impairment in breast cancer patients: A systematic review of longitudinal neuroimaging studies. Neuropsychol. Rev. 2020, 30, 287–309. [Google Scholar] [CrossRef]
- Bai, X.; Zheng, J.; Zhang, B.; Luo, Y. Cognitive dysfunction and neurophysiologic mechanism of breast cancer patients undergoing chemotherapy based on resting state functional magnetic resonance imaging. World Neurosurg. 2021, 149, 406–412. [Google Scholar] [CrossRef]
- Janelsins, M.C.; Heckler, C.E.; Peppone, L.J.; Kamen, C.; Mustian, K.M.; Mohile, S.G.; Magnuson, A.; Kleckner, I.R.; Guido, J.J.; Young, K.L.; et al. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: An analysis from a nationwide, multicenter, prospective longitudinal study. J. Clin. Oncol. 2017, 35, 506. [Google Scholar] [CrossRef]
- Wefel, J.S.; Vardy, J.; Ahles, T.; Schagen, S.B. International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011, 12, 703–708. [Google Scholar] [CrossRef]
- Keil, K.; Kaszniak, A.W. Examining executive function in individuals with brain injury: A review. Aphasiology 2002, 16, 305–335. [Google Scholar] [CrossRef]
- Deprez, S.; Kesler, S.R.; Saykin, A.J.; Silverman, D.H.; De Ruiter, M.B.; McDonald, B.C. International cognition and cancer task force recommendations for neuroimaging methods in the study of cognitive impairment in non-CNS cancer patients. JNCI J. Natl. Cancer Inst. 2018, 110, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Deprez, S.; Amant, F.; Yigit, R.; Porke, K.; Verhoeven, J.; Stock, J.V.D.; Smeets, A.; Christiaens, M.-R.; Leemans, A.; Van Hecke, W.; et al. Chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning in breast cancer patients. Hum. Brain Mapp. 2011, 32, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Deprez, S.; Vandenbulcke, M.; Peeters, R.; Emsell, L.; Smeets, A.; Christiaens, M.-R.; Amant, F.; Sunaert, S. Longitudinal assessment of chemotherapy-induced alterations in brain activation during multitasking and its relation with cognitive complaints. J. Clin. Oncol. 2014, 32, 2031–2038. [Google Scholar] [CrossRef] [PubMed]
- Birn, R.M.; Kenworthy, L.; Case, L.; Caravella, R.; Jones, T.B.; Bandettini, P.A.; Martin, A. Neural systems supporting lexical search guided by letter and semantic category cues: A self-paced overt response fMRI study of verbal fluency. Neuroimage 2010, 49, 1099–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.-L.; Wagner, J.; Heugel, N.; Sugar, J.; Lee, Y.-W.; Conant, L.; Malloy, M.; Heffernan, J.; Quirk, B.; Zinos, A.; et al. Functional near-infrared spectroscopy and its clinical application in the field of neuroscience: Advances and future directions. Front. Neurosci. 2020, 14, 724. [Google Scholar] [CrossRef]
- Hong, K.; Yaqub, M.A. Application of functional near-infrared spectroscopy in the healthcare industry: A review. J. Innov. Opt. Health Sci. 2019, 12, 1930012. [Google Scholar] [CrossRef]
- Wagner, L.I.; Sweet, J.; Butt, Z.; Lai, J.; Cella, D. Measuring patient self-reported cognitive function: Development of the functional assessment of cancer therapy-cognitive function instrument. J. Support. Oncol. 2009, 7, W32–W39. [Google Scholar]
- Jenkins, V.; Shilling, V.; Deutsch, G.; Bloomfield, D.; Morris, R.; Allan, S.J.; Bishop, H.M.; Hodson, N.A.; Mitra, S.; Sadler, G.R.; et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early-stage breast cancer. Br. J. Cancer 2006, 94, 828–834. [Google Scholar] [CrossRef]
- Bray, V.J.; Dhillon, H.M.; Bell, M.L.; Kabourakis, M.; Fiero, M.H.; Yip, D.; Boyle, F.; Price, M.A.; Vardy, J.L. Evaluation of a web-based cognitive rehabilitation program in cancer survivors reporting cognitive symptoms after chemotherapy. J. Clin. Oncol. 2017, 35, 217–225. [Google Scholar] [CrossRef] [Green Version]
- FACIT Questionnaires: FACT-Cog Scoring and Interpretation Materials. Available online: https://www.facit.org/measures/FACT-Cog (accessed on 10 March 2021).
- Costa, D.S.; Loh, V.; Birney, D.; Dhillon, H.; Fardell, J.E.; Gessler, D.; Vardy, J. The structure of the FACT-cog v3 in cancer patients, students, and older adults. J. Pain Symptom Manag. 2018, 55, 1173–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyk, K.V.; Crespi, C.M.; Petersen, L.; Ganz, P.A. Identifying cancer-related cognitive impairment using the FACT-cog perceived cognitive impairment. JNCI Cancer Spectr. 2020, 4, kz099. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Heutte, N.; Rigal, O.; Noal, S.; Kurtz, J.-E.; Lévy, C.; Allouache, D.; Rieux, C.; Lefel, J.; Clarisse, B.; et al. Decline in cognitive function in older adults with early-stage breast cancer after adjuvant treatment. Oncologist 2016, 21, 1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Ah, D.; Carpenter, J.S.; Saykin, A.; Monahan, P.; Wu, J.; Yu, M.; Rebok, G.; Ball, K.; Schneider, B.; Weaver, M.; et al. Advanced cognitive training for breast cancer survivors: A randomized controlled trial. Breast Cancer Res. Treat. 2012, 135, 799–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whittaker, A.L.; George, R.P.; O’Malley, L. Prevalence of cognitive impairment following chemotherapy treatment for breast cancer: A systematic review and meta-analysis. Sci. Rep. 2022, 12, 1–22. [Google Scholar] [CrossRef]
- Dinapoli, L.; Colloca, G.; Di Capua, B.; Valentini, V. Psychological aspects to consider in breast cancer diagnosis and treatment. Curr. Oncol. Rep. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Li, H.; Sereika, S.M.; Marsland, A.L.; Conley, Y.P.; Bender, C.M. Symptom clusters in women with breast cancer during the first 18 months of adjuvant therapy. J. Pain Symptom Manag. 2020, 59, 233–241. [Google Scholar] [CrossRef]
- Kesler, S.R.; Kent, J.S.; O’Hara, R. Prefrontal cortex and executive function impairments in primary breast cancer. Arch. Neurol. 2011, 68, 1447–1453. [Google Scholar] [CrossRef] [Green Version]
- Quesnel, C.; Savard, J.; Ivers, H. Cognitive impairments associated with breast cancer treatments: Results from a longitudinal study. Breast Cancer Res. Treat. 2009, 116, 113–123. [Google Scholar] [CrossRef]
- Hermelink, K.; Untch, M.; Lux, M.P.; Kreienberg, R.; Beck, T.; Bauerfeind, I.; Münzel, K. Cognitive function during neoadjuvant chemotherapy for breast cancer: Results of a prospective, multicenter, longitudinal study. Cancer 2007, 109, 1905–1913. [Google Scholar] [CrossRef]
- Freeman, J.R.; Broshek, D.K. Assessing cognitive dysfunction in breast cancer: What are the tools? Clin. Breast Cancer 2002, 3, S91–S99. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yang, Z.; Dong, B.; Chen, C.; Zhang, M.; Huang, Z.; Chen, Z.; Wang, K. Chemotherapy-induced prospective memory impairment in patients with breast cancer. Psychooncology 2013, 22, 2391–2395. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen, W.; Van de Cavey, J.; Vingerhoets, G.; Vanheule, S.; Van den Broecke, R.; Denys, H. Heterogeneous response of chemotherapy-related cognitive decline in patients with breast cancer: A prospective study. J. Int. Neuropsychol. Soc. 2020, 26, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Andryszak, P.; Wiłkość, M.; Żurawski, B.; Izdebski, P. Verbal fluency in breast cancer patients treated with chemotherapy. Breast Cancer 2017, 24, 376–383. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Li, J.; Ren, J.; Hu, X.; Zhu, C.; Tian, Y.; Hu, P.; Ma, H.; Yu, F.; Wang, K. Selective impairment of attention networks in breast cancer patients receiving chemotherapy treatment. Psychooncology 2014, 23, 1165–1171. [Google Scholar] [CrossRef]
- Yao, C.; Bernstein, L.J.; Rich, J.B. Executive functioning impairment in women treated with chemotherapy for breast cancer: A systematic review. Breast Cancer Res. Treat. 2017, 166, 15–28. [Google Scholar] [CrossRef]
- Bernstein, L.J.; McCreath, G.A.; Komeylian, Z.; Rich, J.B. Cognitive impairment in breast cancer survivors treated with chemotherapy depends on control group type and cognitive domains assessed: A multilevel meta-analysis. Neurosci. Biobehav. Rev. 2017, 83, 417–428. [Google Scholar] [CrossRef]
- Ono, M.; Ogilvie, J.; Wilson, J.S.; Green, H.J.; Chambers, S.K.; Ownsworth, T.; Shum, D.H.K. A meta-analysis of cognitive impairment and decline associated with adjuvant chemotherapy in women with breast cancer. Front. Oncol. 2015, 5, 59. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Bray, S.; Bryant, D.M.; Glover, G.H.; Reiss, A.L. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage 2011, 54, 2808–2821. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, M.; Yoshikawa, E.; Matsuoka, Y.; Sugawara, Y.; Nakano, T.; Akechi, T.; Wada, N.; Imoto, S.; Murakami, K.; Uchitomi, Y.; et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer 2007, 109, 146–156. [Google Scholar] [CrossRef]
- Kesler, S.R.; Bennett, F.C.; Mahaffey, M.L.; Spiegel, D. Regional brain activation during verbal declarative memory in metastatic breast cancer. Clin. Cancer Res. 2009, 15, 6665–6673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, B.C.; Conroy, S.K.; Ahles, T.A.; West, J.D.; Saykin, A.J. Alterations in brain activation during working memory processing associated with breast cancer and treatment: A prospective functional magnetic resonance imaging study. J. Clin. Oncol. 2012, 30, 2500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nudelman, K.N.H.; Wang, Y.; McDonald, B.C.; Conroy, S.; Smith, D.J.; West, J.D.; O’Neill, D.P.; Schneider, B.P.; Saykin, A.J. Altered cerebral blood flow one month after systemic chemotherapy for breast cancer: A prospective study using pulsed arterial spin labeling MRI perfusion. PLoS ONE 2014, 9, 96713. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Lin, H.; Yan, Y.; Xu, X.; Wang, L.; Zhang, J.; Yu, Y. Impairment of the executive function in breast cancer patients receiving chemotherapy treatment: A functional MRI study. Eur. J. Cancer Care 2017, 26, 12553. [Google Scholar] [CrossRef]
- Wefel, J.S.; Lenzi, R.; Theriault, R.L.; Davis, R.N.; Meyers, C.A. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: Results of a prospective, randomized, longitudinal trial. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2004, 100, 2292–2299. [Google Scholar] [CrossRef]
- Eide, S.; Feng, Z. Doxorubicin chemotherapy-induced “chemo-brain”: Meta-analysis. Eur. J. Pharm. 2020, 881, 173078. [Google Scholar] [CrossRef]
- Kitamura, Y.; Hattori, S.; Yoneda, S.; Watanabe, S.; Kanemoto, E.; Sugimoto, M.; Kawai, T.; Machida, A.; Kanzaki, H.; Miyazaki, I.; et al. Doxorubicin and cyclophosphamide treatment produces anxiety-like behavior and spatial cognition impairment in rats: Possible involvement of hippocampal neurogenesis via brain-derived neurotrophic factor and cyclin D1 regulation. Behav. Brain Res. 2015, 292, 184–193. [Google Scholar] [CrossRef]
- Salas-Ramirez, K.Y.; Bagnall, C.; Frias, L.; Abdali, S.A.; Ahles, T.A.; Hubbard, K. Doxorubicin and cyclophosphamide induce cognitive dysfunction and activate the ERK and AKT signaling pathways. Behav. Brain Res. 2015, 292, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Fardell, J.E.; Zhang, J.; De Souza, R.; Vardy, J.; Johnston, I.; Allen, C.; Henderson, J.; Piquette-Miller, M. The impact of sustained and intermittent docetaxel chemotherapy regimens on cognition and neural morphology in healthy mice. Psychopharmacology 2014, 231, 841–852. [Google Scholar] [CrossRef]
- Seigers, R.; Loos, M.; Van Tellingen, O.; Boogerd, W.; Smit, A.B.; Schagen, S.B. Cognitive impact of cytotoxic agents in mice. Psychopharmacology 2015, 232, 17–37. [Google Scholar] [CrossRef]


| N = 90 | N = 90 | ||
|---|---|---|---|
| Variable | Categories | CT+ N (%) | CT− N (%) |
| Marital status | Married | 64 (71.7) | 66 (73.3) |
| Single | 9 (10) | 12 (13.3) | |
| Divorced | 6 (6.7) | 8 (9) | |
| Widowed | 11 (12.2) | 4 (4.4) | |
| Education level | No studies | 9(10) | 5 (5.5) |
| Elementary school | 30 (33.3) | 25 (27.8) | |
| Middle school | 13 (14.4) | 11 (12.2) | |
| High school | 15 (16.7) | 24 (26.7) | |
| Higher education | 23 (25.6) | 25 (27.8) | |
| Employment situation | Currently in employment | 8 (8.9) | 12 (13.3) |
| Temporary sick leave | 43 (47.8) | 31 (34.4) | |
| Permanent sick leave | 9 (10) | 5 (5.6) | |
| Unemployed | 20 (22.2) | 25 (27.8) | |
| Retired | 10 (11.1) | 17 (18.9) | |
| Tumor staging | 0 | 4 (4.4) | 5 (5.6) |
| I | 18 (20) | 39 (43.3) | |
| II | 31 (34.5) | 30 (33.3) | |
| III | 24 (26.7) | 8 (8.9) | |
| IV | 13 (14.4) | 8 (8.9) | |
| Grade | 1 | 18 (20) | 25 (27.8) |
| 2 | 23 (25.6) | 40 (44.4) | |
| 3 | 49 (54.4) | 49 (27.8) | |
| Molecular subtype | Luminal A/Luminal B HER2 negative-like | 47 (52.2) | 60 (66.7) |
| Luminal B HER2 positive-like/HER2-type | 33 (36.7) | 30 (33.3) | |
| Triple negative | 10 (11.1) | 0 (0)3 | |
| Menopause | Natural | 42 (46.7) | 52 (57.8) |
| Drug-induced menopause | 25 (27.8) | 6 (6.7) | |
| Intervention-induced menopause | 3 (3.3) | 9 (10) | |
| Reproductive stage | 20 (22.2) | 23 (25.5) | |
| Surgical treatment | Conservative surgery | 41 (45.6) | 76 (84.5) |
| Uni- or bilateral mastectomy | 23 (25.5) | 11 (12.2) | |
| Without surgical treatment | 26 (28.9) | 3 (3.3) | |
| Chemotherapy cycles | Chemotherapy cycles < 4 | 53 (58.9) | |
| Chemotherapy cycles ≥ 4 | 37 (41.1) |
| L ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS | ||
|---|---|---|
| ATC Classifications | n | (%) |
| L01 ANTINEOPLASTIC AGENTS | ||
| L01A ALKYLATING AGENTS | ||
| L01AA Nitrogen mustard analogues | ||
| 01 Cyclophosphamide | 53 | 58.9 |
| L01B ANTIMETABOLITES | ||
| L01BA Folic acid analogues | ||
| 01 Methotrexate | 1 | 1.1 |
| L01BC Pyrimidine analogues | ||
| 02 Fluorouracil | 1 | 1.1 |
| 06 Capecitabine | 2 | 2.2 |
| L01C PLANT ALKALOIDS AND OTHER NATURAL PRODUCTS | ||
| L01CD Taxanes | ||
| 01 Paclitaxel | 6 | 6.7 |
| 02 Docetaxel | 54 | 60 |
| L01D CYTOTOXIC ANTIBIOTICS AND RELATED SUBSTANCES | ||
| L01DB Anthracyclines and related substances | ||
| 01 Doxorubicin | 36 | 40 |
| 03 Epirubicin | 3 | 3.3 |
| L01X OTHER ANTINEOPLASTIC AGENTS | ||
| L01XA Platinum compounds | ||
| 02 Carboplatin | 9 | 10 |
| L01XC Monoclonal antibodies | ||
| 03 Trastuzumab | 14 | 15.6 |
| 13 Pertuzumab | 6 | 6.7 |
| 14 Trastuzumab emtamsine | 3 | 3.3 |
| L01XX Other antineoplastic agents | ||
| 41 Eribulin | 2 | 2.2 |
| Group | Drug | n | % |
|---|---|---|---|
| 1 | Docetaxel | 10 | 11.1 |
| 2 | Cyclophosphamide + Docetaxel | 14 | 15.5 |
| 3 | Cyclophosphamide + Doxorubicin | 15 | 16.7 |
| 4 | Doxorubicin + Cyclophosphamide+Docetaxel | 16 | 17.8 |
| 5 | Docetaxel + other antineoplastic agents | 15 | 16.7 |
| 6 | Other combinations of antineoplastic agents | 20 | 22.2 |
| Variables of Subjective Neuropsychological Assessment | |||||||
|---|---|---|---|---|---|---|---|
| CT+ | CT− | CT Cycles | Pharmacological Group 4 | ||||
| <4 | ≥4 | Yes | No | ||||
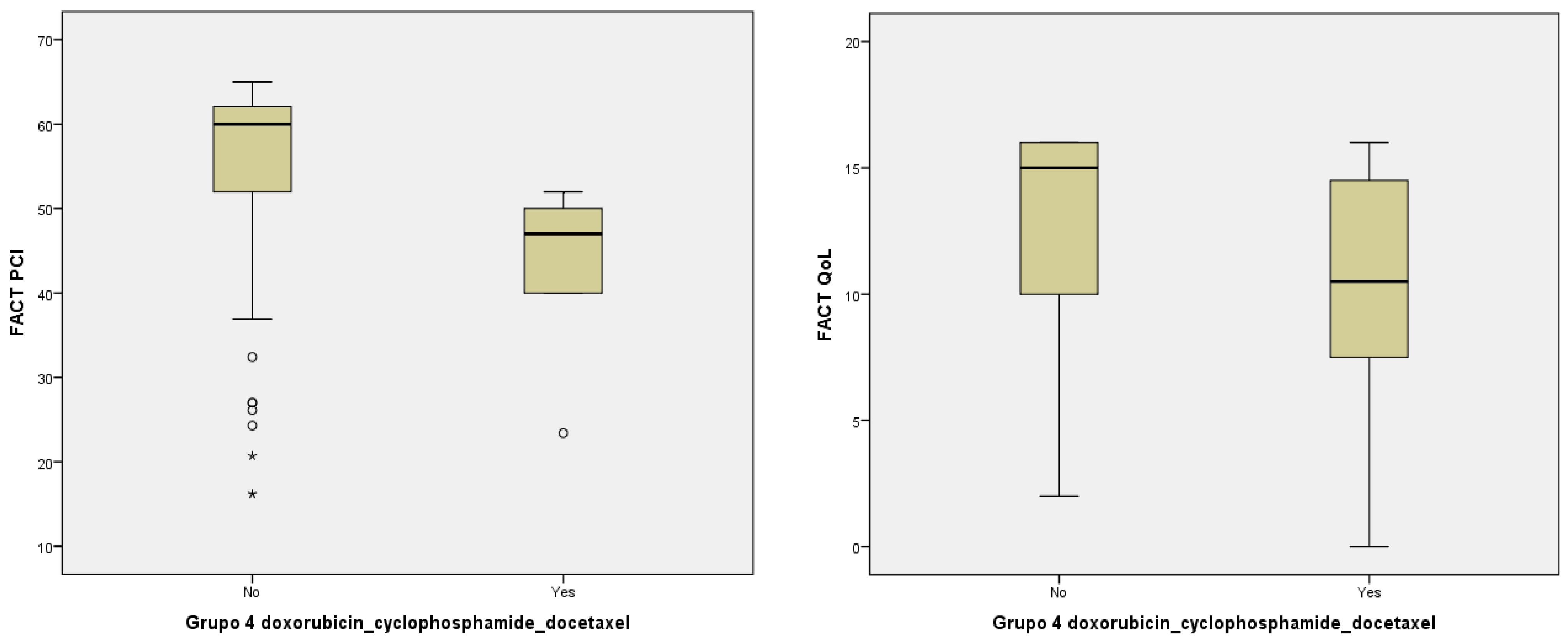
| PCI | Mean | 50.60 ± 15.64 | 55.01 ± 12.10 | 52.92 ± 13.34 | 46.77 ± 14.84 | 44.96 ± 7.19 | 54.79 ± 12.11 |
| Median (IQR) | 55 (22) | 58 (20) | 58.95 (25) | 47 (23) | 47(10) | 60 (11) | |
| p-value | p = 0.005 a | p = 0.002 a | p = 0.013 a | ||||
| Oth | Mean | 14.18 ± 2.56 | 15.66 ± 1.44 | 15.13 ± 1.77 | 13.22 ± 2.88 | 14.81 ± 2.78 | 15.34 ± 1.45 |
| Median (IQR) | 15 (3) | 16 (0) | 16 (1) | 13 (4) | 16 (1) | 16 (0) | |
| p-value | p = 0.026 a | p < 0.001 a | p = 0.071 | ||||
| PCA | Mean | 13.30 ± 4.51 | 15.82 ± 4.22 | 15.01 ± 4.71 | 11.90 ± 3.81 | 13.67 ± 15.17 | 15.17 ± 4.37 |
| Median (IQR) | 13.22 (5) | 15 (7) | 14 (6) | 12 (6) | 12.80 (8) | 16.33 (5) | |
| p-value | p = 0.039 a | p = 0.001 a | p = 0.102 | ||||
| QoL | Mean | 11.73 ± 5.11 | 13.28 ± 4.76 | 10.93 ± 5.06 | 8.82 ± 3.56 | 10.19 ± 5.35 | 12.84 ± 4.12 |
| Median (IQR) | 11 (6) | 16 (4) | 12.50 (10) | 10 (3) | 10.50 (9) | 15 (6) | |
| p-value | p = 0.034 a | p = 0.023 a | p = 0.019 a | ||||
| PCI (<54) | % | 44.4% | 28.9% | 35% | 52% | 49.8% | 56.3% |
| χ2 | χ2 = 19.29 | χ2 = 7.018 | χ2 = 1.44 | ||||
| p-value | p < 0.001 b | p = 0.030 b | p = 0.129 | ||||
| Variables of Objective Neuropsychological Assessment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CT+ | CT− | Number of CT Cycles | CT Cycles | PVF | SVF | rSO2 | |||
| <4 | ≥4 | ||||||||
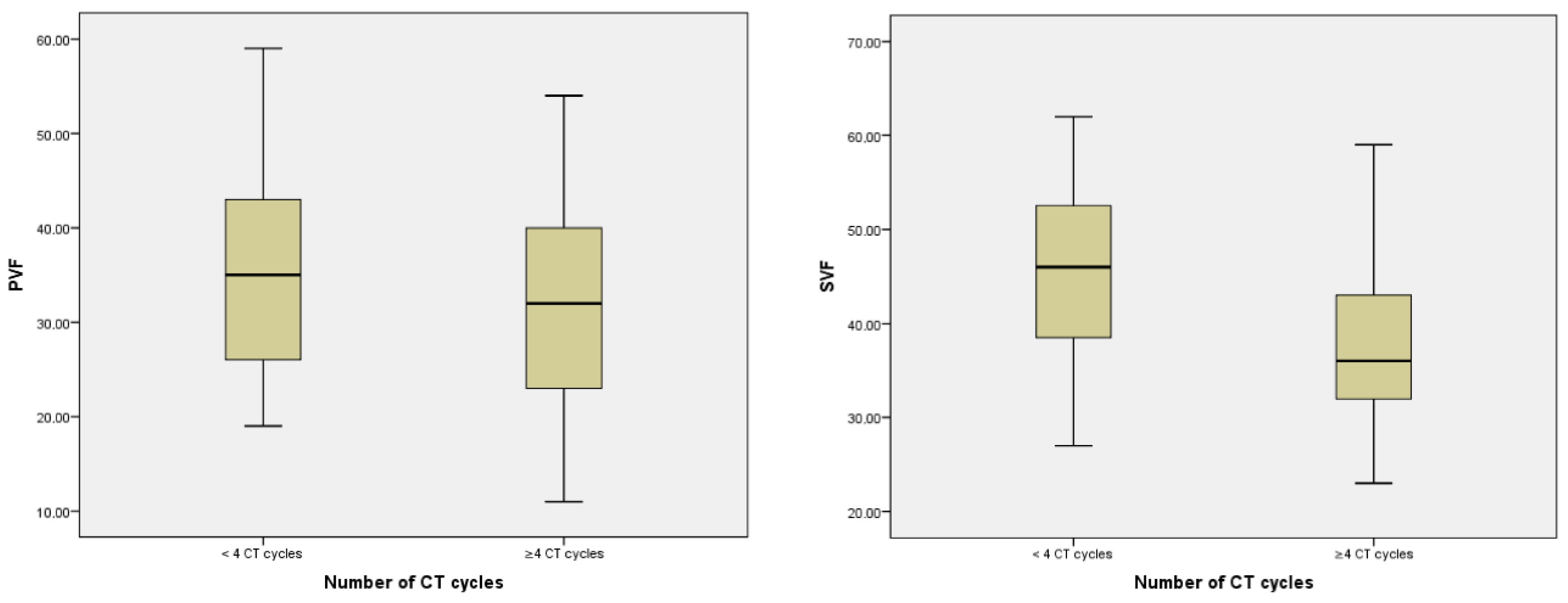
| PVF | Mean | 41.26 ± 9.55 | 47.03 ± 9.31 | - | 45.00 ± 9.32 | 36.91 ± 8.20 | na | - | - |
| Median (IQR) | 40 (14) | 47.50 (14.75) | - | 46 (14.5) | 36 (11) | na | - | - | |
| r and/or p-value | p < 0.001 a | r = −0.332 p < 0.001 b | p < 0.001 a | na | p = 0.592 | r = −0.535 p < 0.001 b | |||
| SVF | Mean | 33.60 ± 10.44 | 36.14 ± 10.68 | 35.31 ± 10.68 | 31.47 ± 10.57 | - | na | ||
| Median (IQR) | 34 (17) | 37.50 (17) | 35 (17) | 32 (17) | - | na | |||
| r and/or p-value | p < 0.001 a | r = −0.154 p = 0.040 b | p = 0.004 a | p = 0.592 | na | r = 0.485 p < 0.001 b | |||
| rSO2 | Mean | 63.30 ± 8.02 | 67.98 ± 7.80 | - | 66.77 ± 7.47 | 62.88 ± 7.60 | - | - | na |
| Median (IQR) | 62.58 (12.25) | 68.66 (10.96) | - | 68.41 (11.5) | 62.66 (8.79) | - | - | na | |
| r and/or p-value | p < 0.001 a | r = −0.225 p = 0.002 b | p < 0.001 a | r = 0.535 p < 0.001 b | r = 0.485 p < 0.001 b | na | |||
| Measures (N = 66) | Left Side | Right Side | Both Sides | |||
|---|---|---|---|---|---|---|
| M ± SD | Correlation a | M ± SD | Correlation a | M ± SD | Correlation b | |
| rSO2-CT−1 | 64.81 ± 7.01 | - | 66.70 ± 8.01 | - | 65.75 ± 7.86 | r = 0.841, p < 0.001 |
| rSO2-CT−2 | 67.84 ± 7.22 | r = 0.958, p < 0.001 | 69.89 ± 8.15 | r = 0.805, p < 0.001 | 68.86 ± 8.52 | r = 0.825, p < 0.001 |
| rSO2-CT−3 | 67.13 ± 7.46 | r = 0.907, p < 0.001 | 69.98 ± 9.11 | r = 0.781, p < 0.001 | 68.55 ± 7.41 | r = 0.795, p < 0.001 |
| rSO2-CT−4 | 68.73 ± 7.62 | r = 0.906, p < 0.001 | 68.85 ± 8.90 | r = 0.730, p < 0.001 | 68.79 ± 7.02 | r = 0.819, p < 0.001 |
| rSO2-CT+1 | 60.69 ± 8.52 | r = 0.616, p < 0.001 | 62.23 ± 9.01 | r = 0.723, p < 0.001 | 61.46 ± 7.35 | r = 0.743, p < 0.001 |
| rSO2-CT+2 | 64.24 ± 7.29 | r = 0.909, p < 0.001 | 65.19 ± 9.23 | r = 0.829, p < 0.001 | 64.71 ± 8.36 | r = 0.505, p < 0.001 |
| rSO2-CT+3 | 63.14 ± 8.01 | r = 0.909, p < 0.001 | 64.61 ± 9.01 | r = 0.825, p < 0.001 | 63.87 ± 8.12 | r = 0.521, p < 0.001 |
| rSO2-CT+4 | 62.80 ± 8.23 | r = 0.820, p < 0.001 | 63.53 ± 9.56 | r = 0.912, p < 0.001 | 63.16 ± 8.62 | r = 0.518, p < 0.001 |
| M rSO2-CT− | 67.12 ± 7.10 | - | 68.85 ± 8.32 | - | 67.98 ± 7.80 | r = 0.821, p < 0.001 |
| M rSO2-CT+ | 62.71 ± 8.01 | r = 0.720, p < 0.001 | 63.89 ± 9.2 | r = 0.628, p < 0.001 | 63.30 ± 8.02 | r = 0.530, p < 0.001 |
| Relation between Subjective and Objective Neuropsychological Assessment Variables | |||
|---|---|---|---|
| PVF | SVF | rSO2 | |
| PCI | r = 0.516 p < 0.001 a | r = 0.630 p < 0.001 a | r = 0.650 p < 0.001 a |
| Oth | r = 0.170 p = 0.023 a | r = 0.159 p = 0.033 a | r = 0702 p < 0.001 a |
| PCA | r = 0.675 p < 0.001 a | r = 0.648 p < 0.001 a | r = 0.395 p < 0.001 a |
| QoL | r = 0.452 p < 0.001 a | r = 0.153 p = 0.041 a | r = 0.405 p < 0.001 a |
| PCI (<54) | p < 0.001 b | p < 0.001 b | p < 0.001 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durán-Gómez, N.; López-Jurado, C.F.; Nadal-Delgado, M.; Pérez-Civantos, D.; Guerrero-Martín, J.; Cáceres, M.C. Chemotherapy-Related Cognitive Impairment in Patients with Breast Cancer Based on Functional Assessment and NIRS Analysis. J. Clin. Med. 2022, 11, 2363. https://doi.org/10.3390/jcm11092363
Durán-Gómez N, López-Jurado CF, Nadal-Delgado M, Pérez-Civantos D, Guerrero-Martín J, Cáceres MC. Chemotherapy-Related Cognitive Impairment in Patients with Breast Cancer Based on Functional Assessment and NIRS Analysis. Journal of Clinical Medicine. 2022; 11(9):2363. https://doi.org/10.3390/jcm11092363
Chicago/Turabian StyleDurán-Gómez, Noelia, Casimiro Fermín López-Jurado, Marta Nadal-Delgado, Demetrio Pérez-Civantos, Jorge Guerrero-Martín, and Macarena C. Cáceres. 2022. "Chemotherapy-Related Cognitive Impairment in Patients with Breast Cancer Based on Functional Assessment and NIRS Analysis" Journal of Clinical Medicine 11, no. 9: 2363. https://doi.org/10.3390/jcm11092363
APA StyleDurán-Gómez, N., López-Jurado, C. F., Nadal-Delgado, M., Pérez-Civantos, D., Guerrero-Martín, J., & Cáceres, M. C. (2022). Chemotherapy-Related Cognitive Impairment in Patients with Breast Cancer Based on Functional Assessment and NIRS Analysis. Journal of Clinical Medicine, 11(9), 2363. https://doi.org/10.3390/jcm11092363







