Combined Stimulation of the Substantia Nigra and the Subthalamic Nucleus for the Treatment of Refractory Gait Disturbances in Parkinson’s Disease: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
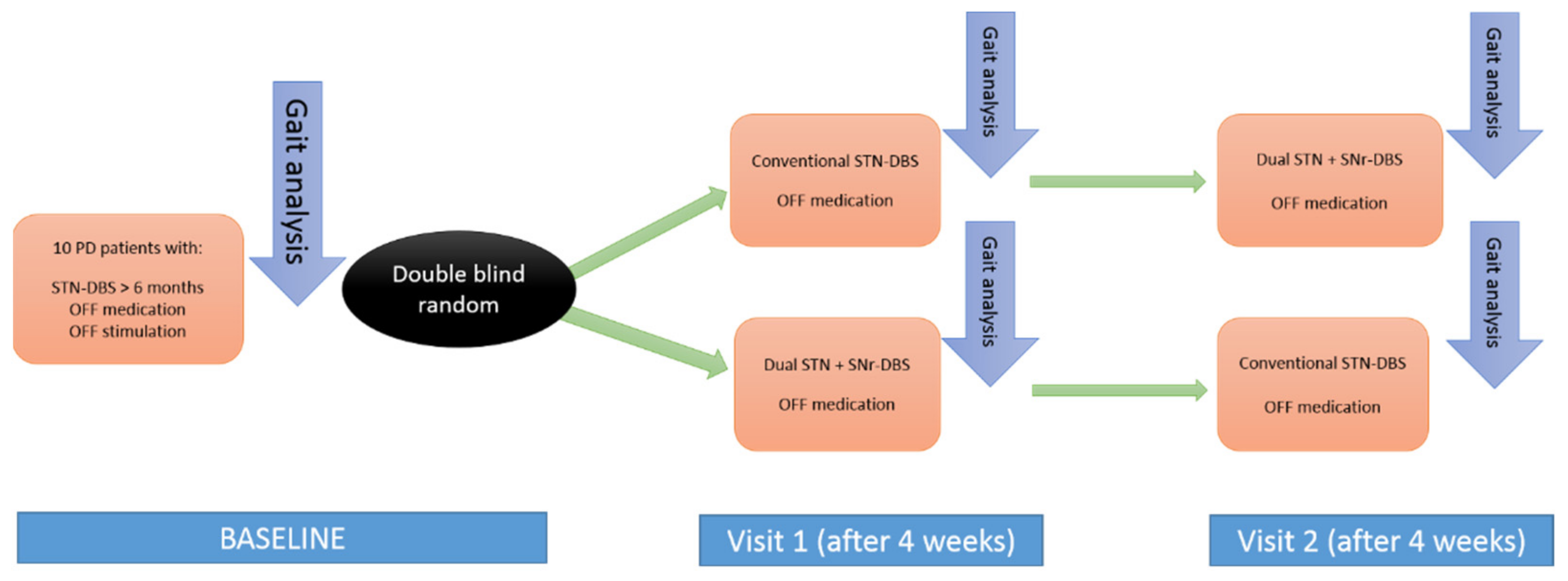
2.1. Design
2.2. Subjects
2.3. Study Protocol
- Baseline: At least 6 months after surgery, the period during which the patients were receiving STN-DBS, quantitative gait analysis was performed. All patients were in off-stimulation (DBS stopped at least 48h before the analysis) and off-medication state (having stopped taking the medication for a minimum of 12 h before). After baseline evaluation, patients were randomized to be programmed in the corresponding stimulation mode (STN-DBS versus STN + SNr-DBS);
- Visit 1: At the end of the first period, after four weeks, quantitative gait analysis in the off-medication state was repeated. Subsequently, the stimulation type was changed (STN + SNr-DBS vs. STN-DBS) for each group (cross-over);
- Visit 2: After the second period, lasting 4 weeks, quantitative gait analysis in the off-medication state was repeated.
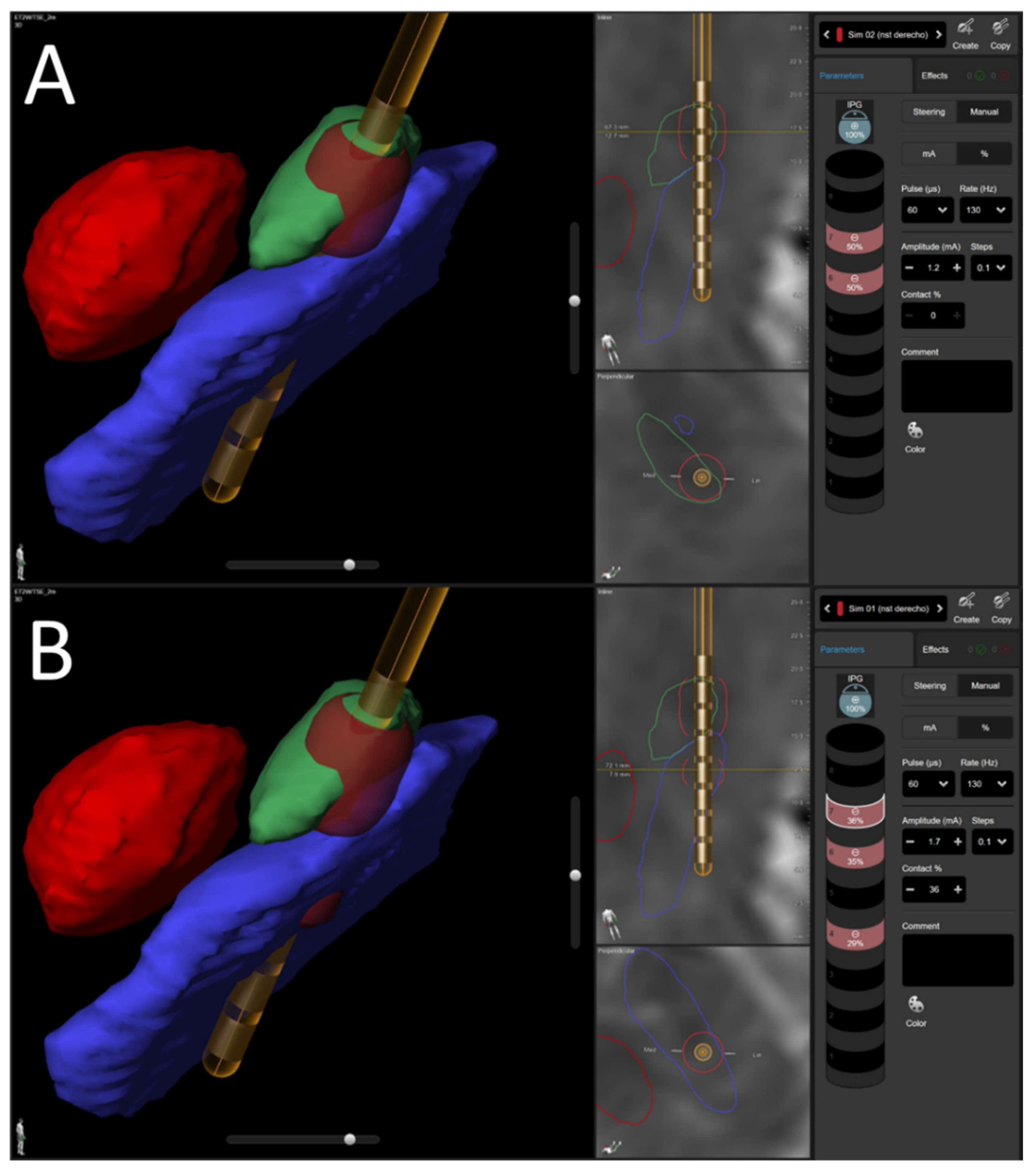
2.4. Locating the Electrodes
2.5. Stimulation Parameters
2.6. Gait Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Limousin, P.; Pollak, P.; Benazzouz, A.; Hoffmann, D.; Le Bas, J.F.; Broussolle, E.; Perret, J.E.; Benabid, A.L. Effect of Parkinsonian Signs and Symptoms of Bilateral Subthalamic Nucleus Stimulation. Lancet 1995, 345, 91–95. [Google Scholar] [CrossRef]
- Weaver, F.M.; Follett, K.; Stern, M.; Hur, K.; Harris, C.; Marks, W.J.; Rothlind, J.; Sagher, O.; Reda, D.; Moy, C.S.; et al. Bilateral Deep Brain Stimulation vs. Best Medical Therapy for Patients with Advanced Parkinson Disease: A Randomized Controlled Trial. JAMA 2009, 301, 63–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Oroz, M.C.; Obeso, J.A.; Lang, A.E.; Houeto, J.-L.; Pollak, P.; Rehncrona, S.; Kulisevsky, J.; Albanese, A.; Volkmann, J.; Hariz, M.I.; et al. Bilateral Deep Brain Stimulation in Parkinson’s Disease: A Multicentre Study with 4 Years Follow-Up. Brain J. Neurol. 2005, 128, 2240–2249. [Google Scholar] [CrossRef]
- Collomb-Clerc, A.; Welter, M.-L. Effects of Deep Brain Stimulation on Balance and Gait in Patients with Parkinson’s Disease: A Systematic Neurophysiological Review. Neurophysiol. Clin. Clin. Neurophysiol. 2015, 45, 371–388. [Google Scholar] [CrossRef] [Green Version]
- Schlenstedt, C.; Shalash, A.; Muthuraman, M.; Falk, D.; Witt, K.; Deuschl, G. Effect of High-Frequency Subthalamic Neurostimulation on Gait and Freezing of Gait in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Eur. J. Neurol. 2017, 24, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Giladi, N.; Treves, T.A.; Simon, E.S.; Shabtai, H.; Orlov, Y.; Kandinov, B.; Paleacu, D.; Korczyn, A.D. Freezing of Gait in Patients with Advanced Parkinson’s Disease. J. Neural Transm. 2001, 108, 53–61. [Google Scholar] [CrossRef]
- Bloem, B.R.; Hausdorff, J.M.; Visser, J.E.; Giladi, N. Falls and Freezing of Gait in Parkinson’s Disease: A Review of Two Interconnected, Episodic Phenomena. Mov. Disord. Off. J. Mov. Disord. Soc. 2004, 19, 871–884. [Google Scholar] [CrossRef]
- Kleiner-Fisman, G.; Herzog, J.; Fisman, D.N.; Tamma, F.; Lyons, K.E.; Pahwa, R.; Lang, A.E.; Deuschl, G. Subthalamic Nucleus Deep Brain Stimulation: Summary and Meta-Analysis of Outcomes. Mov. Disord. Off. J. Mov. Disord. Soc. 2006, 21 (Suppl. S1), S290–S304. [Google Scholar] [CrossRef]
- McNeely, M.E.; Earhart, G.M. Medication and Subthalamic Nucleus Deep Brain Stimulation Similarly Improve Balance and Complex Gait in Parkinson Disease. Parkinsonism Relat. Disord. 2013, 19, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Oroz, M.C.; Moro, E.; Krack, P. Long-Term Outcomes of Surgical Therapies for Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2012, 27, 1718–1728. [Google Scholar] [CrossRef] [Green Version]
- Deuschl, G.; Agid, Y. Subthalamic Neurostimulation for Parkinson’s Disease with Early Fluctuations: Balancing the Risks and Benefits. Lancet Neurol. 2013, 12, 1025–1034. [Google Scholar] [CrossRef]
- Vallabhajosula, S.; Haq, I.U.; Hwynn, N.; Oyama, G.; Okun, M.; Tillman, M.D.; Hass, C.J. Low-Frequency versus High-Frequency Subthalamic Nucleus Deep Brain Stimulation on Postural Control and Gait in Parkinson’s Disease: A Quantitative Study. Brain Stimulat. 2015, 8, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Seger, A.; Gulberti, A.; Vettorazzi, E.; Braa, H.; Buhmann, C.; Gerloff, C.; Hamel, W.; Moll, C.K.E.; Pötter-Nerger, M. Short Pulse and Conventional Deep Brain Stimulation Equally Improve the Parkinsonian Gait Disorder. J. Park. Dis. 2021, 11, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Ren, Z.; Guo, S.; Li, J.; Li, Y. Effects of Pedunculopontine Nucleus Deep Brain Stimulation on Gait Disorders in Parkinson’s Disease: A Meta-Analysis of the Literature. Clin. Neurol. Neurosurg. 2020, 198, 106108. [Google Scholar] [CrossRef] [PubMed]
- Pötter-Nerger, M.; Volkmann, J. Deep Brain Stimulation for Gait and Postural Symptoms in Parkinson’s Disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2013, 28, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.; Walach, M.; Meisner, C.; Fritz, M.; Scholten, M.; Breit, S.; Plewnia, C.; Bender, B.; Gharabaghi, A.; Wächter, T.; et al. Nigral Stimulation for Resistant Axial Motor Impairment in Parkinson’s Disease? A Randomized Controlled Trial. Brain J. Neurol. 2013, 136, 2098–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, T.Z.; Davies, L.; Stein, J.; France, S. The Role of Descending Basal Ganglia Connections to the Brain Stem in Parkinsonian Akinesia. Br. J. Neurosurg. 1998, 12, 245–249. [Google Scholar] [CrossRef]
- Valldeoriola, F.; Muñoz, E.; Rumià, J.; Roldán, P.; Cámara, A.; Compta, Y.; Martí, M.J.; Tolosa, E. Simultaneous Low-Frequency Deep Brain Stimulation of the Substantia Nigra Pars Reticulata and High-Frequency Stimulation of the Subthalamic Nucleus to Treat Levodopa Unresponsive Freezing of Gait in Parkinson’s Disease: A Pilot Study. Parkinsonism Relat. Disord. 2019, 60, 153–157. [Google Scholar] [CrossRef] [Green Version]
- Wojtecki, L.; Vesper, J.; Schnitzler, A. Interleaving Programming of Subthalamic Deep Brain Stimulation to Reduce Side Effects with Good Motor Outcome in a Patient with Parkinson’s Disease. Parkinsonism Relat. Disord. 2011, 17, 293–294. [Google Scholar] [CrossRef]
- Benedetti, M.G.; Agostini, V.; Knaflitz, M.; Gasparroni, V.; Boschi, M.; Piperno, R. Self-Reported Gait Unsteadiness in Mildly Impaired Neurological Patients: An Objective Assessment through Statistical Gait Analysis. J. Neuroeng. Rehabil. 2012, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Agostini, V.; Balestra, G.; Knaflitz, M. Segmentation and Classification of Gait Cycles. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 946–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agostini, V.; Nascimbeni, A.; Gaffuri, A.; Imazio, P.; Benedetti, M.G.; Knaflitz, M. Normative EMG Activation Patterns of School-Age Children During Gait. Gait Posture 2010, 32, 285–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villadoniga, M.; San Millan, A.; Cabanes-Martinez, L.; Aviles-Olmos, I.; Del Alamo-De Pedro, M.; Regidor, I. Quantitative gait analysis in patients with advanced Parkinson’s disease. Rev. Neurol. 2016, 63, 97–102. [Google Scholar] [PubMed]
- Horn, M.A.; Gulberti, A.; Hidding, U.; Gerloff, C.; Hamel, W.; Moll, C.K.E.; Pötter-Nerger, M. Comparison of Shod and Unshod Gait in Patients with Parkinson’s Disease with Subthalamic and Nigral Stimulation. Front. Hum. Neurosci. 2022, 15, 751242. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.; Breit, S.; Wächter, T.; Plewnia, C.; Gharabaghi, A.; Krüger, R. Combined Stimulation of the Substantia Nigra Pars Reticulata and the Subthalamic Nucleus Is Effective in Hypokinetic Gait Disturbance in Parkinson’s Disease. J. Neurol. 2011, 258, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Scholten, M.; Klemt, J.; Heilbronn, M.; Plewnia, C.; Bloem, B.R.; Bunjes, F.; Krüger, R.; Gharabaghi, A.; Weiss, D. Effects of Subthalamic and Nigral Stimulation on Gait Kinematics in Parkinson’s Disease. Front. Neurol. 2017, 8, 543. [Google Scholar] [CrossRef]
- Hidding, U.; Gulberti, A.; Pflug, C.; Choe, C.; Horn, A.; Prilop, L.; Braaß, H.; Fründt, O.; Buhmann, C.; Weiss, D.; et al. Modulation of Specific Components of Sleep Disturbances by Simultaneous Subthalamic and Nigral Stimulation in Parkinson’s Disease. Parkinsonism Relat. Disord. 2019, 62, 141–147. [Google Scholar] [CrossRef]
- Pflug, C.; Nienstedt, J.C.; Gulberti, A.; Müller, F.; Vettorazzi, E.; Koseki, J.-C.; Niessen, A.; Flügel, T.; Hidding, U.; Buhmann, C.; et al. Impact of Simultaneous Subthalamic and Nigral Stimulation on Dysphagia in Parkinson’s Disease. Ann. Clin. Transl. Neurol. 2020, 7, 628–638. [Google Scholar] [CrossRef] [Green Version]
- Agostini, V.; Nascimbeni, A.; Gaffuri, A.; Knaflitz, M. Multiple Gait Patterns within the Same Winters Class in Children with Hemiplegic Cerebral Palsy. Clin. Biomech. 2015, 30, 908–914. [Google Scholar] [CrossRef] [Green Version]
- Moreau, C.; Defebvre, L.; Destée, A.; Bleuse, S.; Clement, F.; Blatt, J.L.; Krystkowiak, P.; Devos, D. STN-DBS Frequency Effects on Freezing of Gait in Advanced Parkinson Disease. Neurology 2008, 71, 80–84. [Google Scholar] [CrossRef]
- Xie, T.; Padmanaban, M.; Bloom, L.; MacCracken, E.; Bertacchi, B.; Dachman, A.; Warnke, P. Effect of Low versus High Frequency Stimulation on Freezing of Gait and Other Axial Symptoms in Parkinson Patients with Bilateral STN DBS: A Mini-Review. Transl. Neurodegener. 2017, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Chastan, N.; Westby, G.W.M.; Yelnik, J.; Bardinet, E.; Do, M.C.; Agid, Y.; Welter, M.L. Effects of Nigral Stimulation on Locomotion and Postural Stability in Patients with Parkinson’s Disease. Brain J. Neurol. 2009, 132, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Georgiades, M.J.; Shine, J.M.; Gilat, M.; McMaster, J.; Owler, B.; Mahant, N.; Lewis, S.J.G. Hitting the Brakes: Pathological Subthalamic Nucleus Activity in Parkinson’s Disease Gait Freezing. Brain 2019, 142, 3906–3916. [Google Scholar] [CrossRef] [PubMed]
- Nandi, D.; Aziz, T.Z.; Liu, X.; Stein, J.F. Brainstem Motor Loops in the Control of Movement. Mov. Disord. 2002, 17, S22–S27. [Google Scholar] [CrossRef] [PubMed]

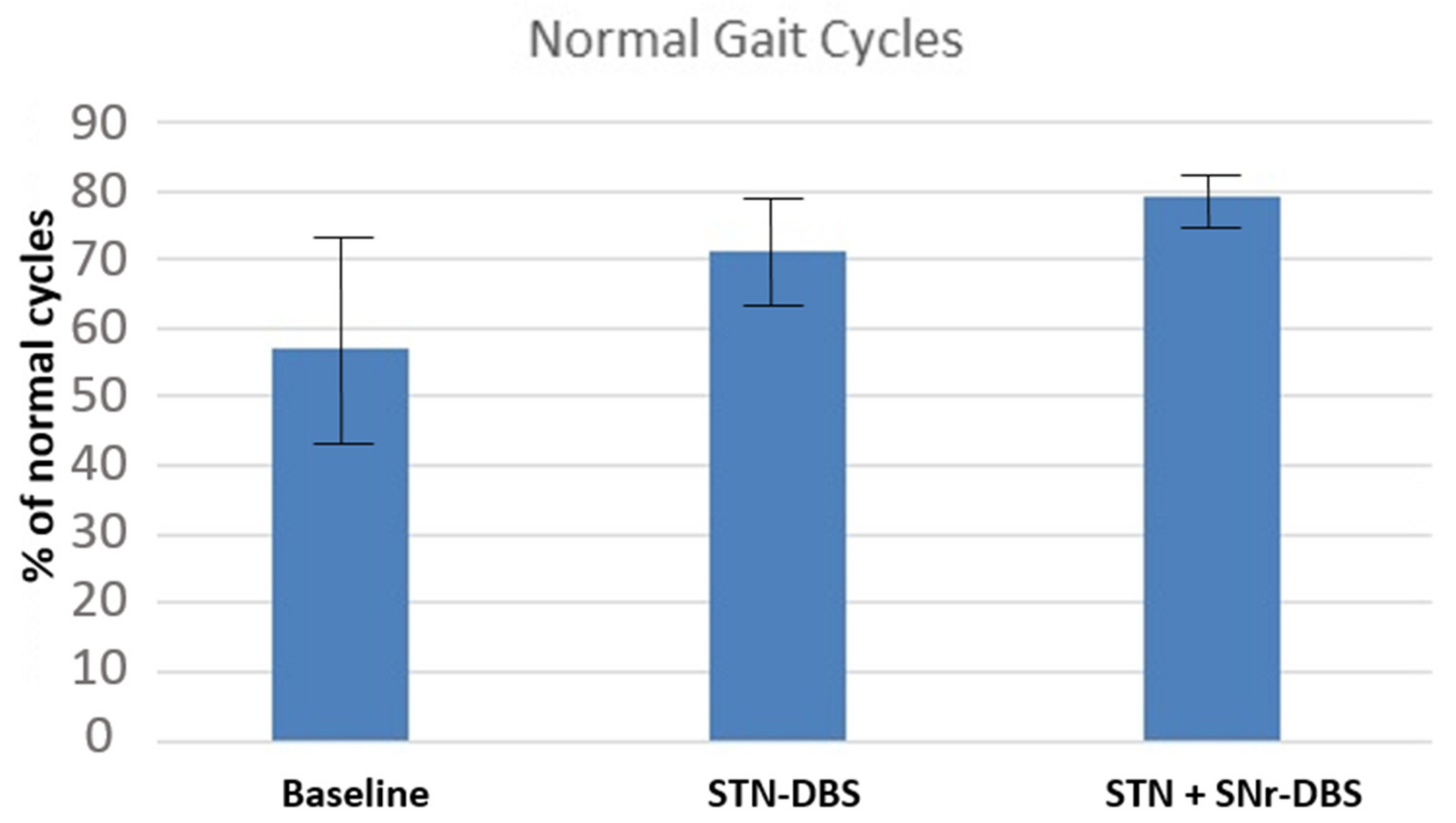
| HFPS Cycles | Baseline | STN | STN + SNr |
|---|---|---|---|
| Mean (± SEM) | 0.57 (0.41; 0.73) | 0.71 (0.63; 0.79) | 0.79 (0.74; 0.84) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villadóniga, M.; Cabañes-Martínez, L.; López-Viñas, L.; Fanjul, S.; del Álamo, M.; Regidor, I. Combined Stimulation of the Substantia Nigra and the Subthalamic Nucleus for the Treatment of Refractory Gait Disturbances in Parkinson’s Disease: A Preliminary Study. J. Clin. Med. 2022, 11, 2269. https://doi.org/10.3390/jcm11082269
Villadóniga M, Cabañes-Martínez L, López-Viñas L, Fanjul S, del Álamo M, Regidor I. Combined Stimulation of the Substantia Nigra and the Subthalamic Nucleus for the Treatment of Refractory Gait Disturbances in Parkinson’s Disease: A Preliminary Study. Journal of Clinical Medicine. 2022; 11(8):2269. https://doi.org/10.3390/jcm11082269
Chicago/Turabian StyleVilladóniga, Marta, Lidia Cabañes-Martínez, Laura López-Viñas, Samira Fanjul, Marta del Álamo, and Ignacio Regidor. 2022. "Combined Stimulation of the Substantia Nigra and the Subthalamic Nucleus for the Treatment of Refractory Gait Disturbances in Parkinson’s Disease: A Preliminary Study" Journal of Clinical Medicine 11, no. 8: 2269. https://doi.org/10.3390/jcm11082269
APA StyleVilladóniga, M., Cabañes-Martínez, L., López-Viñas, L., Fanjul, S., del Álamo, M., & Regidor, I. (2022). Combined Stimulation of the Substantia Nigra and the Subthalamic Nucleus for the Treatment of Refractory Gait Disturbances in Parkinson’s Disease: A Preliminary Study. Journal of Clinical Medicine, 11(8), 2269. https://doi.org/10.3390/jcm11082269







