Prediction of Poststroke Depression Based on the Outcomes of Machine Learning Algorithms
Abstract
1. Introduction
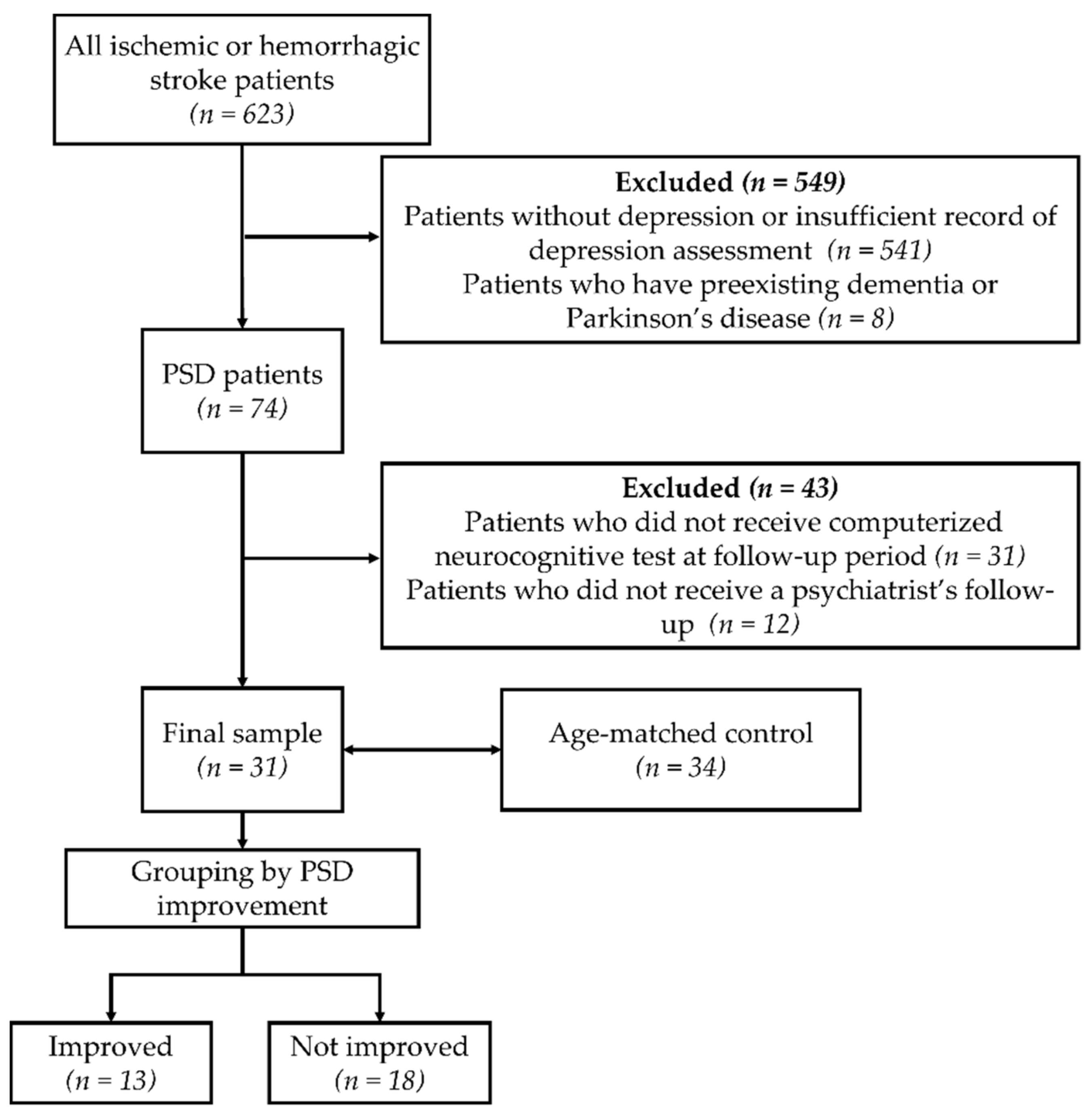
2. Subjects and Methods
2.1. Subjects
2.2. Measurements
2.2.1. Neurological Assessment
2.2.2. Psychological Assessment
2.2.3. Cognitive Assessments
2.2.4. Functional Assessments
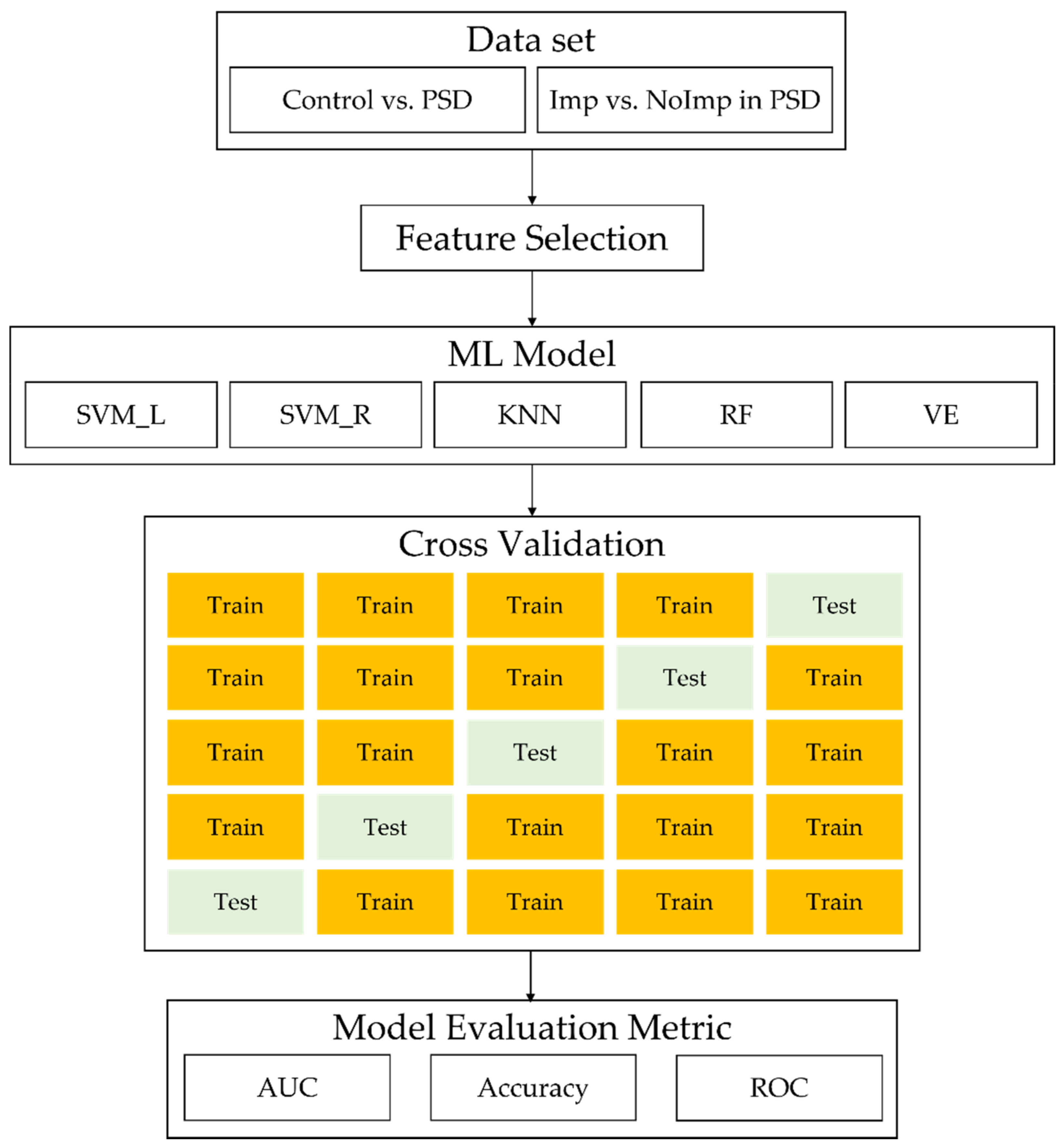
2.3. ML Analysis
2.4. Statistics
3. Results
3.1. Baseline Characteristics
3.2. Cognitive and Functional Analysis Using Statistical Methods
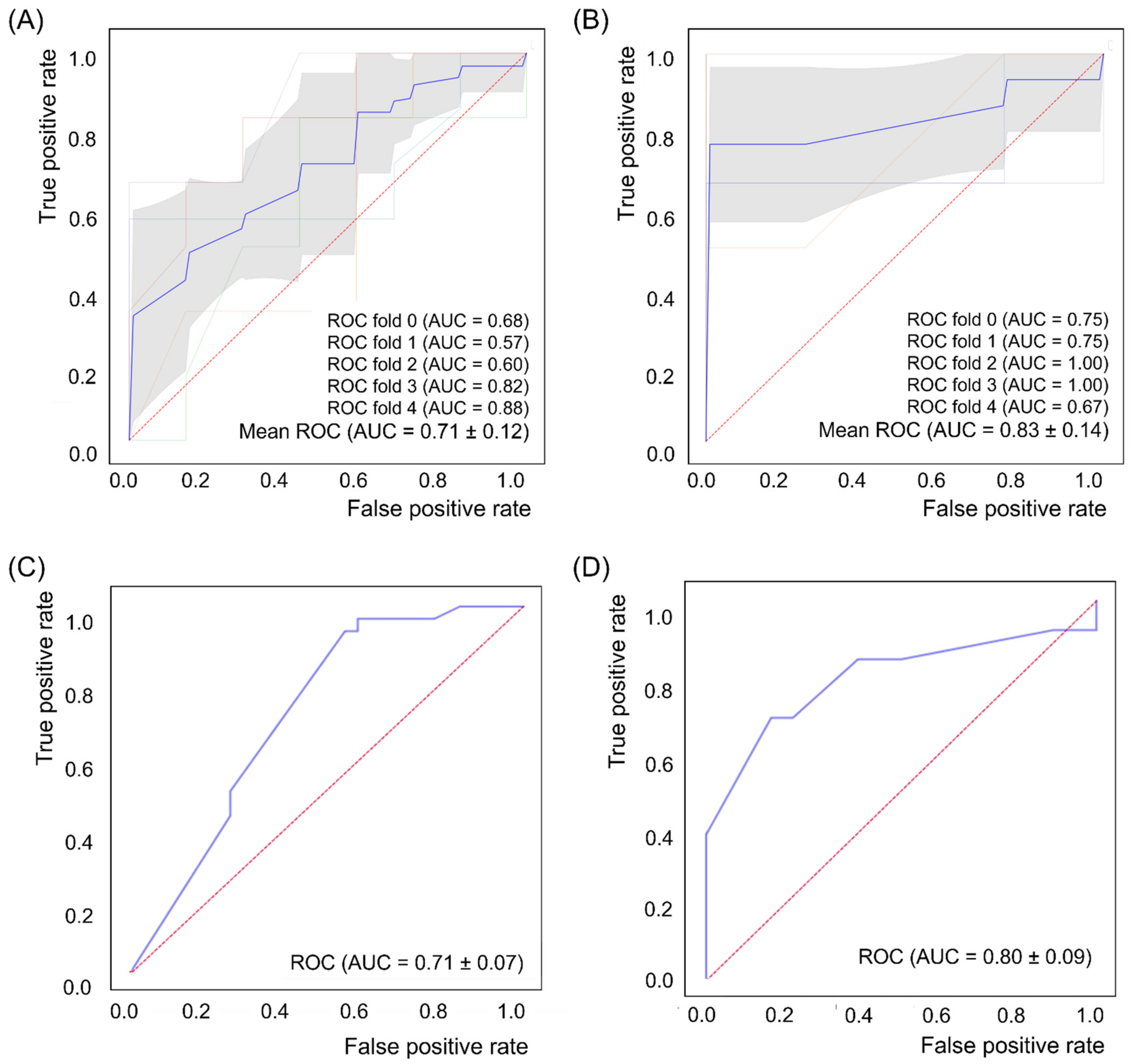
3.3. ML Analysis
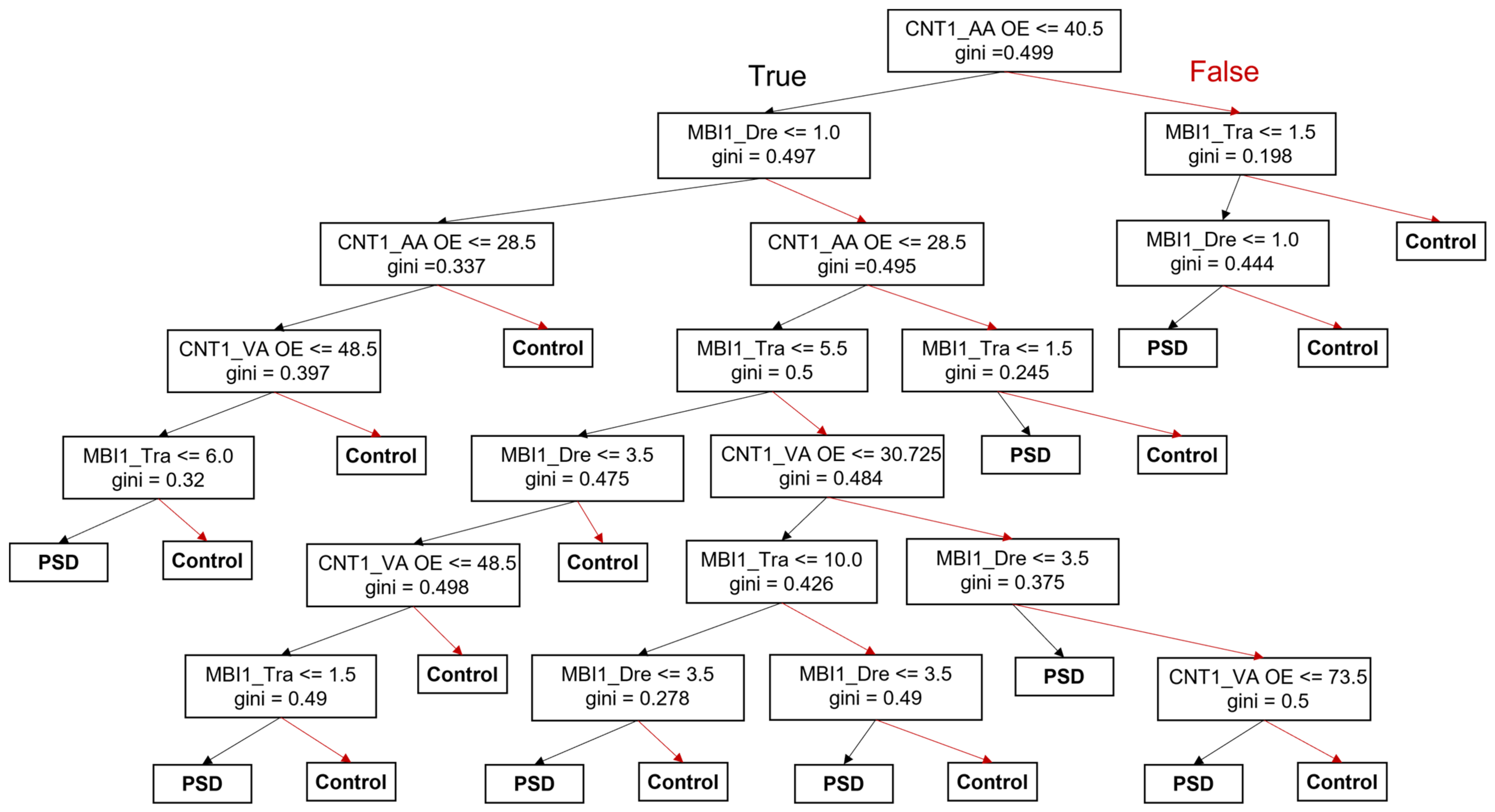
3.4. Decision-Making Model for the Prediction of PSD Occurrence and Prognosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burvill, P.W.; Johnson, G.A.; Jamrozik, K.D.; Anderson, C.S.; Stewart-Wynne, E.G.; Chakera, T.M. Prevalence of depression after stroke: The Perth Community Stroke Study. Br. J. Psychiatry 1995, 166, 320–327. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, G.E.; Scarpina, F.; Mauro, A.; Mori, I.; Castelnuovo, G.; Bigoni, M.; Baudo, S.; Molinari, E. Characteristics of anxiety and psychological well-being in chronic post-stroke patients. J. Neurol. Sci. 2014, 338, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.; Lonnqvist, J.; Palomaki, H.; Kaste, M. Assessment of depression after stroke: A comparison of different screening instruments. Stroke 2009, 40, 523–529. [Google Scholar] [CrossRef]
- Robinson, R.G. Poststroke depression: Prevalence, diagnosis, treatment, and disease progression. Biol. Psychiatry 2003, 54, 376–387. [Google Scholar] [CrossRef]
- Whyte, E.M.; Mulsant, B.H. Post stroke depression: Epidemiology, pathophysiology, and biological treatment. Biol. Psychiatry 2002, 52, 253–264. [Google Scholar] [CrossRef]
- Ayerbe, L.; Ayis, S.; Wolfe, C.D.; Rudd, A.G. Natural history, predictors and outcomes of depression after stroke: Systematic review and meta-analysis. Br. J. Psychiatry 2013, 202, 14–21. [Google Scholar] [CrossRef]
- Hellmann-Regen, J.; Piber, D.; Hinkelmann, K.; Gold, S.M.; Heesen, C.; Spitzer, C.; Endres, M.; Otte, C. Depressive syndromes in neurological disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263 (Suppl. S2), S123–S136. [Google Scholar] [CrossRef]
- Allan, L.M.; Rowan, E.N.; Thomas, A.J.; Polvikoski, T.M.; O’Brien, J.T.; Kalaria, R.N. Long-term incidence of depression and predictors of depressive symptoms in older stroke survivors. Br. J. Psychiatry 2013, 203, 453–460. [Google Scholar] [CrossRef]
- Jiang, X.G.; Lin, Y.; Li, Y.S. Correlative study on risk factors of depression among acute stroke patients. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 1315–1323. [Google Scholar]
- Astrom, M.; Adolfsson, R.; Asplund, K. Major depression in stroke patients. A 3-year longitudinal study. Stroke 1993, 24, 976–982. [Google Scholar] [CrossRef]
- Kauhanen, M.; Korpelainen, J.T.; Hiltunen, P.; Brusin, E.; Mononen, H.; Maatta, R.; Nieminen, P.; Sotaniemi, K.A.; Myllyla, V.V. Poststroke depression correlates with cognitive impairment and neurological deficits. Stroke 1999, 30, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.K.; Ng, T.P.; Koh, G.; Yong, D.; Fong, N.P. Is improvement in impaired cognition and depressive symptoms in post-stroke patients associated with recovery in activities of daily living? Acta. Neurol. Scand. 2007, 115, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Schottke, H.; Gerke, L.; Dusing, R.; Mollmann, A. Post-stroke depression and functional impairments—A 3-year prospective study. Compr. Psychiatry 2020, 99, 152171. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, S.; Iosa, M.; Coiro, P.; Venturiero, V.; Savo, A.; De Angelis, D.; Morone, G. Post-stroke Depression Increases Disability More Than 15% in Ischemic Stroke Survivors: A Case-Control Study. Front. Neurol. 2019, 10, 926. [Google Scholar] [CrossRef]
- Andersen, G.; Vestergaard, K.; Riis, J.O.; Ingeman-Nielsen, M. Dementia of depression or depression of dementia in stroke? Acta Psychiatr. Scand. 1996, 94, 272–278. [Google Scholar] [CrossRef]
- Robinson, R.G.; Schultz, S.K.; Castillo, C.; Kopel, T.; Kosier, J.T.; Newman, R.M.; Curdue, K.; Petracca, G.; Starkstein, S.E. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: A placebo-controlled, double-blind study. Am. J. Psychiatry 2000, 157, 351–359. [Google Scholar] [CrossRef]
- Heo, J.; Yoon, J.G.; Park, H.; Kim, Y.D.; Nam, H.S.; Heo, J.H. Machine Learning-Based Model for Prediction of Outcomes in Acute Stroke. Stroke 2019, 50, 1263–1265. [Google Scholar] [CrossRef]
- Jang, S.K.; Chang, J.Y.; Lee, J.S.; Lee, E.J.; Kim, Y.H.; Han, J.H.; Chang, D.I.; Cho, H.J.; Cha, J.K.; Yu, K.H.; et al. Reliability and Clinical Utility of Machine Learning to Predict Stroke Prognosis: Comparison with Logistic Regression. J. Stroke 2020, 22, 403–406. [Google Scholar] [CrossRef]
- Lin, C.H.; Hsu, K.C.; Johnson, K.R.; Fann, Y.C.; Tsai, C.H.; Sun, Y.; Lien, L.M.; Chang, W.L.; Chen, P.L.; Lin, C.L.; et al. Evaluation of machine learning methods to stroke outcome prediction using a nationwide disease registry. Comput. Methods Programs Biomed. 2020, 190, 105381. [Google Scholar] [CrossRef]
- Hama, S.; Yoshimura, K.; Yanagawa, A.; Shimonaga, K.; Furui, A.; Soh, Z.; Nishino, S.; Hirano, H.; Yamawaki, S.; Tsuji, T. Relationships between motor and cognitive functions and subsequent post-stroke mood disorders revealed by machine learning analysis. Sci. Rep. 2020, 10, 19571. [Google Scholar] [CrossRef]
- Rauwenhoff, J.C.C.; Bronswijk, S.C.; Peeters, F.; Bol, Y.; Geurts, A.C.H.; van Heugten, C.M. Personalized predictions of treatment outcome in patients with post-stroke depressive symptoms. J. Rehabil. Med. 2020, 52, jrm00120. [Google Scholar] [CrossRef] [PubMed]
- First, M.B.; Spitzer, R.L.; Gibbon, M.W.J.B.; Williams, J.B. Structured Clinical Interview for DSM-IV Clinical Version (SCID-I/CV); American Psychiatric Press: Washington, DC, USA, 1997. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 1980; Volume 3. [Google Scholar]
- Kunitz, S.C.; Gross, C.R.; Heyman, A.; Kase, C.S.; Mohr, J.P.; Price, T.R.; Wolf, P.A. The pilot Stroke Data Bank: Definition, design, and data. Stroke 1984, 15, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Turner-Stokes, L.; Hassan, N. Depression after stroke: A review of the evidence base to inform the development of an integrated care pathway. Part 2: Treatment alternatives. Clin. Rehabil. 2002, 16, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M. The Hamilton Depression Scale—accelerator or break on antidepressant drug discovery. Psychiatry 1960, 23, 56–62. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Kang, Y.W.; Na, D.L.; Hahn, S.H. A validity study on the korean mini-mental state examination (K-MMSE) in dementia patients. J. Korean Neurol. Ass. 1997, 15, 300–308. [Google Scholar]
- Thomas Gualtieri, C. Computerized neurocognitive testing and its potential for modern psychiatry. Psychiatry 2004, 1, 29–36. [Google Scholar]
- Han, C.; Jo, S.A.; Jo, I.; Kim, E.; Park, M.H.; Kang, Y. An adaptation of the Korean mini-mental state examination (K-MMSE) in elderly Koreans: Demographic influence and population-based norms (the AGE study). Arch. Gerontol. Geriatr. 2008, 47, 302–310. [Google Scholar] [CrossRef]
- Katz, N.; Erez, A.B.-H.; Livni, L.; Averbuch, S. Dynamic Lowenstein Occupational Therapy Cognitive Assessment: Evaluation of potential to change in cognitive performance. Am. J. Occup. Ther. 2012, 66, 207–214. [Google Scholar] [CrossRef][Green Version]
- Keith, R.A.; Granger, C.V.; Hamilton, B.B.; Sherman, F.S. Advances in Clinical Rehabilitation; Springer: New York, NY, USA, 1987; Volume 2, pp. 6–18. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Mitchell, N.; Hewitt, C.; Adamson, J.; Parrott, S.; Torgerson, D.; Ekers, D.; Holmes, J.; Lester, H.; McMillan, D.; Richards, D.; et al. A randomised evaluation of CollAborative care and active surveillance for Screen-Positive EldeRs with sub-threshold depression (CASPER): Study protocol for a randomized controlled trial. Trials 2011, 12, 225. [Google Scholar] [CrossRef] [PubMed]
- Barker-Collo, S.L. Depression and anxiety 3 months post stroke: Prevalence and correlates. Arch. Clin. Neuropsychol. 2007, 22, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Semkovska, M.; Quinlivan, L.; O’Grady, T.; Johnson, R.; Collins, A.; O’Connor, J.; Knittle, H.; Ahern, E.; Gload, T. Cognitive function following a major depressive episode: A systematic review and meta-analysis. Lancet Psychiatry 2019, 6, 851–861. [Google Scholar] [CrossRef]
- Villa, R.F.; Ferrari, F.; Moretti, A. Post-stroke depression: Mechanisms and pharmacological treatment. Pharmacol. Ther. 2018, 184, 131–144. [Google Scholar] [CrossRef]
- Woranush, W.; Moskopp, M.L.; Sedghi, A.; Stuckart, I.; Noll, T.; Barlinn, K.; Siepmann, T. Preventive Approaches for Post-Stroke Depression: Where Do We Stand? A Systematic Review. Neuropsychiatr. Dis. Treat. 2021, 17, 3359–3377. [Google Scholar] [CrossRef]
- Allida, S.; Cox, K.L.; Hsieh, C.F.; House, A.; Hackett, M.L. Pharmacological, psychological and non-invasive brain stimulation interventions for preventing depression after stroke. Cochrane Database Sys. Rev. 2020, 5, CD003689. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, D.; Zeng, Y.; Wu, W. Risk Factors for Post-stroke Depression: A Meta-analysis. Front. Aging Neurosci. 2017, 9, 218. [Google Scholar] [CrossRef]
- Robinson, R.G.; Jorge, R.E. Post-Stroke Depression: A Review. Am. J. Psychiatry 2016, 173, 221–231. [Google Scholar] [CrossRef]
- Schooling, C.M.; Jones, H.E. Clarifying questions about “risk factors”: Predictors versus explanation. Emerg. Themes Epidemiol. 2018, 15, 10. [Google Scholar] [CrossRef]
- Bzdok, D.; Altman, N.; Krzywinski, M. Statistics versus machine learning. Nat. Methods 2018, 15, 233–234. [Google Scholar] [CrossRef]
- Sirsat, M.S.; Ferme, E.; Camara, J. Machine Learning for Brain Stroke: A Review. J. Stroke Cerebrovasc. Dis. 2020, 29, 105162. [Google Scholar] [CrossRef] [PubMed]
- Que, Q.; Belkin, M. Back to the Future: Radial Basis Function Network Revisited. IEEE Trans. Pattern Anal. Mach. Intell. 2020, 42, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Robinson, R.G.; Kosier, J.T. Treatment of cognitive impairment after poststroke depression: A double-blind treatment trial. Stroke 2000, 31, 1482–1486. [Google Scholar] [CrossRef] [PubMed]
- Lincoln, N.B.; Nicholl, C.R.; Flannaghan, T.; Leonard, M.; Van der Gucht, E. The validity of questionnaire measures for assessing depression after stroke. Clin. Rehabil. 2003, 17, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Jorge, R.E.; Acion, L.; Moser, D.; Adams, H.P., Jr.; Robinson, R.G. Escitalopram and enhancement of cognitive recovery following stroke. Arch. Gen. Psychiatry 2010, 67, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, I.; Krokstad, S.; Mykletun, A.; Dahl, A.A.; Tell, G.S.; Tambs, K. Does a higher educational level protect against anxiety and depression? The HUNT study. Soc. Sci. Med. 2008, 66, 1334–1345. [Google Scholar] [CrossRef]
- Baccaro, A.; Wang, Y.P.; Candido, M.; Conforto, A.B.; Brunoni, A.R.; Leite, C.D.C.; Busatto Filho, G.; Lotufo, P.A.; Bensenor, I.M.; Goulart, A.C. Post-stroke depression and cognitive impairment: Study design and preliminary findings in a Brazilian prospective stroke cohort (EMMA study). J. Affect. Disord. 2019, 245, 72–81. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, M.; Su, Z.; Guan, B.; Wang, A.; Wang, Y.; Zhang, N.; Wang, C. Post-stroke depression: Different characteristics based on follow-up stage and gender-a cohort perspective study from Mainland China. Neurol. Res. 2017, 39, 996–1005. [Google Scholar] [CrossRef]
- Whyte, E.M.; Mulsant, B.H.; Vanderbilt, J.; Dodge, H.H.; Ganguli, M. Depression after stroke: A prospective epidemiological study. J. Am. Geriatr. Soc. 2004, 52, 774–778. [Google Scholar] [CrossRef]
- Ogundimu, E.O.; Altman, D.G.; Collins, G.S. Adequate sample size for developing prediction models is not simply related to events per variable. J. Clin. Epidemiol. 2016, 76, 175–182. [Google Scholar] [CrossRef]
- Fruehwald, S.; Gatterbauer, E.; Rehak, P.; Baumhackl, U. Early fluoxetine treatment of post-stroke depression--a three-month double-blind placebo-controlled study with an open-label long-term follow up. J. Neurol. 2003, 250, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Wiart, L.; Petit, H.; Joseph, P.A.; Mazaux, J.M.; Barat, M. Fluoxetine in early poststroke depression: A double-blind placebo-controlled study. Stroke 2000, 31, 1829–1832. [Google Scholar] [CrossRef] [PubMed]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic minority over-sampling technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Kohavi, R. A study of cross-validation and bootstrap for accuracy estimation and model selection. In Proceedings of the International Joint Conference on Artificial Intelligence, Montreal, QC, Canada, 20–25 August 1995; pp. 1137–1145. [Google Scholar]
| Controls | PSD Patients | p Value 2 | ||||
|---|---|---|---|---|---|---|
| NoImp | Imp | p Value 1 | All | |||
| No. of subjects | 34 | 18 | 13 | 0.798 | 31 | |
| Age (years) | 64.6 ± 15.9 | 62.3 ± 11.5 | 63.5 ± 13.9 | 0.779 | 62.8 ± 15.9 | 0.563 |
| Female, no. (%) | 15 (44) | 7 (39) | 8 (61) | 0.285 | 21 (58) | 0.546 |
| Educational period (years) | 9.2 ± 5.0 | 7.0 ± 3.4 | 9.8 ± 4.0 | 0.027 * | 8.2 ± 3.9 | 0.406 |
| Onset (days) | 74.3 ± 68.9 | 71.2 ± 41.3 | 69.5 ± 25.4 | 0.890 | 70.5 ± 35.0 | 0.250 |
| No. of depressive symptoms | ||||||
| Initial | - | 6.06 ± 1.39 | 6.69 ± 1.60 | 0.260 | 6.32 ± 1.49 | - |
| Follow-up | - | 6.67 ± 1.53 | 2.08 ± 2.25 | 0.000 * | 4.74 ± 2.94 | - |
| Type of stroke, no. (%) | ||||||
| Hemorrhagic stroke | 15 (44) | 3 (23) | 9 (50) | 0.833 | 12 (39) | 0.661 |
| Ischemic stroke | 19 (56) | 10 (77) | 9 (50) | 0.833 | 19 (61) | 0.661 |
| Side of hemiplegia (Rt:Lt:both) | 14 (42):12 (35):8 (23) | 6 (33):7 (39):5 (28) | 3 (23):8 (61):2 (16) | 0.462 | 9 (29):15 (48):7 (23) | 0.518 |
| Family Hx. (medical disorders) | 12 (35.3) | 6 (33) | 6 (46) | 0.470 | 12 (39) | 0.777 |
| Family Hx. (mental disorders) | 0 (0) | 1 (5) | 0 (0) | 0.388 | 1 (3) | 0.295 |
| Smoking (years) | 8.2 ± 15.8 | 6.1 ± 14.4 | 9.6 ± 17.1 | 0.567 | 7.5 ± 15.4 | 0.880 |
| Diabetes mellitus | 8 (23) | 4 (22) | 6 (46) | 0.160 | 10 (30) | 0.436 |
| Hypertension | 10 (56) | 10 (55) | 6 (46) | 0.605 | 16 (52) | 0.288 |
| NIHSS score | 7.6 ± 5.6 | 7.3 ± 7.0 | 8.0 ± 4.3 | 0.535 | 6.8 ± 3.4 | 0.826 |
| Controls | PSD Patients | p Value 2 | ||||
|---|---|---|---|---|---|---|
| NoImp | Imp | p Value 1 | All | |||
| K-MMSE | ||||||
| Initial | 14.0 ± 8.4 | 12.4 ± 7.6 | 16.3 ± 7.5 | 0.206 | 14.1 ± 7.7 | 0.787 |
| Follow-up | 19.3 ± 9.3 | 16.1 ± 8.2 | 21.6 ± 6.8 | 0.037 * | 18.7 ± 8.0 | 0.491 |
| Gain | 5.4 ± 6.8 | 4.3 ± 3.2 | 5.4 ± 3.1 | 0.395 | 4.7 ± 3.1 | 0.535 |
| p value 3 | 0.000 * | 0.001 * | 0.000 * | 0.000 * | ||
| CNT | ||||||
| Initial | 419.1 ± 180.2 | 371.5 ± 114.4 | 415.8 ± 145.1 | 0.603 | 390.1 ± 127.8 | 0.427 |
| Follow-up | 449.5 ± 220.7 | 422.9 ± 191.7 | 492.3 ± 230.0 | 0.526 | 449.9 ± 203.7 | 0.980 |
| Gain | 33.6 ± 70.9 | 49.1 ± 64.5 | 36.0 ± 57.7 | 0.618 | 44.0 ± 60.5 | 0.580 |
| p value 3 | 0.119 | 0.241 | 0.237 | 0.088 | ||
| K-MBI | ||||||
| Initial | 25.7 ± 25.2 | 19.8 ± 15.5 | 25.7 ± 25.2 | 0.718 | 19.8 ± 15.5 | 0.103 |
| Follow-up | 46.5 ± 28.6 | 40.1 ± 19.7 | 46.5 ± 28.6 | 0.330 | 40.1 ± 19.7 | 0.006 * |
| Gain | 18.7 ± 12.7 | 20.2 ± 15.5 | 18.7 ± 12.7 | 0.703 | 20.2 ± 15.5 | 0.276 |
| p value 3 | 0.000 * | 0.001 * | 0.002 * | 0.000 * | ||
| FIM | ||||||
| Initial | 46.3 ± 22.9 | 44.1± 15.5 | 46.3 ± 22.9 | 0.904 | 44.1 ± 15.5 | 0.285 |
| Follow-up | 64.7 ± 28.2 | 59.1 ± 17.5 | 64.7 ± 28.2 | 0.525 | 59.1 ± 17.5 | 0.013 * |
| Gain | 17.0 ± 11.9 | 16.5 ± 8.2 | 17.0 ± 11.9 | 0.925 | 16.5 ± 8.2 | 0.132 |
| p value 3 | 0.000 * | 0.000 * | 0.005 * | 0.000 * | ||
| Controls | PSD Patients | p Value 2 | ||||
|---|---|---|---|---|---|---|
| NoImp | Imp | p Value 1 | All | |||
| Orientation | ||||||
| Initial | 5.1 ± 3.1 | 4.2 ± 3.5 | 5.5 ± 3.4 | 0.224 | 4.7 ± 3.5 | 0.630 |
| Follow-up | 6.8 ± 3.4 | 5.3 ± 3.3 | 7.5 ± 2.6 | 0.143 | 6.2 ± 3.2 | 0.355 |
| Gain | 1.7 ± 2.8 | 1.4 ± 2.1 | 1.4 ± 2.2 | 0.688 | 1.4 ± 2.1 | 0.916 |
| p value 3 | 0.003 * | 0.020 * | 0.077 | 0.003 * | ||
| Registration | ||||||
| Initial | 2.3 ± 1.1 | 2.3 ± 1.2 | 2.9 ± 0.3 | 0.138 | 2.6 ± 1.0 | 0.254 |
| Follow-up | 2.5 ± 1.2 | 2.6 ± 1.4 | 3.0 ± 0.0 | 0.701 | 2.8 ± 1.1 | 0.200 |
| Gain | 0.2 ± 0.9 | 0.4 ± 1.0 | 0.1 ± 0.3 | 0.544 | 0.3 ± 0.8 | 0.983 |
| p value 3 | 0.196 | 0.131 | 0.343 | 0.084 | ||
| Recall | ||||||
| Initial | 0.9 ± 1.8 | 0.5 ± 0.6 | 1.2 ± 1.5 | 0.364 | 0.8 ± 1.1 | 0.446 |
| Follow-up | 1.9 ± 2.1 | 0.9 ± 1.0 | 2.8 ± 2.2 | 0.027 * | 1.6 ± 1.8 | 0.894 |
| Gain | 0.9 ± 1.5 | 0.4 ± 0.7 | 1.6 ± 1.6 | 0.026 * | 0.9 ± 1.2 | 0.601 |
| p value 3 | 0.003 * | 0.038 * | 0.011 * | 0.002 * | ||
| Attention and calculation | ||||||
| Initial | 1.1 ± 1.3 | 0.5 ± 0.8 | 1.2 ± 1.2 | 0.063 | 0.8 ± 1.0 | 0.495 |
| Follow-up | 1.7 ± 1.3 | 1.4 ± 1.4 | 2.0 ± 0.9 | 0.374 | 1.7 ± 1.2 | 0.946 |
| Gain | 0.5 ± 1.3 | 0.9 ± 1.0 | 0.7 ± 1.7 | 0.695 | 0.8 ± 1.3 | 0.439 |
| p value 3 | 0.013 * | 0.010 * | 0.226 | 0.007 * | ||
| Language and complex commands | ||||||
| Initial | 4.6 ± 2.7 | 4.9 ± 2.7 | 5.5 ± 2.6 | 0.762 | 5.2 ± 2.6 | 0.341 |
| Follow-up | 6.3 ± 3.0 | 5.8 ± 2.8 | 7.4 ± 1.5 | 0.080 | 6.4 ± 2.5 | 0.696 |
| Gain | 1.4 ± 2.2 | 1.1 ± 1.1 | 1.6 ± 1.8 | 0.571 | 1.3 ± 1.4 | 0.659 |
| p value 3 | 0.000 * | 0.003 * | 0.022 * | 0.000 * | ||
| Controls | PSD Patients | p Value 2 | ||||
|---|---|---|---|---|---|---|
| NoImp | Imp | p Value 1 | All | |||
| Language memory digit span forward | ||||||
| Initial | 30.4 ± 5.1 | 29.2 ± 4.2 | 32.2 ± 7.3 | 0.090 | 30.9 ± 5.9 | 0.869 |
| Follow-up | 31.5 ± 6.9 | 29.0 ± 3.2 | 35.1 ± 5.2 | 0.017 * | 31.5 ± 6.9 | 0.592 |
| Gain | 2.3 ± 3.3 | 0.5 ± 2.2 | 1.4 ± 5.2 | 0.728 | 0.8 ± 3.3 | 0.105 |
| p value 3 | 0.005 * | 0.465 | 1.00 | 0.483 | ||
| Language memory digit span backward | ||||||
| Initial | 29.9 ± 5.6 | 28.3 ± 2.5 | 30.4 ± 7.4 | 0.689 | 29.2 ± 5.1 | 0.934 |
| Follow-up | 33.0 ± 11.2 | 29.0 ± 4.8 | 35.6 ± 8.2 | 0.013 * | 32.0 ± 7.8 | 1.000 |
| Gain | 3.9 ± 8.4 | 1.1 ± 2.8 | 7.0 ± 8.3 | 0.090 | 3.4 ± 6.1 | 0.847 |
| p value 3 | 0.005 * | 0.180 | 0.046 * | 0.017 * | ||
| Visual memory visual span forward | ||||||
| Initial | 30.7 ± 7.5 | 28.9 ± 3.0 | 33.5 ± 9.3 | 0.201 | 30.8 ± 6.7 | 0.820 |
| Follow-up | 32.0 ± 7.8 | 29.6 ± 3.4 | 32.6 ± 7.6 | 0.496 | 30.8 ± 5.4 | 0.684 |
| Gain | 2.7 ± 6.3 | 1.1 ± 2.9 | −0.9 ± 5.6 | 0.619 | 0.3 ± 6.3 | 0.373 |
| p value 3 | 0.016 * | 0.225 | 1.00 | 0.440 | ||
| Visual memory visual span backward | ||||||
| Initial | 30.6 ± 6.1 | 28.8 ± 3.0 | 32.9 ± 8.0 | 0.215 | 30.5 ± 5.9 | 0.650 |
| Follow-up | 32.0 ± 5.9 | 29.8 ± 4.3 | 34.7 ± 10.6 | 0.230 | 31.7 ± 7.5 | 0.593 |
| Gain | 2.5 ± 4.6 | 0.6 ± 4.8 | 1.4 ± 5.7 | 0.814 | 0.9 ± 5.0 | 0.424 |
| p value 3 | 0.012 * | 0.439 | 0.465 | 0.323 | ||
| Auditory attention correct response | ||||||
| Initial | 29.8 ± 6.6 | 27.8 ± 3.2 | 29.0 ± 3.2 | 0.399 | 28.3 ± 4.1 | 0.320 |
| Follow-up | 31.9 ± 11.2 | 31.9 ± 7.0 | 36.1 ± 18.3 | 1.000 | 33.3 ± 12.3 | 0.361 |
| Gain | 3.7 ± 7.7 | 3.6 ± 5.0 | 6.7 ± 12.1 | 0.826 | 4.9 ± 8.4 | 0.465 |
| p value 3 | 0.027 * | 0.068 | 0.102 | 0.027 * | ||
| Auditory attention commission error | ||||||
| Initial | 29.8 ± 6.6 | 27.8 ± 3.2 | 29.0 ± 3.2 | 0.399 | 28.3 ± 4.1 | 0.320 |
| Follow-up | 31.9 ± 11.2 | 31.9 ± 7.0 | 36.1 ± 18.3 | 1.000 | 33.6 ± 12.6 | 0.361 |
| Gain | 3.7 ± 7.7 | 3.6 ± 5.0 | 6.7 ± 12.1 | 0.826 | 4.9 ± 8.4 | 0.465 |
| p value 3 | 0.027 * | 0.068 | 0.102 | 0.027 * | ||
| Auditory attention omission error | ||||||
| Initial | 38.1 ± 17.2 | 34.5 ± 14.8 | 33.6 ± 13.7 | 0.723 | 28.7 ± 5.7 | 0.481 |
| Follow-up | 36.0 ± 15.1 | 41.3 ± 21.3 | 36.3 ± 18.5 | 0.711 | 31.2 ± 9.4 | 0.892 |
| Gain | −5.9 ± 22.5 | 5.5 ± 10.5 | 5.7 ± 16.0 | 0.585 | 1.4 ± 10.7 | 0.052 |
| p value 3 | 0.221 | 0.109 | 0.414 | 0.075 | ||
| Auditory attention correct time SD | ||||||
| Initial | 38.1 ± 17.2 | 34.5 ± 14.8 | 33.6 ± 13.7 | 0.773 | 34.1 ± 14.1 | 0.232 |
| Follow-up | 36.1 ± 15.1 | 41.3 ± 21.3 | 36.3 ± 18.5 | 0.740 | 39.2 ± 19.8 | 0.924 |
| Gain | −7.5 ± 21.7 | 6.1 ± 10.9 | 5.7 ± 16.0 | 0.521 | 5.9 ± 12.9 | 0.026 * |
| p value 3 | 0.026 * | 0.068 | 0.414 | 0.085 | ||
| Visual attention correct response | ||||||
| Initial | 33.2 ± 12.7 | 30.1 ± 9.9 | 35.4 ± 11.9 | 0.308 | 31.4 ± 11.1 | 0.617 |
| Follow-up | 38.7 ± 14.5 | 37.7 ± 19.2 | 34.1 ± 16.0 | 0.669 | 36.2 ± 17.5 | 0.202 |
| Gain | 9.0 ± 13.2 | 11.6 ± 19.2 | −2.4 ± 5.8 | 0.041 * | 5.4 ± 16.2 | 0.174 |
| p value 3 | 0.006 * | 0.078 | 0.197 | 0.026 * | ||
| Visual attention commission error | ||||||
| Initial | 33.4 ± 13.1 | 30.1 ± 10.7 | 32.4 ± 12.0 | 0.381 | 31.1 ± 11.1 | 0.223 |
| Follow-up | 38.7 ± 14.5 | 37.7 ± 19.2 | 34.1 ± 16.0 | 0.606 | 36.2 ± 17.5 | 0.072 |
| Gain | 9.3 ± 13.9 | 10.1 ± 18.7 | −1.3 ± 5.3 | 0.138 | 5.4 ± 15.5 | 0.049 * |
| p value 3 | 0.006 * | 0.078 | 0.414 | 0.288 | ||
| Visual attention omission error | ||||||
| Initial | 38.2 ± 18.2 | 30.1 ± 12.5 | 30.9 ± 11.9 | 0.481 | 30.5 ± 12.0 | 0.027 * |
| Follow-up | 40.4 ± 20.5 | 31.0 ± 6.8 | 33.1 ± 16.3 | 0.396 | 31.9 ± 11.2 | 0.303 |
| Gain | 1.0 ± 20.7 | 4.0 ± 6.8 | −1.1 ± 22.6 | 0.253 | 1.9 ± 15.0 | 0.677 |
| p value 3 | 0.671 | 0.066 | 0.655 | 0.344 | ||
| Visual attention correct time SD | ||||||
| Initial | 29.6 ± 5.8 | 29.3 ± 4.1 | 30.6 ± 6.4 | 0.651 | 29.9 ± 5.2 | 0.640 |
| Follow-up | 30.3 ± 4.6 | 29.5 ± 3.1 | 31.6 ± 6.3 | 0.622 | 30.5 ± 4.8 | 0.885 |
| Gain | 3.8 ± 7.7 | 0.4 ± 6.3 | 1.3 ± 2.9 | 0.763 | 0.8 ± 4.9 | 0.438 |
| p value 3 | 0.010 * | 0.468 | 0.257 | 0.205 | ||
| Controls | PSD Patients | p Value 2 | ||||
|---|---|---|---|---|---|---|
| NoImp | Imp | p Value 1 | All | |||
| Hygiene | ||||||
| Initial | 2.1 ± 1.8 | 1.8 ± 1.5 | 1.6 ± 2.0 | 0.507 | 1.7 ± 1.7 | 0.369 |
| Follow-up | 3.3 ± 1.6 | 2.6 ± 1.5 | 3.2 ± 1.3 | 0.414 | 2.9 ± 1.4 | 0.166 |
| Gain | 1.2 ± 1.7 | 0.9 ± 1.5 | 1.6 ± 1.7 | 0.277 | 1.2 ± 1.6 | 0.628 |
| p value 3 | 0.001 * | 0.027 * | 0.017 * | 0.001 * | ||
| Bathing | ||||||
| Initial | 1.1 ± 1.2 | 0.6 ± 0.5 | 0.3 ± 0.5 | 0.101 | 0.5 ± 0.5 | 0.075 |
| Follow-up | 2.4 ± 1.6 | 1.4 ± 1.2 | 1.7 ± 1.5 | 0.567 | 1.5 ± 1.3 | 0.030 * |
| Gain | 1.4 ± 1.6 | 0.7 ± 1.0 | 1.4 ± 1.4 | 0.164 | 1.0 ± 1.2 | 0.324 |
| p value 3 | 0.000 * | 0.015 * | 0.016 * | 0.001 * | ||
| Eating | ||||||
| Initial | 3.7 ± 3.5 | 3.0 ± 3.0 | 3.3 ± 3.9 | 0.950 | 3.1 ± 3.3 | 0.543 |
| Follow-up | 6.6 ± 2.9 | 5.2 ± 3.1 | 5.7 ± 3.4 | 0.662 | 5.4 ± 3.2 | 0.146 |
| Gain | 2.9 ± 3.4 | 2.1 ± 2.6 | 2.5 ± 3.0 | 0.658 | 2.3 ± 2.7 | 0.531 |
| p value 3 | 0.000 * | 0.011 * | 0.024 * | 0.001 * | ||
| Toileting | ||||||
| Initial | 2.9 ± 3.5 | 1.2 ± 2.2 | 2.3 ± 3.5 | 0.526 | 1.6 ± 2.8 | 0.097 |
| Follow-up | 5.5 ± 3.7 | 3.4 ± 2.8 | 4.3 ± 3.8 | 0.679 | 3.8 ± 3.2 | 0.047 * |
| Gain | 2.8 ± 3.9 | 2.6 ± 2.8 | 2.0 ± 3.1 | 0.455 | 2.4 ± 2.9 | 0.773 |
| p value 3 | 0.001 * | 0.006 * | 0.058 | 0.001 * | ||
| Stair-climbing | ||||||
| Initial | 0.3 ± 1.4 | 0.0 ± 0.0 | 0.4 ± 1.4 | 0.239 | 0.2 ± 0.9 | 0.613 |
| Follow-up | 3.2 ± 3.7 | 0.3 ± 1.2 | 1.5 ± 3.2 | 0.273 | 0.8 ± 2.3 | 0.002 * |
| Gain | 2.9 ± 3.6 | 0.3 ± 1.2 | 1.0 ± 2.5 | 0.314 | 0.6 ± 1.8 | 0.003 * |
| p value 3 | 0.001 * | 0.317 | 0.180 | 0.109 | ||
| Dressing | ||||||
| Initial | 3.4 ± 2.7 | 2.2 ± 1.7 | 1.6 ± 2.1 | 0.230 | 2.0 ± 1.9 | 0.030 * |
| Follow-up | 6.1 ± 3.2 | 3.9 ± 2.5 | 4.9 ± 3.1 | 0.453 | 4.3 ± 2.8 | 0.029 * |
| Gain | 2.8 ± 3.2 | 1.9 ± 2.1 | 3.0 ± 3.3 | 0.388 | 2.3 ± 2.6 | 0.694 |
| p value 3 | 0.000 * | 0.008 * | 0.028 * | 0.001 * | ||
| Bowel control | ||||||
| Initial | 5.3 ± 4.4 | 4.6 ± 4.5 | 4.8 ± 5.1 | 0.831 | 4.7 ± 4.7 | 0.448 |
| Follow-up | 8.4 ± 3.3 | 7.4 ± 3.7 | 6.7 ± 4.2 | 0.734 | 7.1 ± 3.9 | 0.190 |
| Gain | 3.2 ± 4.2 | 3.1 ± 4.1 | 2.0 ± 3.2 | 0.558 | 2.6 ± 3.8 | 0.921 |
| p value 3 | 0.001 * | 0.013 * | 0.039 * | 0.002 * | ||
| Bladder control | ||||||
| Initial | 4.8 ± 4.5 | 3.1 ± 4.1 | 4.9 ± 4.9 | 0.169 | 3.9 ± 4.5 | 0.291 |
| Follow-up | 8.2 ± 3.4 | 5.8 ± 4.3 | 6.5 ± 4.5 | 0.548 | 6.1 ± 4.3 | 0.047 * |
| Gain | 3.4 ± 4.3 | 2.5 ± 4.2 | 1.6 ± 2.8 | 0.724 | 2.1 ± 3.6 | 0.314 |
| p value 3 | 0.000 * | 0.031 * | 0.066 | 0.007 * | ||
| Transfer | ||||||
| Initial | 6.2 ± 5.0 | 3.0 ± 3.7 | 5.4 ± 5.9 | 0.408 | 4.0 ± 4.8 | 0.050 |
| Follow-up | 10.1 ± 4.4 | 6.9 ± 4.0 | 7.5 ± 5.7 | 0.697 | 7.1 ± 4.6 | 0.013 * |
| Gain | 4.1 ± 4.5 | 4.5 ± 4.1 | 2.2 ± 3.5 | 0.137 | 3.6 ± 4.0 | 0.630 |
| p value 3 | 0.000 * | 0.003 * | 0.058 | 0.001 * | ||
| Ambulation | ||||||
| Initial | 2.4 ± 4.1 | 0.3 ± 0.5 | 1.1 ± 2.4 | 0.669 | 0.6 ± 1.6 | 0.058 |
| Follow-up | 6.8 ± 5.7 | 2.6 ± 3.5 | 3.4 ± 4.9 | 0.770 | 2.9 ± 4.0 | 0.009 * |
| Gain | 4.5 ± 5.0 | 2.4 ± 3.4 | 2.1 ± 3.2 | 0.749 | 2.3 ± 3.3 | 0.129 |
| p value 3 | 0.000 * | 0.006 * | 0.042 * | 0.001 * | ||
| Controls | PSD Patients | p Value 2 | ||||
|---|---|---|---|---|---|---|
| NoImp | Imp | p Value 1 | All | |||
| Self-care | ||||||
| Initial | 15.4 ± 8.0 | 12.4 ± 5.0 | 11.6 ± 6.0 | 0.468 | 12.1 ± 5.4 | 0.102 |
| Follow-up | 23.2 ± 10.2 | 17.0 ± 6.2 | 19.0 ± 8.8 | 0.588 | 17.8 ± 7.3 | 0.033 * |
| Gain | 7.8 ± 8.8 | 4.9 ± 3.9 | 6.5 ± 6.0 | 0.759 | 5.5 ± 4.8 | 0.783 |
| p value 3 | 0.000 * | 0.000 * | 0.005 * | 0.000 * | ||
| Sphincter control | ||||||
| Initial | 7.8 ± 5.1 | 6.1 ± 4.2 | 7.6 ± 5.7 | 0.606 | 6.7 ± 4.9 | 0.350 |
| Follow-up | 11.4 ± 4.2 | 9.9 ± 4.2 | 9.6 ± 5.3 | 0.921 | 9.8 ± 4.6 | 0.200 |
| Gain | 3.6 ± 4.4 | 4.0 ± 4.0 | 2.0 ± 2.9 | 0.090 | 3.2 ± 3.7 | 0.847 |
| p value 3 | 0.000 * | 0.001 * | 0.017 * | 0.000 * | ||
| Transfer | ||||||
| Initial | 8.3 ± 5.2 | 5.2 ± 3.1 | 7.2 ± 4.5 | 0.296 | 6.0 ± 3.8 | 0.052 |
| Follow-up | 12.2 ± 5.5 | 7.9 ± 2.5 | 8.9 ± 5.4 | 0.757 | 8.3 ± 3.9 | 0.004 * |
| Gain | 3.9 ± 4.4 | 3.3 ± 2.2 | 1.7 ± 2.8 | 0.110 | 2.7 ± 2.5 | 0.530 |
| p value 3 | 0.000 * | 0.001 * | 0.074 | 0.000 * | ||
| Locomotion | ||||||
| Initial | 3.2 ± 2.5 | 2.3 ± 0.8 | 2.8 ± 1.5 | 0.497 | 2.5 ± 1.1 | 0.231 |
| Follow-up | 5.9 ± 3.7 | 4.1 ± 2.8 | 4.6 ± 4.2 | 0.541 | 4.3 ± 3.3 | 0.032 * |
| Gain | 2.7 ± 3.2 | 1.8 ± 2.8 | 1.7 ± 2.9 | 0.315 | 1.8 ± 2.8 | 0.159 |
| p value 3 | 0.000 * | 0.012 * | 0.066 | 0.002 * | ||
| Communication | ||||||
| Initial | 7.9 ± 3.3 | 8.1 ± 3.5 | 7.7 ± 3.9 | 0.777 | 7.9 ± 3.6 | 0.925 |
| Follow-up | 10.2 ± 3.4 | 8.8 ± 3.0 | 9.6 ± 3.4 | 0.668 | 9.1 ± 3.1 | 0.169 |
| Gain | 2.3 ± 2.3 | 1.0 ± 1.8 | 1.9 ± 2.0 | 0.194 | 1.4 ± 1.9 | ,138 |
| p value 3 | 0.000 * | 0.034 * | 0.016 * | 0.001 * | ||
| Social | ||||||
| Initial | 10.2 ± 5.1 | 10.1 ± 4.7 | 9.2 ± 5.3 | 0.627 | 9.7 ± 4.9 | 0.808 |
| Follow-up | 13.7 ± 5.4 | 11.5 ± 4.8 | 13.0 ± 5.5 | 0.654 | 12.1 ± 5.1 | 0.177 |
| Gain | 3.5 ± 4.1 | 1.6 ± 2.7 | 3.3 ± 2.8 | 0.152 | 2.3 ± 2.8 | 0.370 |
| p value 3 | 0.000 * | 0.000 * | 0.000 * | 0.000 * | ||
| Classification | F-Value | p-Value | Effect Size | AUC | Accuracy | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|---|---|
| Control vs. PSD | 12.975 | 0.002 | CNT1_AA OE | 0.933 | 0.706 | 0.696 | 0.967 | 0.419 |
| MBI1_Bat | 0.426 | |||||||
| Imp vs. NoImp | 13.296 | 0.001 | Edu_Per | 1.616 | 0.797 | 0.778 | 0.692 | 0.833 |
| Rank | Parameter Name |
|---|---|
| (A) Control vs. PSD groups | |
| 1 | Initial subscore of ambulation on the K-MBI (MBI1_Amb) |
| 2 | Initial subscore of auditory attention omission error on the CNT (CNT1_AA OE) |
| 3 | Initial subscore of recall on the K-MMSE (MMSE1_Rec) |
| 4 | Initial subscore of dressing on the K-MBI (MBI1_Dre) |
| 5 | Initial subscore of locomotion on the FIM (FIM1_Loc) |
| (B) Imp vs. NoImp groups | |
| 1 | Initial subscore of bladder control on the K-MBI (MBI1_Bla) |
| 2 | Initial subscore of bowel control on the K-MBI (MBI1_Bow) |
| 3 | Initial subscore of transfer on the FIM (FIM1_Tra) |
| 4 | Initial subscore of visual memory visual span backward on the CNT (CNT1_VM VSB) |
| 5 | Initial subscore of communication on the FIM (FIM1_Com) |
| ML Models | 5-Fold Cross-Validation | 10-Fold Cross-Validation | ||||||
|---|---|---|---|---|---|---|---|---|
| AUC | Accuracy | Sensitivity | Specificity | AUC | Accuracy | Sensitivity | Specificity | |
| Control vs. PSD | ||||||||
| SVM_L | 0.690 | 0.600 | 0.748 | 0.557 | 0.706 | 0.636 | 0.775 | 0.542 |
| SVM_R | 0.708 | 0.646 | 0.681 | 0.495 | 0.711 | 0.700 | 0.742 | 0.517 |
| KNN | 0.659 | 0.538 | 0.743 | 0.352 | 0.681 | 0.579 | 0.742 | 0.425 |
| RF | 0.685 | 0.538 | 0.619 | 0.557 | 0.696 | 0.560 | 0.767 | 0.600 |
| VE | 0.675 | 0.615 | 0.676 | 0.552 | 0.646 | 0.650 | 0.708 | 0.517 |
| Imp vs. NoImp | ||||||||
| SVM_L | 0.830 | 0.771 | 0.600 | 0.883 | 0.797 | 0.775 | 0.650 | 0.950 |
| SVM_R | 0.496 | 0.648 | 0.267 | 0.817 | 0.722 | 0.708 | 0.300 | 0.800 |
| KNN | 0.635 | 0.681 | 0.300 | 0.950 | 0.674 | 0.742 | 0.300 | 0.850 |
| RF | 0.760 | 0.743 | 0.467 | 0.867 | 0.624 | 0.717 | 0.500 | 0.950 |
| VE | 0.784 | 0.743 | 0.533 | 0.867 | 0.747 | 0.733 | 0.450 | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, Y.H.; Kim, S.Y.; Kim, T.U.; Lee, S.J.; Park, S.J.; Jung, H.-Y.; Hyun, J.K. Prediction of Poststroke Depression Based on the Outcomes of Machine Learning Algorithms. J. Clin. Med. 2022, 11, 2264. https://doi.org/10.3390/jcm11082264
Ryu YH, Kim SY, Kim TU, Lee SJ, Park SJ, Jung H-Y, Hyun JK. Prediction of Poststroke Depression Based on the Outcomes of Machine Learning Algorithms. Journal of Clinical Medicine. 2022; 11(8):2264. https://doi.org/10.3390/jcm11082264
Chicago/Turabian StyleRyu, Yeong Hwan, Seo Young Kim, Tae Uk Kim, Seong Jae Lee, Soo Jun Park, Ho-Youl Jung, and Jung Keun Hyun. 2022. "Prediction of Poststroke Depression Based on the Outcomes of Machine Learning Algorithms" Journal of Clinical Medicine 11, no. 8: 2264. https://doi.org/10.3390/jcm11082264
APA StyleRyu, Y. H., Kim, S. Y., Kim, T. U., Lee, S. J., Park, S. J., Jung, H.-Y., & Hyun, J. K. (2022). Prediction of Poststroke Depression Based on the Outcomes of Machine Learning Algorithms. Journal of Clinical Medicine, 11(8), 2264. https://doi.org/10.3390/jcm11082264






