Monitoring of Anticoagulant Activity of Dabigatran and Rivaroxaban in the Presence of Heparins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plasma Samples
2.3. TT Test
2.4. Anti-Factor Xa Activity Assay
2.5. Statistical Analysis
3. Results
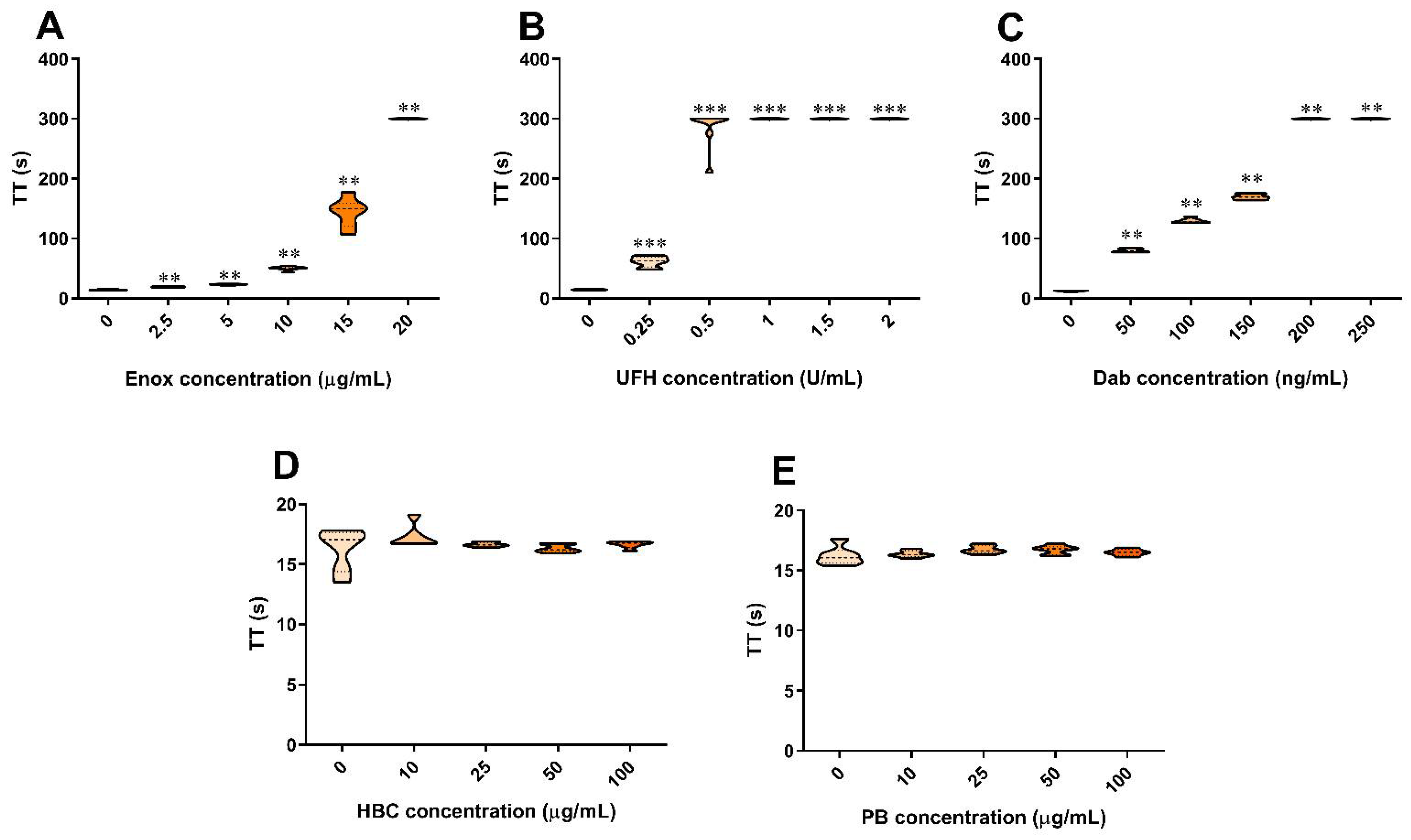
3.1. The Effect of Anticoagulants, HBC, and Polybrene on the TT Test in Human Plasma
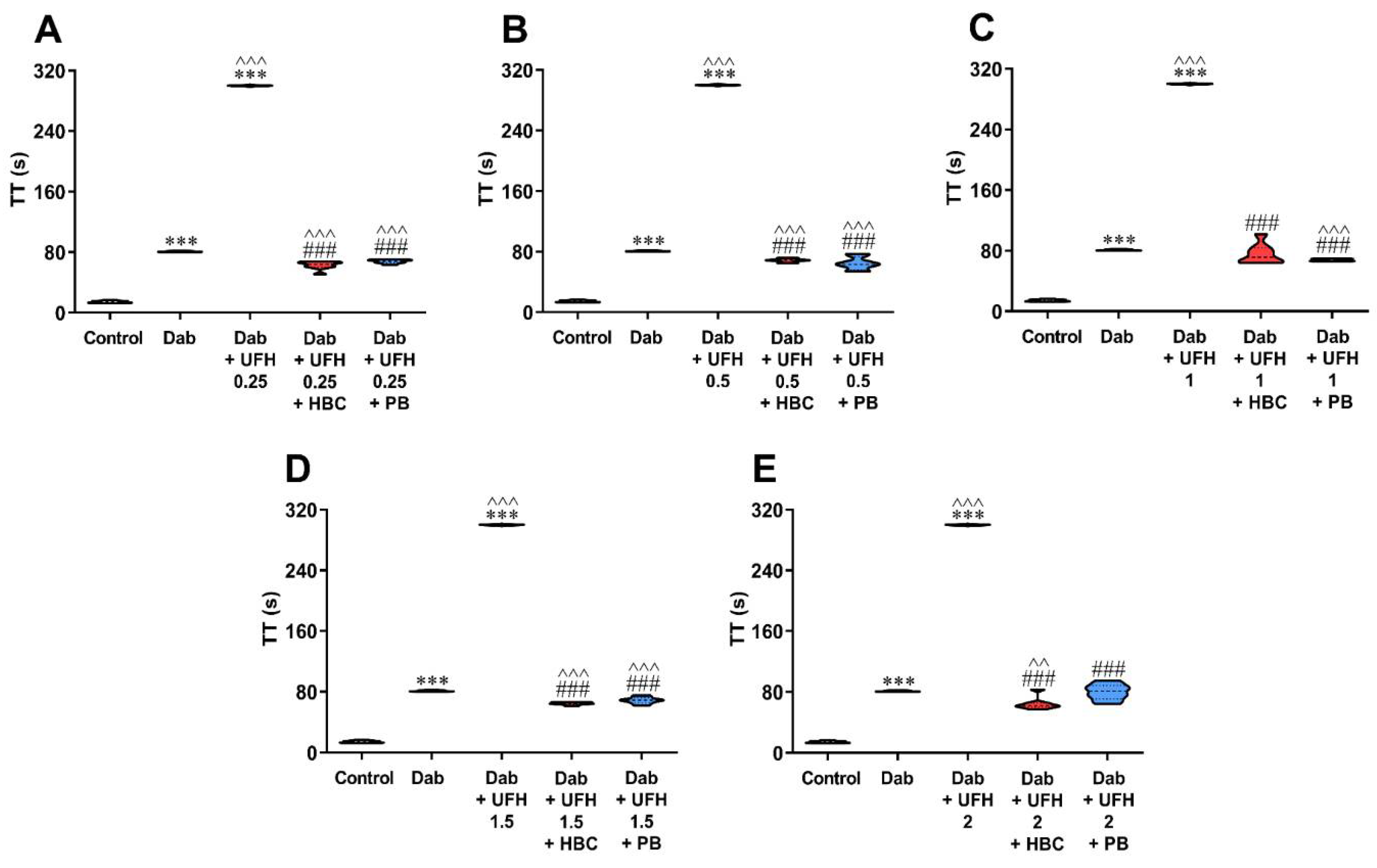
3.2. Inhibition of UFH by HBC or Polybrene in the Presence of Dabigatran in the TT Test Measured in Human Plasma
3.3. Inhibition of Enoxaparin by HBC or Polybrene in the Presence of Dabigatran in the TT Test Measured in Human Plasma
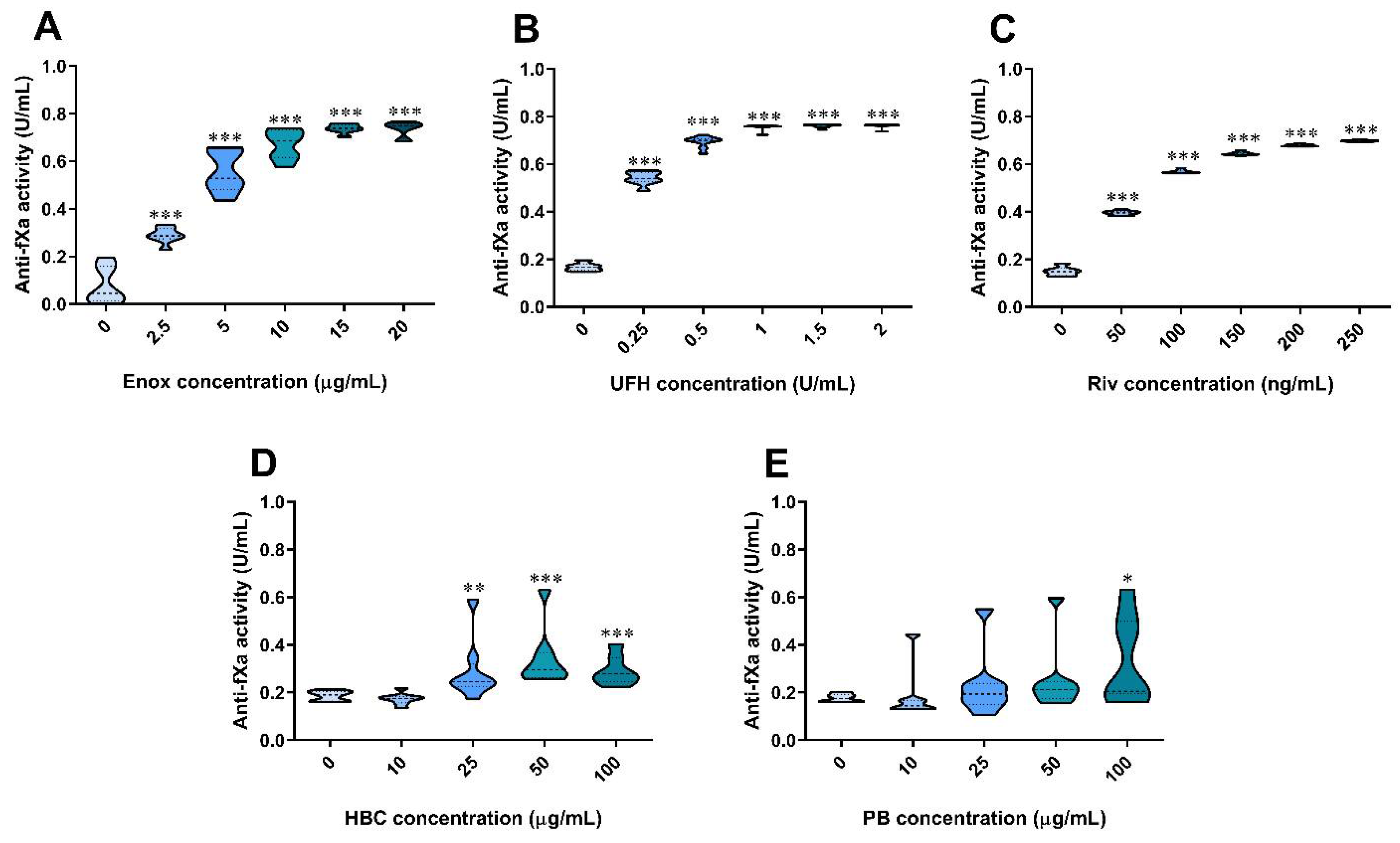
3.4. The Effect of Anticoagulants, HBC, and Polybrene on the Anti-Factor Xa Assay in Human Plasma
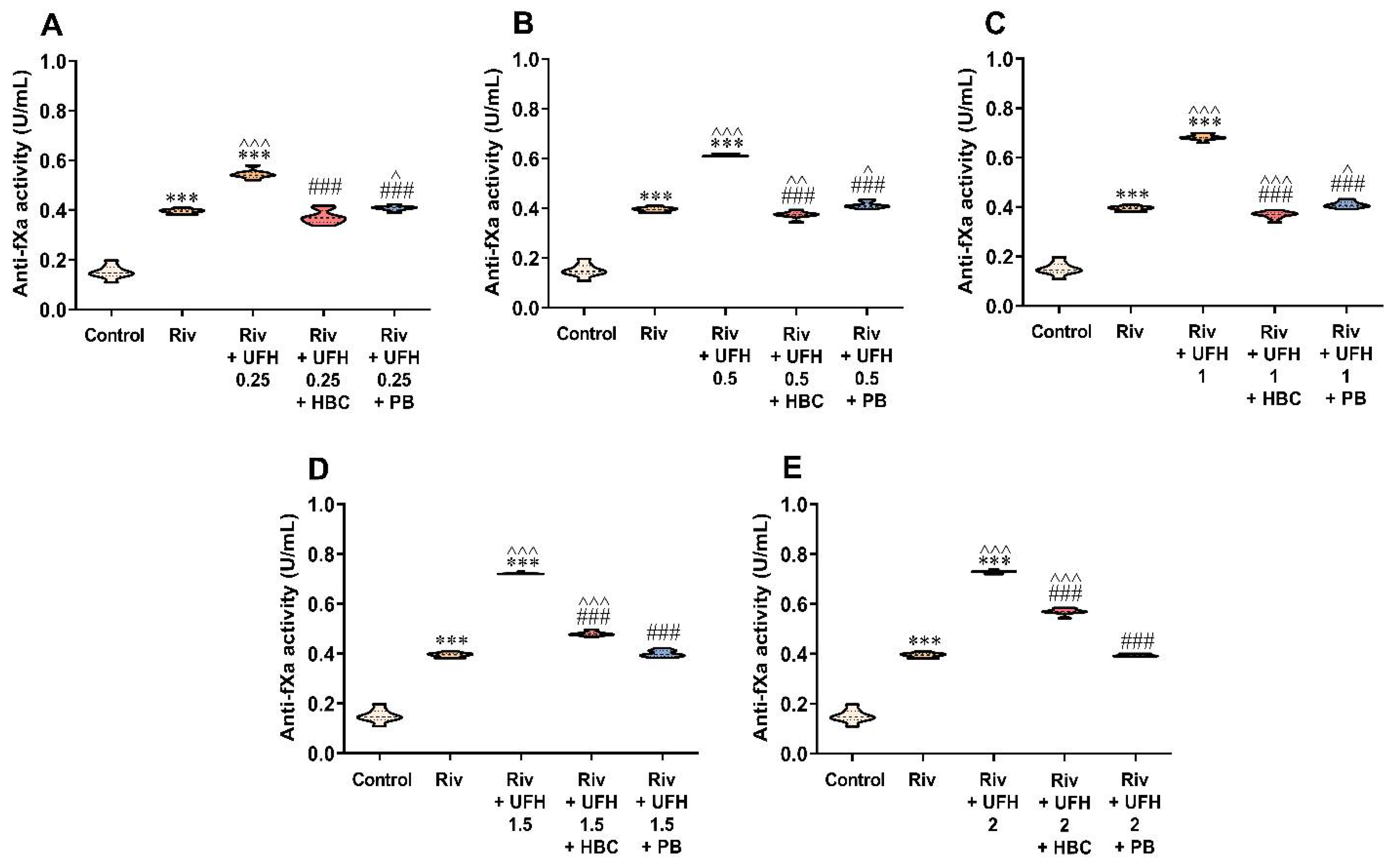
3.5. Inhibition of UFH by HBC or Polybrene in the Presence of Rivaroxaban in the Anti-Factor Xa Activity Test Measured in Human Plasma
3.6. Inhibition of Enoxaparin by HBC or Polybrene in the Presence of Rivaroxaban in the Anti-Factor Xa Activity Test Measured in Human Plasma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitchen, S.; Gray, E.; Mackie, I.; Baglin, T.; Makris, M.; BCSH committee. Measurement of Non-Coumarin Anticoagulants and Their Effects on Tests of Haemostasis: Guidance from the British Committee for Standards in Haematology. Br. J. Haematol. 2014, 166, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Tripodi, A.; Ageno, W.; Ciaccio, M.; Legnani, C.; Lippi, G.; Manotti, C.; Marcucci, R.; Moia, M.; Morelli, B.; Poli, D.; et al. Position Paper on Laboratory Testing for Patients on Direct Oral Anticoagulants. A Consensus Document from the SISET, FCSA, SIBioC and SIPMeL. Blood Transfus. 2018, 16, 462–470. [Google Scholar] [PubMed]
- Moner-Banet, T.; Alberio, L.; Bart, P.-A. Does One Dose Really Fit All? On the Monitoring of Direct Oral Anticoagulants: A Review of the Literature. Hamostaseologie 2020, 40, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Lessire, S.; Douxfils, J.; Pochet, L.; Dincq, A.-S.; Larock, A.-S.; Gourdin, M.; Dogné, J.-M.; Chatelain, B.; Mullier, F. Estimation of Rivaroxaban Plasma Concentrations in the Perioperative Setting in Patients With or Without Heparin Bridging. Clin. Appl. Thromb. Hemost. 2018, 24, 129–138. [Google Scholar] [CrossRef]
- da Silva, S.R.; Reichembach, M.T.; Pontes, L.; de Souza, G.d.P.e.S.C.M.; Kusma, S. Heparin Solution in the Prevention of Occlusions in Hickman® Catheters a Randomized Clinical Trial. Rev. Lat. Am. Enfermagem 2021, 29, 3385. [Google Scholar] [CrossRef]
- Jeon, M.; Han, A.; Kang, H.; Lee, K.-H.; Lee, J.-H.; Lee, J.-H. A Comparison of Coagulation Test Results from Heparinized Central Venous Catheter and Venipuncture. Blood Coagul. Fibrinolysis 2020, 31, 145–151. [Google Scholar] [CrossRef]
- Strickland, S.W.; Palkimas, S.; Acker, M.; Bazydlo, L.A.L. A Novel Laboratory Assay to Monitor Unfractionated Heparin Dosing in Patients Taking Apixaban Prior to Hospital Admission. J. Appl. Lab. Med. 2021, 6, 378–386. [Google Scholar] [CrossRef]
- Hu, A.; Niu, J.; Winkelmayer, W.C. Oral Anticoagulation in Patients With End-Stage Kidney Disease on Dialysis and Atrial Fibrillation. Semin. Nephrol. 2018, 38, 618–628. [Google Scholar] [CrossRef]
- Cini, M.; Legnani, C.; Testa, S.; Tripodi, A.; Cosmi, B.; Palareti, G. An in Vitro Study to Investigate the Interference of Enoxaparin on Plasma Levels of Direct Oral Factor Xa Inhibitors Measured by Chromogenic Assays. Int. J. Lab. Hematol. 2019, 41, 309–315. [Google Scholar] [CrossRef]
- Bauersachs, R.; Agnelli, G.; Gitt, A.K.; Monreal, M.; Mismetti, P.; Willich, S.N.; Laeis, P.; Fronk, E.-M.; Bramlage, P.; Cohen, A.T. The Role of Heparin Lead-in in the Real-World Management of Acute Venous Thromboembolism: The PREFER in VTE Registry. Thromb. Res. 2017, 157, 181–188. [Google Scholar] [CrossRef]
- Racanelli, A.; Hoppensteadt, D.; Fareed, J. In Vitro Protamine Neutralization Profiles of Heparins Differing in Source and Molecular Weight. Semin. Thromb. Hemost. 1989, 15, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Tientadakul, P.; Kongkan, C.; Chinswangwatanakul, W. Use of an Automated Coagulation Analyzer to Perform Heparin Neutralization with Polybrene in Blood Samples for Routine Coagulation Testing: Practical, Rapid, and Inexpensive. Arch. Pathol. Lab. Med. 2013, 137, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Hardy, M.; Michaux, I.; Lessire, S.; Douxfils, J.; Dogné, J.-M.; Bareille, M.; Horlait, G.; Bulpa, P.; Chapelle, C.; Laporte, S.; et al. Prothrombotic Hemostasis Disturbances in Patients with Severe COVID-19: Individual Daily Data. Data Brief 2020, 33, 106519. [Google Scholar] [CrossRef]
- Kikura, M.; Lee, M.K.; Levy, J.H. Heparin Neutralization with Methylene Blue, Hexadimethrine, or Vancomycin after Cardiopulmonary Bypass. Anesth. Analg. 1996, 83, 223–227. [Google Scholar] [CrossRef]
- Hardy, M.; Douxfils, J.; Morimont, L.; Didembourg, M.; Carlo, A.; de Maistre, E.; Lecompte, T.; Mullier, F. Study of in Vitro Thrombin Generation after Neutralization of Heparin. Int. J. Lab. Hematol. 2022, 44, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Sokolowska, E.; Kalaska, B.; Miklosz, J.; Mogielnicki, A. The Toxicology of Heparin Reversal with Protamine: Past, Present and Future. Expert Opin. Drug Metab. Toxicol. 2016, 12, 897–909. [Google Scholar] [CrossRef]
- Miklosz, J.; Kalaska, B.; Podlasz, P.; Chmielewska-Krzesińska, M.; Zajączkowski, M.; Kosiński, A.; Pawlak, D.; Mogielnicki, A. Cardiovascular and Respiratory Toxicity of Protamine Sulfate in Zebrafish and Rodent Models. Pharmaceutics 2021, 13, 359. [Google Scholar] [CrossRef]
- Miklosz, J.; Kalaska, B.; Kaminski, K.; Rusak, M.; Szczubialka, K.; Nowakowska, M.; Pawlak, D.; Mogielnicki, A. The Inhibitory Effect of Protamine on Platelets Is Attenuated by Heparin without Inducing Thrombocytopenia in Rodents. Marine Drugs 2019, 17, 539. [Google Scholar] [CrossRef]
- Jacobsen, E.M.; Trettenes, E.J.; Wisløff, F.; Abildgaard, U. Detection and Quantification of Lupus Anticoagulants in Plasma from Heparin Treated Patients, Using Addition of Polybrene. Thrombosis J. 2006, 4, 3. [Google Scholar] [CrossRef][Green Version]
- BIOPHEN™ DiXaI; REF No. 221030 [Online]; Hyphen BioMed: Neuville sur Oise, France, September 2018; Available online: https://www.endotell.ch/shop/mediafiles/pdf/Hyphen%20inserts%20E%20and%20FR/Heparin%20Kits%20E/221030_DiXaI_v6_2018-09%20EN.pdf (accessed on 20 February 2022).
- Benoit, R.; Nougier, C.; Desmurs-Clavel, H.; Simon, M.; Dargaud, Y. The Modification of the Thrombin Generation Assay for the Clinical Assessment of Hypercoagulability in Patients Receiving Heparin Therapy. Int. J. Lab. Hematol. 2022, 44, 371–378. [Google Scholar] [CrossRef]
- Kalaska, B.; Kaminski, K.; Miklosz, J.; Yusa, S.I.; Sokolowska, E.; Blazejczyk, A.; Wietrzyk, J.; Kasacka, I.; Szczubialka, K.; Pawlak, D.; et al. Heparin-binding copolymer reverses effects of unfractionated heparin, enoxaparin, and fondaparinux in rats and mice. Transl. Res. 2016, 177, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Kalaska, B.; Miklosz, J.; Kamiński, K.; Swieton, J.; Jakimczuk, A.; Yusa, S.-I.; Pawlak, D.; Nowakowska, M.; Szczubiałka, K.; Mogielnicki, A. Heparin-Binding Copolymer as a Complete Antidote for Low-Molecular-Weight Heparins in Rats. J. Pharmacol. Exp. Ther. 2020, 373, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Kalaska, B.; Kamiński, K.; Miklosz, J.; Nakai, K.; Yusa, S.-I.; Pawlak, D.; Nowakowska, M.; Mogielnicki, A.; Szczubiałka, K. Anticoagulant Properties of Poly(Sodium 2-(Acrylamido)-2-Methylpropanesulfonate)-Based Di- and Triblock Polymers. Biomacromolecules 2018, 19, 3104–3118. [Google Scholar] [CrossRef] [PubMed]
- Swieton, J.; Miklosz, J.; Yusa, S.-I.; Szczubialka, K.; Pawlak, D.; Mogielnicki, A.; Kalaska, B. Reversal Activity and Toxicity of Heparin-Binding Copolymer after Subcutaneous Administration of Enoxaparin in Mice. Int. J. Mol. Sci. 2021, 22, 11149. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, R.C.; Adcock, D.M.; Bates, S.M.; Douxfils, J.; Favaloro, E.J.; Gouin-Thibault, I.; Guillermo, C.; Kawai, Y.; Lindhoff-Last, E.; Kitchen, S. International Council for Standardization in Haematology (ICSH) Recommendations for Laboratory Measurement of Direct Oral Anticoagulants. Thromb. Haemost. 2018, 118, 437–450. [Google Scholar] [CrossRef]
- Douxfils, J.; Adcock, D.M.; Bates, S.M.; Favaloro, E.J.; Gouin-Thibault, I.; Guillermo, C.; Kawai, Y.; Lindhoff-Last, E.; Kitchen, S.; Gosselin, R.C. 2021 Update of the International Council for Standardization in Haematology Recommendations for Laboratory Measurement of Direct Oral Anticoagulants. Thromb. Haemost. 2021, 121, 1008–1020. [Google Scholar] [CrossRef]
- Paul, C.; Baby, M.; Anthraper, A.R. NOACs: An Emerging Class of Oral Anticoagulants-a Review Article. Future J. Pharm. Sci. 2020, 6, 95. [Google Scholar] [CrossRef]
- Dale, B.J.; Chan, N.C.; Eikelboom, J.W. Laboratory Measurement of the Direct Oral Anticoagulants. Br. J. Haematol. 2016, 172, 315–336. [Google Scholar] [CrossRef]
- Lessire, S.; Douxfils, J.; Baudar, J.; Bailly, N.; Dincq, A.-S.; Gourdin, M.; Dogné, J.-M.; Chatelain, B.; Mullier, F. Is Thrombin Time Useful for the Assessment of Dabigatran Concentrations? An in Vitro and Ex Vivo Study. Thromb. Res. 2015, 136, 693–696. [Google Scholar] [CrossRef]
- Dager, W.E.; Gosselin, R.C.; Kitchen, S.; Dwyre, D. Dabigatran Effects on the International Normalized Ratio, Activated Partial Thromboplastin Time, Thrombin Time, and Fibrinogen: A Multicenter, in Vitro Study. Ann. Pharmacother. 2012, 46, 1627–1636. [Google Scholar] [CrossRef]
- Agee, K.R.; Balk, R.A. Central Venous Catheterization in the Critically Ill Patient. Crit. Care Clin. 1992, 8, 677–686. [Google Scholar] [CrossRef]
- Gorski, L.A. The 2016 Infusion Therapy Standards of Practice. Home Healthc. Now 2017, 35, 10–18. [Google Scholar] [CrossRef] [PubMed]
- an Pelt, L.J.; Lukens, M.V.; Testa, S.; Chatelain, B.; Douxfils, J.; Mullier, F. The DaXa-Inhibition Assay: A Concept for a Readily Available, Universal AXa Assay That Measures the Direct Inhibitory Effect of All Anti-Xa Drugs. Thromb. Res. 2018, 168, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef]
- Koscielny, J.; von Heymann, C.; Bauersachs, R.; Mouret, P.; Antz, M. Perioperative Antikoagulation Mit NOAK Am Beispiel Rivaroxaban. MMW Fortschr. Med. 2017, 159, 18–23. [Google Scholar] [CrossRef]
- Guillet, B.; Simon, N.; Sampol, J.J.; Lorec-Penet, A.-M.; Portugal, H.; Berland, Y.; Dussol, B.; Brunet, P. Pharmacokinetics of the Low Molecular Weight Heparin Enoxaparin during 48 h after Bolus Administration as an Anticoagulant in Haemodialysis. Nephrol. Dial. Transplant. 2003, 18, 2348–2353. [Google Scholar] [CrossRef]
- Frydman, A. Low-Molecular-Weight Heparins: An Overview of Their Pharmacodynamics, Pharmacokinetics and Metabolism in Humans. Pathophysiol. Haemos. Thromb. 1996, 26, 24–38. [Google Scholar] [CrossRef]
- Brophy, D.F.; Carr, M.E.; Martin, E.J.; Venitz, J.; Gehr, T.W.B. The Pharmacokinetics of Enoxaparin Do Not Correlate with Its Pharmacodynamic Effect in Patients Receiving Dialysis Therapies. J. Clin. Pharmacol. 2006, 46, 887–894. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakimczuk, A.; Kalaska, B.; Kamiński, K.; Miklosz, J.; Yusa, S.-I.; Pawlak, D.; Szczubiałka, K.; Mogielnicki, A. Monitoring of Anticoagulant Activity of Dabigatran and Rivaroxaban in the Presence of Heparins. J. Clin. Med. 2022, 11, 2236. https://doi.org/10.3390/jcm11082236
Jakimczuk A, Kalaska B, Kamiński K, Miklosz J, Yusa S-I, Pawlak D, Szczubiałka K, Mogielnicki A. Monitoring of Anticoagulant Activity of Dabigatran and Rivaroxaban in the Presence of Heparins. Journal of Clinical Medicine. 2022; 11(8):2236. https://doi.org/10.3390/jcm11082236
Chicago/Turabian StyleJakimczuk, Aleksandra, Bartlomiej Kalaska, Kamil Kamiński, Joanna Miklosz, Shin-Ichi Yusa, Dariusz Pawlak, Krzysztof Szczubiałka, and Andrzej Mogielnicki. 2022. "Monitoring of Anticoagulant Activity of Dabigatran and Rivaroxaban in the Presence of Heparins" Journal of Clinical Medicine 11, no. 8: 2236. https://doi.org/10.3390/jcm11082236
APA StyleJakimczuk, A., Kalaska, B., Kamiński, K., Miklosz, J., Yusa, S.-I., Pawlak, D., Szczubiałka, K., & Mogielnicki, A. (2022). Monitoring of Anticoagulant Activity of Dabigatran and Rivaroxaban in the Presence of Heparins. Journal of Clinical Medicine, 11(8), 2236. https://doi.org/10.3390/jcm11082236







