An Elevated FIB-4 Score Is Associated with an Increased Incidence of Depression among Outpatients in Germany
Abstract
:1. Introduction
2. Materials and Methods
2.1. Database
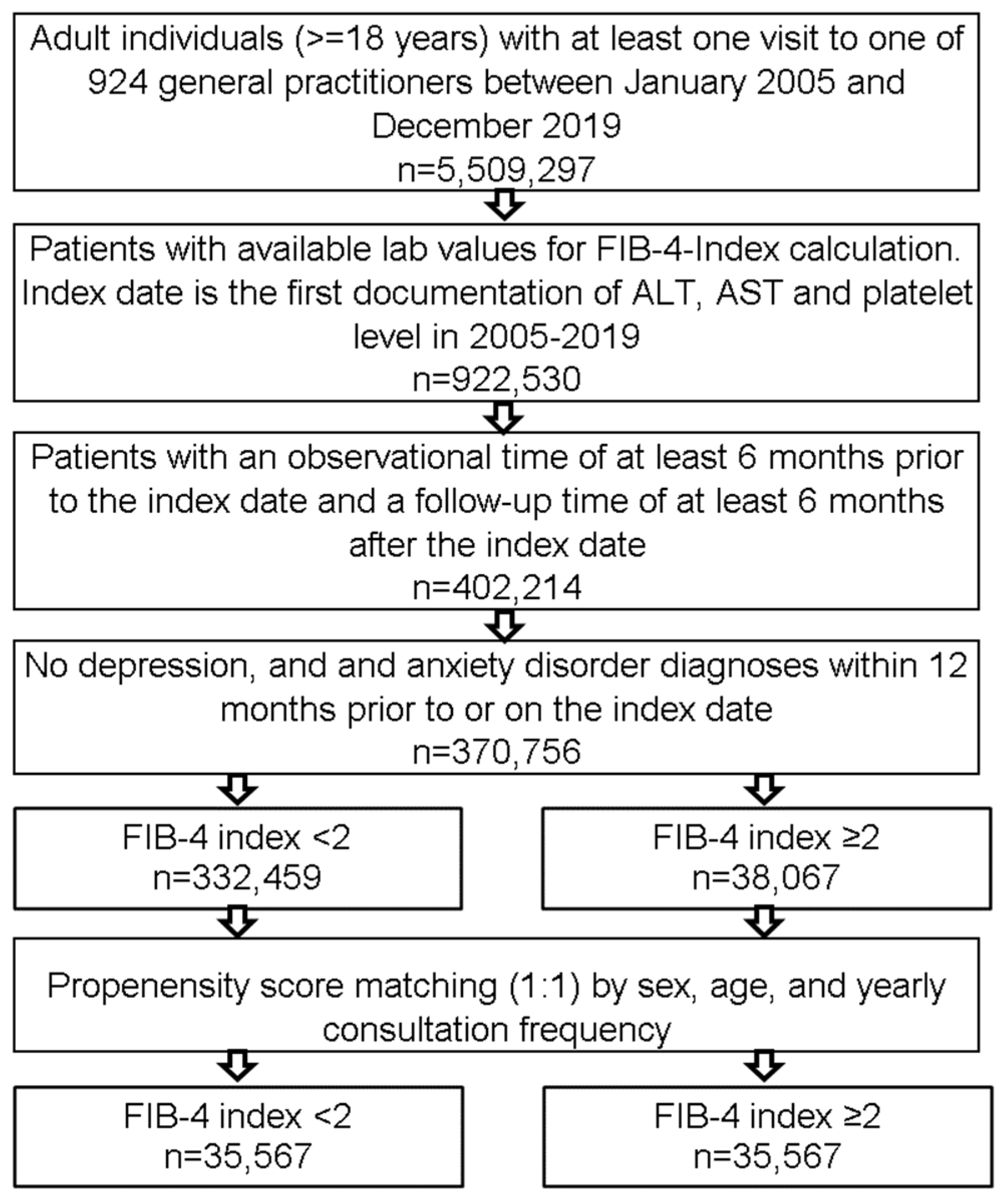
2.2. Study Population
2.3. Study Outcomes and Statistical Analyses
3. Results
3.1. Preliminary Analyses
3.2. Basic Characteristics of the Study Sample
3.3. A FIB-4 Score ≥2.0 Is Associated with an Increased Incidence of Depression
3.4. Association of FIB-4 Index ≥2.0 and Anxiety Disorders
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahl, K.G.; Krüger, T.; Eckermann, G.; Wedemeyer, H. Depressionen und Lebererkrankungen: Die Rolle von Mikrobiom und Inflammation. Fortschr. Neurol. Psychiatr. 2018, 87, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Boden, J.; Fergusson, D.M. Alcohol and depression. Addiction 2011, 106, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Ishino, R. Liver injury associated with antidepressants. Curr. Drug Saf. 2013, 8, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.N.; Abbassi, M.A.; Gandapur, A.; Alam, A.; Haroon, M.Z.; Hussain, J. Frequency of Depression Among Patients With Chronic Liver Disease. J. Ayub Med. Coll. Abbottabad. 2020, 32, 535–539. [Google Scholar]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.-J.; Fan, S.-H.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Peirce, J.M.; Alviña, K. The role of inflammation and the gut microbiome in depression and anxiety. J. Neurosci. Res. 2019, 97, 1223–1241. [Google Scholar] [CrossRef] [Green Version]
- D’Mello, C.; Swain, M.G. Immune-to-brain communication pathways in inflammation-associated sickness and depression. Curr. Top. Behav. Neurosci. 2016, 31, 73–94. [Google Scholar] [CrossRef]
- Lv, W.-J.; Wu, X.-L.; Chen, W.-Q.; Li, Y.-F.; Zhang, G.-F.; Chao, L.-M.; Zhou, J.-H.; Guo, A.; Liu, C.; Guo, S.-N. The Gut Microbiome Modulates the Changes in Liver Metabolism and in Inflammatory Processes in the Brain of Chronic Unpredictable Mild Stress Rats. Oxidative Med. Cell. Longev. 2019, 2019, 7902874. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Wu, X.; Hu, X.; Wang, T.; Jin, F. Recognizing Depression from the Microbiota–Gut–Brain Axis. Int. J. Mol. Sci. 2018, 19, 1592. [Google Scholar] [CrossRef] [Green Version]
- Foster, J.A.; McVey Neufeld, K.-A. Gut–brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Cramb, R.; Davison, S.M.; DIllon, J.F.; Foulerton, M.; Godfrey, E.M.; Hall, R.; Harrower, U.; Hudson, M.; Langford, A.; et al. Guidelines on the management of abnormal liver blood tests. Gut. 2018, 67, 6–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Gilman, S.E.; Sucha, E.; Kingsbury, M.; Horton, N.J.; Murphy, J.M.; Colman, I. Depression and mortality in a longitudinal study: 1952–2011. Can. Med. Assoc. J. 2017, 189, E1304–E1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathmann, W.; Bongaerts, B.; Carius, H.-J.; Kruppert, S.; Kostev, K. Basic characteristics and representativeness of the German Disease Analyzer database. Int. J. Clin. Pharmacol. Ther. 2018, 56, 459–466. [Google Scholar] [CrossRef]
- Loosen, S.H.; Kostev, K.; Keitel, V.; Tacke, F.; Roderburg, C.; Luedde, T. An elevated FIB-4 score predicts liver cancer development: A longitudinal analysis from 29,999 patients with NAFLD. J. Hepatol. 2021, 76, 247–248. [Google Scholar] [CrossRef]
- Loosen, S.H.; Roderburg, C.; Jahn, J.K.; Joerdens, M.; Luedde, T.; Kostev, K.; Luedde, M. Heart failure and depression: A comparative analysis with different types of cancer. Eur. J. Prev. Cardiol. 2022, 29, e112–e114. [Google Scholar] [CrossRef]
- Mössinger, H.; Kostev, K. Age effects on treatment patterns in 138,097 patients with unipolar depression followed in general practices in Germany. J. Psychiatr. Res. 2021, 144, 208–216. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.-C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2017 (GBD 2017) Results; Institute for Health Metrics and Evaluation (IHME): Seattle, WA, USA, 2018; Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 8 December 2021).
- Evans-Lacko, S.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Benjet, C.; Bruffaerts, R.; Chiu, W.T.; Florescu, S.; de Girolamo, G.; Gureje, O.; et al. Socio-economic variations in the mental health treatment gap for people with anxiety, mood, and substance use disorders: Results from the WHO World Mental Health (WMH) surveys. Psychol. Med. 2017, 48, 1560–1571. [Google Scholar] [CrossRef] [Green Version]
- Goldman, L.S.; Nielsen, N.H.; Champion, H.C. Awareness, diagnosis, and treatment of depression. J. Gen. Intern. Med. 1999, 14, 569–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feightner, J.W.; Worrall, G. Early detection of depression by primary care physicians. Can. Med. Assoc. J. 1990, 142, 1215–1220. [Google Scholar]
- Garland, J.; Solomons, K. Early detection of depression in young and elderly people. Br. Columbia Med. J. 2002, 44, 469–472. [Google Scholar]
- Picardi, A.; Lega, I.; Tarsitani, L.; Caredda, M.; Matteucci, G.; Zerella, M.; Miglio, R.; Gigantesco, A.; Cerbo, M.; Gaddini, A.; et al. A randomised controlled trial of the effectiveness of a program for early detection and treatment of depression in primary care. J. Affect. Disord. 2016, 198, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Richter, T.; Fishbain, B.; Richter-Levin, G.; Okon-Singer, H. Machine Learning-Based Behavioral Diagnostic Tools for Depression: Advances, Challenges, and Future Directions. J. Pers. Med. 2021, 11, 957. [Google Scholar] [CrossRef]
- Cacheda, F.; Fernandez, D.; Novoa, F.J.; Carneiro, V. Early Detection of Depression: Social Network Analysis and Random Forest Techniques. J. Med. Internet Res. 2019, 21, e12554. [Google Scholar] [CrossRef]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Kroenke, K.; Strine, T.W.; Spitzer, R.L.; Williams, J.B.; Berry, J.T.; Mokdad, A.H. The PHQ-8 as a measure of current depression in the general population. J. Affect. Disord. 2009, 114, 163–173. [Google Scholar] [CrossRef]
- Ding, J.-H.; Jin, Z.; Yang, X.-X.; Lou, J.; Shan, W.-X.; Hu, Y.-X.; Du, Q.; Liao, Q.-S.; Xie, R.; Xu, J.-Y. Role of gut microbiota via the gut-liver-brain axis in digestive diseases. World J. Gastroenterol. 2020, 26, 6141–6162. [Google Scholar] [CrossRef]
- Gupta, H.; Suk, K.T.; Kim, D.J. Gut Microbiota at the Intersection of Alcohol, Brain, and the Liver. J. Clin. Med. 2021, 10, 541. [Google Scholar] [CrossRef]
- Lee, J.S.; O’Connell, E.M.; Pacher, P.; Lohoff, F.W. PCSK9 and the Gut-Liver-Brain Axis: A Novel Therapeutic Target for Immune Regulation in Alcohol Use Disorder. J. Clin. Med. 2021, 10, 1758. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C. Imaging the role of inflammation in mood and anxiety-related disorders. Curr. Neuropharmacol. 2017, 16, 533–558. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, K.G.; Casolaro, C.; Brown, J.A.; Edwards, D.A.; Wikswo, J.P. The Microbiome and the Gut-Liver-Brain Axis for Central Nervous System Clinical Pharmacology: Challenges in Specifying and Integrating In Vitro and In Silico Models. Clin. Pharmacol. Ther. 2020, 108, 929–948. [Google Scholar] [CrossRef]
- Duan, Y.; Llorente, C.; Lang, S.; Brandl, K.; Chu, H.; Jiang, L.; White, R.C.; Clarke, T.H.; Nguyen, K.; Torralba, M.; et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019, 575, 505–511. [Google Scholar] [CrossRef]
- Du, Y.; Gao, X.-R.; Peng, L.; Ge, J.-F. Crosstalk between the microbiota-gut-brain axis and depression. Heliyon 2020, 6, e04097. [Google Scholar] [CrossRef]
- Long-Smith, C.; O’Riordan, K.J.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 477–502. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Shen, X.; Hao, Y.; Li, J.; Li, H.; Xu, H.; Yin, L.; Kuang, W. Gut Microbiome: A Potential Indicator for Differential Diagnosis of Major Depressive Disorder and General Anxiety Disorder. Front. Psychiatry 2021, 12, 1576. [Google Scholar] [CrossRef]
- Dennerstein, L.; Soares, C.N. The unique challenges of managing depression in mid-life women. World Psychiatry 2008, 7, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Unsal, A.; Tozun, M.; Ayranci, U. Prevalence of depression among postmenopausal women and related characteristics. Climacteric 2010, 14, 244–251. [Google Scholar] [CrossRef]
- Comijs, H.C.; Van Marwijk, H.W.; Van Der Mast, R.C.; Naarding, P.; Oude Voshaar, R.C.; Beekman, A.T.F.; Boshuisen, M.; Dekker, J.; Kok, R.; De Waal, M.W.M.; et al. The Netherlands study of depression in older persons (NESDO); A prospective cohort study. BMC Res. Notes 2011, 4, 524. [Google Scholar] [CrossRef] [PubMed]
- Siegmann, E.-M.; Müller, H.H.O.; Luecke, C.; Philipsen, A.; Kornhuber, J.; Grömer, T.W. Association of Depression and Anxiety Disorders With Autoimmune Thyroiditis. JAMA Psychiatry 2018, 75, 577–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Lu, C.; Li, W.; Huang, Y.; Chen, L. Impact of age on the diagnostic performances and cut-offs of APRI and FIB-4 for significant fibrosis and cirrhosis in chronic hepatitis B. Oncotarget 2017, 8, 45768–45776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Ruan, M.; Chen, J.; Fang, Y. Major Depressive Disorder: Advances in Neuroscience Research and Translational Applications. Neurosci. Bull. 2021, 37, 863–880. [Google Scholar] [CrossRef] [PubMed]
- Hyde, C.L.; Nagle, M.W.; Tian, C.; Chen, X.; Paciga, S.A.; Wendland, J.R.; Tung, J.Y.; Hinds, D.A.; Perlis, R.H.; Winslow, A.R. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat. Genet. 2016, 48, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Nobis, A.; Zalewski, D.; Waszkiewicz, N. Peripheral Markers of Depression. J. Clin. Med. 2020, 9, 3793. [Google Scholar] [CrossRef] [PubMed]
| FIB-4 Index Cut-Off Values | Incidence Rate Ratio (95% CI) | p-Value |
|---|---|---|
| Depression | ||
| ≥1.0 vs. <1.0 (n = 141,436) | 0.99 (0.96–1.02) | 0.641 |
| ≥1.3 vs. <1.3 (n = 128.902) | 1.03 (1.00–1.07) | 0.076 |
| ≥1.7 vs. <1.7 (n = 97,608) | 1.04 (1.00–1.08) | 0.039 |
| ≥2.0 vs. <2.0 (n = 71.134) | 1.12 (1.06–1.17) | <0.001 |
| Anxiety disorder | ||
| ≥1.0 vs. <1.0 (n = 141,436) | 0.94 (0.88–0.99) | 0.031 |
| ≥1.3 vs. <1.3 (n = 128.902) | 1.00 (0.94–1.07) | 0.968 |
| ≥1.7 vs. <1.7 (n = 97,608) | 1.10 (1.01–1.19) | 0.021 |
| ≥2.0 vs. <2.0 (n = 71.134) | 1.07 (0.98–1.18) | 0.138 |
| Variable | Proportion of Patients with FIB-4 < 2 (%) N = 35,567 | Proportion of Patients with FIB-4 ≥ 2 (%) N = 35,567 | p-Value |
|---|---|---|---|
| Age (Mean, SD) | 71.8 (11.8) | 71.9 (11.8) | 0.628 |
| Age ≤ 50 | 5.7 | 5.7 | 0.802 |
| Age 51–60 | 10.8 | 10.9 | |
| Age 61–70 | 22.0 | 21.8 | |
| Age 71–80 | 37.9 | 37.7 | |
| Age > 80 | 23.7 | 23.9 | |
| Women | 44.8 | 44.6 | 0.702 |
| Men | 55.2 | 55.4 | |
| Charlson comorbidity Index (Mean, SD) | 2.9 (2.9) | 2.8 (2.9) | 0.571 |
| Yearly consultation frequency during the follow-up time (Mean, SD) | 5.1 (5.9) | 5.0 (5.5) | 0.159 |
| Patients with FIB-4 <2; Incidence per 1000 Person-Years | Patients with FIB-4 ≥2; Incidence per 1000 Person-Years | Incidence Rate Ratio (FIB-4-Index ≥2.0 vs. <2.0) (95% CI) | p-Value | |
|---|---|---|---|---|
| Depression | ||||
| Total | 22.0 | 24.6 | 1.12 (1.06–1.17) | <0.001 |
| Age ≤ 50 | 22.3 | 31.7 | 1.42 (1.19–1.71) | <0.001 |
| Age 51–60 | 23.2 | 31.0 | 1.34 (1.18–1.51) | <0.001 |
| Age 61–70 | 18.0 | 18.9 | 1.05 (0.94–1.16) | 0.386 |
| Age 71–80 | 21.7 | 22.9 | 1.05 (0.98–1.14) | 0.178 |
| Age >80 | 28.3 | 29.9 | 1.06 (0.95–1.17) | 0.293 |
| Women | 29.1 | 32.1 | 1.10 (1.03–1.17) | 0.004 |
| Men | 16.9 | 19.4 | 1.15 (1.07–1.23) | <0.001 |
| Anxiety disorder | ||||
| Total | 5.4 | 5.8 | 1.07 (0.98–1.18) | 0.138 |
| Age ≤ 50 | 6.9 | 8.0 | 1.15 (0.83–1.60) | 0.390 |
| Age 51–60 | 5.1 | 6.6 | 1.31 (1.00–1.71) | 0.046 |
| Age 61–70 | 4.4 | 4.9 | 1.14 (0.93–1.39) | 0.224 |
| Age 71–80 | 5.5 | 5.6 | 1.02 (0.88–1.20) | 0.764 |
| Age >80 | 6.7 | 6.3 | 0.94 (0.76–1.16) | 0.576 |
| Women | 7.0 | 7.6 | 1.09 (0.96–1.24) | 0.182 |
| Men | 4.3 | 4.6 | 1.07 (0.93–1.23) | 0.371 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schöler, D.; Kostev, K.; Demir, M.; Luedde, M.; Konrad, M.; Luedde, T.; Roderburg, C.; Loosen, S.H. An Elevated FIB-4 Score Is Associated with an Increased Incidence of Depression among Outpatients in Germany. J. Clin. Med. 2022, 11, 2214. https://doi.org/10.3390/jcm11082214
Schöler D, Kostev K, Demir M, Luedde M, Konrad M, Luedde T, Roderburg C, Loosen SH. An Elevated FIB-4 Score Is Associated with an Increased Incidence of Depression among Outpatients in Germany. Journal of Clinical Medicine. 2022; 11(8):2214. https://doi.org/10.3390/jcm11082214
Chicago/Turabian StyleSchöler, David, Karel Kostev, Münevver Demir, Mark Luedde, Marcel Konrad, Tom Luedde, Christoph Roderburg, and Sven H. Loosen. 2022. "An Elevated FIB-4 Score Is Associated with an Increased Incidence of Depression among Outpatients in Germany" Journal of Clinical Medicine 11, no. 8: 2214. https://doi.org/10.3390/jcm11082214
APA StyleSchöler, D., Kostev, K., Demir, M., Luedde, M., Konrad, M., Luedde, T., Roderburg, C., & Loosen, S. H. (2022). An Elevated FIB-4 Score Is Associated with an Increased Incidence of Depression among Outpatients in Germany. Journal of Clinical Medicine, 11(8), 2214. https://doi.org/10.3390/jcm11082214









