Contrast-Associated Acute Kidney Injury
Abstract
1. Introduction
2. Epidemiology
3. Definition and Diagnosis
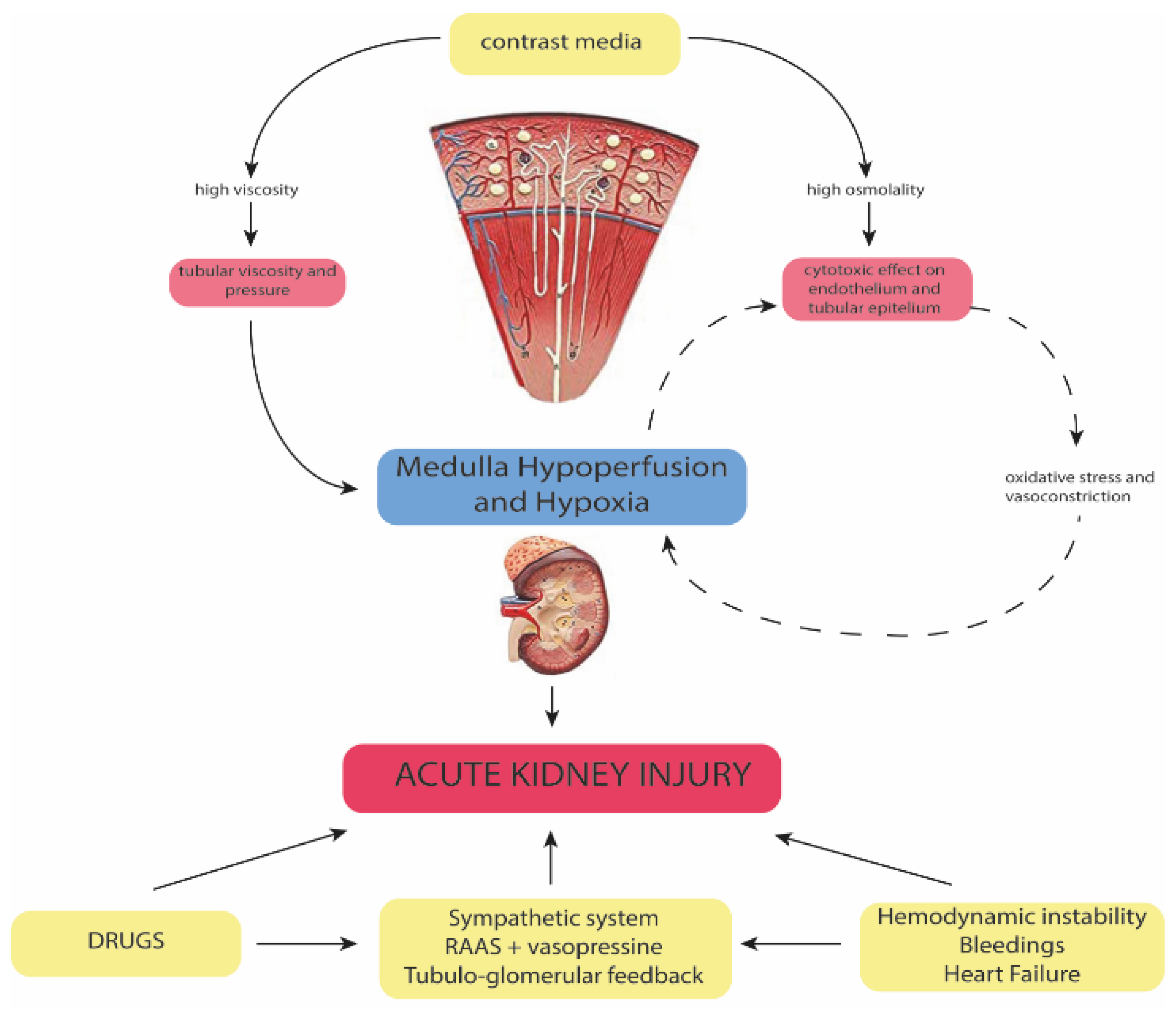
4. Pathophysiology
5. Clinical Implications
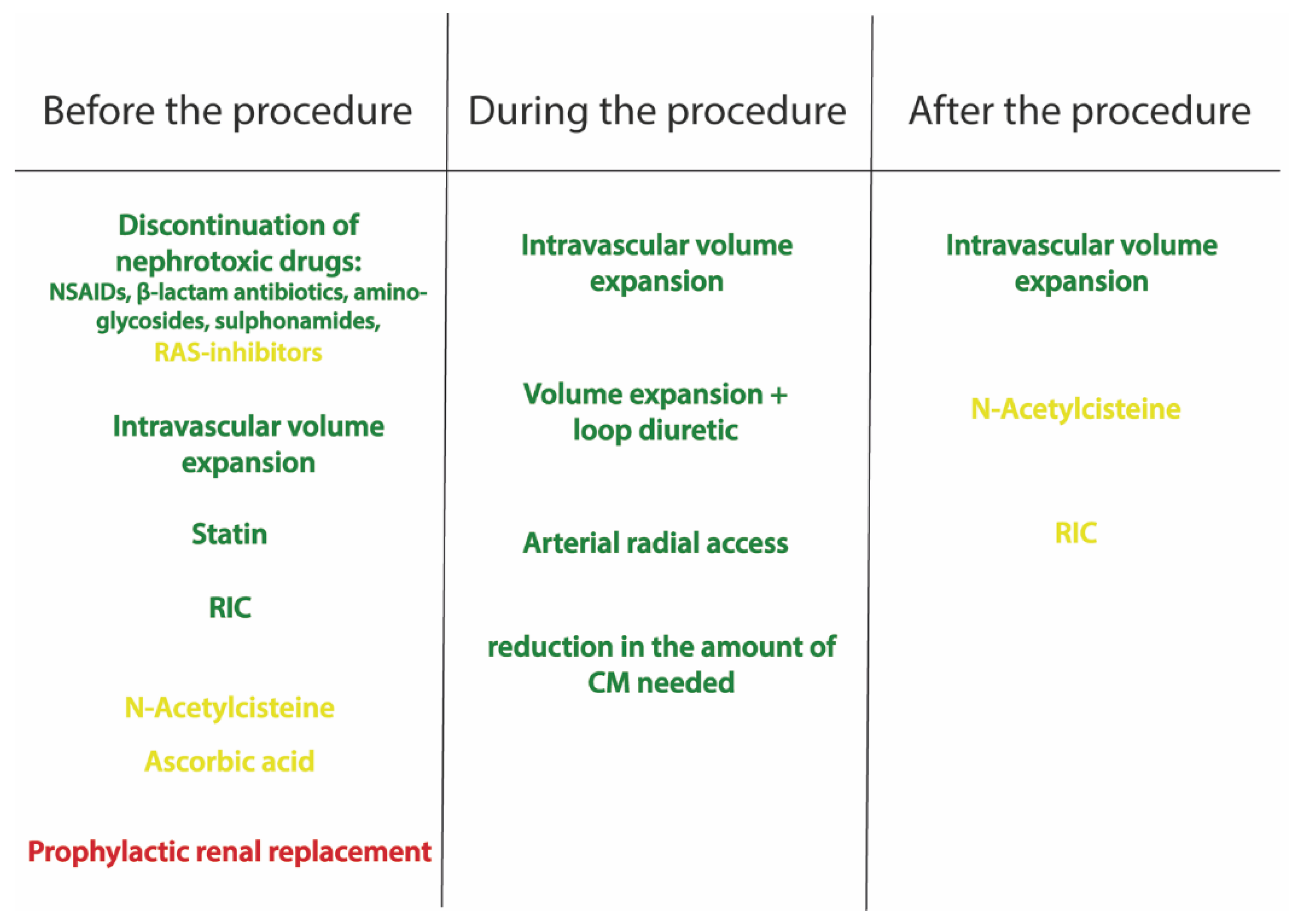
6. Prevention and Management
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- James, M.T.; Samuel, S.M.; Manning, M.A.; Tonelli, M.; Ghali, W.A.; Faris, P.; Knudtson, M.L.; Pannu, N.; Hemmelgarn, B.R. Contrast-induced acute kidney injury and risk of adverse clinical outcomes after coronary angiography: A systematic review and meta-analysis. Circ. Cardiovasc. Interv. 2013, 6, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kini, A.S.; Sarkar, K.; Rafael, O.C.; Jakkula, M.; Kaplish, D.; Lee, P.; Suleman, J.; Krishnan, P.; Kim, M.C.; Sharma, S.K. Serum creatinine ratio: A novel predictor of mortality after percutaneous coronary intervention in patients with normal and abnormal renal function. Catheter. Cardiovasc. Interv. 2009, 74, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Dangas, G.; Iakovou, I.; Nikolsky, E.; Aymong, E.D.; Mintz, G.S.; Kipshidze, N.N.; Lansky, A.J.; Moussa, I.; Stone, G.W.; Moses, J.W.; et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am. J. Cardiol. 2005, 95, 13–19. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; McDonald, J.S.; Bida, J.P.; Carter, R.W.; Fleming, C.J.; Misra, S.; Williamson, E.E.; Kallmse, D.F. Intravenous contrast material-induced nephropathy: Causal or coincident phenomenon? Radiology 2013, 267, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, M.R.; Berns, J.S.; Cohen, R.M.; Goldfarb, S. Nephrotoxic risks of renal angiography: Contrast media-associated nephrotoxicity and atheroembolism—A critical review. Am. J. Kidney Dis. 1994, 24, 713–727. [Google Scholar] [CrossRef]
- Weisbord, S.D.; Mor, M.K.; Resnick, A.L.; Hartwig, K.C.; Sonel, A.F.; Fine, M.J.; Palevsky, P.M. Prevention, incidence, and outcomes of contrast-induced acute kidney injury. Arch. Intern. Med. 2008, 168, 1325–1332. [Google Scholar] [CrossRef]
- Tsai, T.T.; Patel, U.D.; Chang, T.I.; Kennedy, K.F.; Masoudi, F.A.; Matheny, M.E.; Kosiborod, M.; Amin, A.p.; Messenger, J.C.; Rumsfeld, J.S.; et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: Insights from the NCDR Cath-PCI Registry. J. Am. Coll. Cardiol. Intv. 2014, 7, 1–9. [Google Scholar] [CrossRef]
- Solomon, R.; Dauerman, H.L. Contrast-induced acute kidney injury. Circulation 2010, 122, 2451–2455. [Google Scholar] [CrossRef]
- McCullough, P.A.; Adam, A.; Becker, C.R.; Davidson, C.; Lameire, N.; Stacul, F.; Tumlin, J.; CIN Consensus Working Panel. Epidemiology and prognostic implications of contrast-induced nephropathy. Am. J. Cardiol. 2006, 98, 5k–13k. [Google Scholar] [CrossRef]
- McCullough, P.A.; Wolyn, R.; Rocher, L.L.; Levin, R.N.; O’Neill, W.W. Acute renal failure after coronary intervention: Incidence, risk factors, and relationship to mortality. Am. J. Med. 1997, 103, 368–375. [Google Scholar] [CrossRef]
- Stacul, F.; van der Molen, A.J.; Reimer, P.; Webb, J.A.W.; Thomsen, H.S.; Morcos, S.K.; Almén, T.; Aspelin, P.; Bellin, M.F.; Clement, O.; et al. Contrast induced nephropathy: Updated ESUR contrast media safety committee guidelines. Eur. Radiol. 2011, 21, 2527–2541. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Maekawa, Y.; Miyata, H.; Inoue, S.; Ishikawa, S.; Sueyoshi, K.; Noma, S.; Kawamura, A.; Kohsaka, S.; Fukuda, K.; et al. Impact of periprocedural bleeding on incidence of contrast induced acute kidney injury in patients treated with percutaneous coronary intervention. J. Am. Coll. Cardiol. 2013, 62, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Mandurino-Mirizzi, A.; Kajana, V.; Cornara, S.; Somaschini, A.; Demarchi, A.; Galazzi, M.; Crimi, G.; Ferlini, M.; Camporotondo, R.; Gnecchi, M.; et al. Elevated serum uric acid is a predictor of contrast associated acute kidney injury in patient with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Nutr. Met. Cardiovasc. Dis. 2021, 31, 2140–2143. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, M.R.; Goldfarb, S.; Wexler, L.; Ludbrook, P.A.; Murphy, M.J.; Halpern, E.F.; Hill, J.A.; Winniford, M.; Cohen, M.B.; VanFossen, D.B. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: A randomized trial: The Iohexol Cooperative Study. Kidney Int. 1995, 47, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.; Poh, K.K.; Liang, S.; Soon, C.Y.; Tan, H.C. Comparison of risksand clinical predictors of contrast-induced nephropathy in patients undergoing emergency versus nonemergency percutaneous coronaryinterventions. J. Interv. Cardiol. 2010, 23, 451–459. [Google Scholar] [CrossRef]
- Owen, R.J.; Hiremath, S.; Myers, A.; Fraser-Hill, M.; Barrett, B.J. Canadian association of radiologists consensus guidelines for the prevention ofcontrast-induced nephropathy: Update 2012. Can. Assoc. Radiol. J. 2014, 65, 96–105. [Google Scholar] [CrossRef]
- Seeliger, E.; Sendeski, M.; Rihal, C.S.; Persson, P.B. Contrast-induced kidney injury: Mechanisms, risk factors, and prevention. Eur. Heart J. 2012, 33, 2007–2015. [Google Scholar] [CrossRef]
- Marenzi, G.; Lauri, G.; Assanelli, E.; Campodonico, J.; De Metrio, M.; Marana, I.; Grazi, M.; Veglia, F.; Bartorelli, A.L. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J. Am. Coll. Cardiol. 2004, 44, 1780–1785. [Google Scholar] [CrossRef]
- Mehran, R.; Aymong, E.D.; Nikolsky, E.; Lasic, Z.; Iakovou, I.; Fahy, M.; Mintz, G.S.; Lansky, A.J.; Moses, J.W.; Stone, G.W.; et al. A simple riskscore for prediction of contrast-induced nephropathy after percutaneous coronary intervention: Development and initial validation. J. Am. Coll. Cardiol. 2004, 44, 1393–1399. [Google Scholar]
- Bartholomew, B.A.; Harjai, K.J.; Dukkipati, S.; Boura, J.A.; Yerkey, M.W.; Glazier, S.; Grines, C.L.; O’Neill, W.W. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am. J. Cardiol. 2004, 93, 1515–1519. [Google Scholar] [CrossRef]
- Tziakas, D.; Chalikias, G.; Stakos, D.; Apostolakis, S.; Adina, T.; Kikas, P.; Alexoudis, A.; Passadakis, P.; Thodis, E.; Vargemezis, V.; et al. Development ofan easily applicable risk score model for contrast-induced nephropathy prediction after percutaneous coronary intervention: A novel approach tailored to current practice. Int. J. Cardiol. 2013, 163, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.M.; Li, D.; Cheng, H.; Chen, Y.P. Derivation and validation of a risk score for contrast-induced nephropathy after cardiac catheterization in Chinese patients. Clin. Exp. Nephrol. 2014, 18, 892–898. [Google Scholar] [CrossRef] [PubMed]
- Gurm, H.S.; Seth, M.; Kooiman, J.; Share, D. A novel tool for reliable and accurate prediction of renal complications in patients undergoing percutaneous coronary intervention. J. Am. Coll. Cardiol. 2013, 61, 2242–2248. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.T.; Patel, U.D.; Chang, T.I.; Kennedy, K.F.; Masoudi, F.A.; Matheny, M.E.; Kosiborod, M.; Amin, A.P.; Weintraub, W.S.; Curtis, J.P.; et al. Validated contemporary risk model of acute kidney injury in patients undergoing percutaneous coronary interventions: Insights from the National Cardiovascular Data Registry Cath-PCI Registry. J. Am. Heart Assoc. 2014, 3, e001380. [Google Scholar] [CrossRef] [PubMed]
- Silver, S.A.; Shah, P.M.; Chertow, G.M.; Harel, S.; Wald, R.; Harel, Z. Risk prediction models for contrast induced nephropathy: Systematic review. BMJ 2015, 351, 4395. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Ruth Owen, R.; Chiarito, M.; Baber, U.; Sartori, S.; Cao, D.; Nicolas, J.; Pivato, C.A.; Nardin, M.; Krishnan, P.; et al. A contemporary simple risk score for prediction of contrast-associated acute kidney injury after percutaneous coronary intervention: Derivation and validation from an observational registry. Lancet 2021, 398, 1974–1983. [Google Scholar] [CrossRef]
- McCullough, P.A. Radiocontrast-induced acute kidney injury. Nephron Physiol. 2008, 109, 61–72. [Google Scholar] [CrossRef]
- McCullough, P.A. Contrast-induced acute kidney injury. J. Am. Coll. Cardiol. 2008, 51, 1419–1428. [Google Scholar] [CrossRef]
- Thomsen, H.S. Guidelines for contrast media from the European Society of Urogenital Radiology. AJR Am. J. Roentgenol. 2003, 181, 1463–1471. [Google Scholar] [CrossRef]
- Fliser, D.; Laville, M.; Covic, A.; Fouque, D.; Vanholder, R.; Juillard, L.; Van Biesen, W.; Ad-hoc working group of ERBP. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: Part 1, Definitions, conservative management and contrast-induced nephropathy. Nephrol. Dial. Transplant. 2012, 27, 4263–4272. [Google Scholar]
- Solomon, R.; Deray, G. How to prevent contrast-induced nephropathy and manage risk patients: Practical recommendations. Kidney Int. Suppl. 2006, 100, S51–S53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Slocum, N.K.; Grossman, P.M.; Moscucci, M.; Smith, D.S.; Aronow, H.D.; Dixon, S.R.; Share, D.; Gurm, H.S. The changing definition of contrast induced nephropathy and its clinical implications: Insights from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2). Am. Heart J. 2012, 163, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Budano, C.; Levis, M.; D’Amico, M.; Usmiani, T.; Fava, A.; Sbarra, P.; Burdese, M.; Segoloni, G.P.; Colombo, A.; Marra, S. Impact of contrast-induced acute kidney injury definition on clinical outcomes. Am. Heart J. 2011, 161, 963–971. [Google Scholar] [CrossRef]
- Moran, S.M.; Myers, B.D. Course of acute renal failure studied by a model of creatinine kinetics. Kidney Int. 1985, 27, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Waikar, S.S.; Bonventre, J.V. Creatinine kinetics and the definition of acute kidney injury. J. Am. Soc. Nephrol. 2009, 20, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Fernandez, H.; Shashaty, M.G.; Negoianu, D.; Testani, J.M.; Berns, J.S.; Parikh, C.R.; Wilson, F.P. False-positive rate of AKI using consensus creatinine-based criteria. Clin. J. Am. Soc. Nephrol. 2015, 10, 1723–1731. [Google Scholar] [CrossRef]
- Haase, M.; Bellomo, R.; Devarajan, P.; Schlattmann, P.; Haase-Fielitz, A.; NGAL Meta-analysis Investigator Group. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: A systematic review and meta-analysis. Am. J. Kidney Dis. 2009, 54, 1012–1024. [Google Scholar] [CrossRef]
- Maisel, A.S.; Wettersen, N.; van Veldhuisen, D.J.; Mueller, C.; Filippatos, G.; Nowak, R.; Hogan, C.; Kontos, M.C.; Cannon, C.M.; Muller, G.A.; et al. Neutrophil gelatinase-associated lipocalin for acute kidney injury during acute heart failure hospitalizations: The AKINESIS study. J. Am. Coll. Cardiol. 2016, 68, 1420–1431. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Matsushita, K.; Arnlov, J.; Inker, L.A.; Katz, R.; Polkinghorne, K.R.; Rothenbacher, D.; Sarnak, M.J.; Astor, B.C.; Coresh, J.; et al. Cystatin Cversus creatinine in determining risk based on kidney function. N. Engl. J. Med. 2013, 369, 932–943. [Google Scholar] [CrossRef]
- Sendeski, M.M. The pathophysiology of renal tissue damage by iodinated contrast media. Clin. Exp. Pharmacol. Physiol. 2011, 38, 292–299. [Google Scholar] [CrossRef]
- Thomsen, H.S.; Morcos, S.K.; Barrett, B.J. Contrast-induced nephropathy: The wheel has turned 360 degrees. Acta Radiol. 2008, 49, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Hardiek, K.; Katholi, R.E.; Ramkumar, V.; Deitrick, C. Proximal tubule cell response to radiographic contrast media. Am. J. Physiol. Renal Physiol. 2001, 280, F61–F70. [Google Scholar] [CrossRef] [PubMed]
- Katholi, R.E.; Taylor, G.J.; McCann, W.P.; Wookds, W.T.; Womack, K.A.; McCoy, C.D.; Katholi, C.R.; Moses, H.W.; Mishkel, G.J.; Lucore, C.L.; et al. Nephrotoxicity from contrast media: Attenuation with theophylline. Radiology 1995, 195, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Schnackenberg, C.G. Physiological and pathophysiological roles of oxygen radicals in the renal microvasculature. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R335–R342. [Google Scholar] [CrossRef] [PubMed]
- Sendeski, M.; Patzak, A.; Pallone, T.L.; Cao, C.; Persson, A.E.; Persson, P.B. Iodixanol, constriction of medullary descending vasa recta, and risk for contrast medium-induced nephropathy. Radiology 2009, 251, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, E.; Flemming, B.; Wronski, T.; Ladwig, M.; Arakelyan, K.; Godes, M.; Mockel, M.; Persson, P.B. Viscosity of contrast media perturbs renal hemodynamics. J. Am. Soc. Nephrol. 2007, 18, 2912–2920. [Google Scholar] [CrossRef]
- Bartorelli, A.L.; Marenzi, G. Contrast-induced nephropathy. J. Interv. Cardiol. 2008, 21, 74–85. [Google Scholar] [CrossRef]
- Schrier, R.W.; Wang, W. Acute renal failure and sepsis. N. Engl. J. Med. 2004, 351, 159–169. [Google Scholar] [CrossRef]
- Bentley, M.L.; Corwin, H.L.; Dasta, J. Drug-induced acute kidney injury in the critically ill adult: Recognition and prevention strategies. Crit. Care Med. 2010, 38, S169–S174. [Google Scholar] [CrossRef]
- Kiss, N.; Hamar, P. Histopathological evaluation of contrast-induced acute kidney injury rodent models. Biomed Res. Int. 2016, 3763, 250. [Google Scholar] [CrossRef]
- Crimi, G.; Leonardi, S.; Costa, F.; Ariotti, S.; Tebaldi, M.; Biscaglia, S.; Valgimigli, M. Incidence, prognostic impact, and optimal definition of contrast-induced acute kidney injury in consecutive patients with stable or unstable coronary artery disease undergoing percutaneous coronary intervention insights from the all-comer PRODIGY trial. Catheter. Cardiovasc. Interv. 2015, 86, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, N.; Iwasaki, M.; Nakanishi, M.; Araki, T.; Utsunomiya, M.; Hori, M.; Ikeda, N.; Makino, K.; Itaya, H.; Iijima, R.; et al. Impact of continuous deterioration of kidney function 6 to 8 months after percutaneous coronary intervention for acute coronary syndrome. Am. J. Cardiol. 2014, 113, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Wickenbrock, I.; Perings, C.; Maagh, P.; Quack, I.; Van Bracht, M.; Prull, M.W.; Plehn, G.; Trappe, H.J.; Meissner, A. Contrast medium induced nephropathy in patients undergoing percutaneous coronary intervention for acute coronary syndrome: Differences in STEMI and NSTEMI. Clin. Res. Cardiol. 2009, 98, 765–772. [Google Scholar] [CrossRef][Green Version]
- Coca, S.G.; Zabetian, A.; Ferket, B.S.; Zhou, J.; Testani, J.M.; Garg, A.X.; Parikh, C.R. Evaluation of short-term changes in serum creatinine level as a meaningful end point in randomized clinical trials. J. Am. Soc. Nephrol. 2016, 27, 2529–2542. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa, U.V.A.M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization: The task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [PubMed]
- Fried, L.F.; Duckworth, W.; Zhang, J.H.; O’Connor, T.; Brophy, M.; Emanuele, N.; Huang, G.D.; McCullough, P.A.; Palevsky, P.A.; Seliger, S.; et al. Design of combination angiotensin receptor blocker and angiotensin converting enzyme inhibitor for treatment of diabetic nephropathy (VA NEPHRON-D). Clin. J. Am. Soc. Nephrol. 2009, 4, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Parving, H.H.; Brenner, B.M.; McMurray, J.J.; De Zeeuw, D.; Haffner, S.M.; Solomon, S.D.; Chaturvedi, N.; Persson, F.; Desai, A.S.; Nicolaides, M.; et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N. Engl. J. Med. 2012, 367, 2204–2213. [Google Scholar] [CrossRef]
- Bainey, K.R.; Rahim, S.; Etherington, K.; Rokoss, M.L.; Natarajan, M.K.; Velianou, J.L.; Brons, S.; Mehta, S.R.; CAPTAIN Investigators. Effects of withdrawing vs continuing renin-angiotensin blockers on incidence of acute kidney injury in patients with renal insufficiency undergoing cardiac catheterization: Results from the angiotensin converting enzyme inhibitor/angiotensin receptor blocker and contrast induced nephropathy in patients receiving cardiac catheterization (CAPTAIN) trial. Am. Heart J. 2015, 170, 110–116. [Google Scholar]
- Solomon, R.; Werner, C.; Mann, D.; D’Elia, J.; Silva, P. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N. Engl. J. Med. 1994, 331, 1416–1420. [Google Scholar] [CrossRef]
- Trivedi, H.S.; Moore, H.; Nasr, S.; Aggarwal, K.; Agrawal, A.; Goel, P.; Hewett, J. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron. Clin. Pract. 2003, 93, C29–C34. [Google Scholar] [CrossRef]
- Mueller, C.; Buerkle, G.; Buettner, H.J.; Petersen, J.; Perruchoud, A.P.; Eriksson, U.; Marsch, S.; Roskamm, H. Prevention of contrast media-associated nephropathy: Randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch. Intern. Med. 2002, 162, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Brar, S.S.; Aharonian, V.; Mansukhani, P.; Moore, N.; Shen, A.Y.J.; Jorgensen, M.; Dua, A.; Short, L.; Kane, K. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: The POSEIDON randomized controlled trial. Lancet 2014, 383, 1814–1823. [Google Scholar] [CrossRef]
- Qian, G.; Fu, Z.; Guo, J.; Cao, F.; Chen, Y. Prevention of contrast-induced nephropathy by central venous pressure-guided fluid administration in chronic kidney disease and congestive heart failure patients. JACC Cardiovasc. Interv. 2016, 9, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Solomon, R.; Gordon, P.; Manoukian, S.V.; Abbott, J.D.; Kereiakes, D.J.; Jeremias, A.; Kim, M.; Dauerman, H.L.; BOSS Trial Invetigators. Randomized trial of bicarbonate or saline study for the prevention of contrast-induced nephropathy in patients with CKD. Clin. J. Am. Soc. Nephrol. 2015, 10, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Brar, S.S.; Shen, A.Y.; Jorgensen, M.B.; Kotlewski, A.; Aharonian, V.J.; Desai, N.; Ree, M.; Shah, A.I.; Burchette, R.J. Sodium bicarbonate vs. sodium chloride for the prevention of contrast medium-induced nephropathy in patients undergoing coronary angiography: A randomized trial. JAMA 2008, 300, 1038–1046. [Google Scholar] [CrossRef]
- Weisbord, S.D.; Gallagher, M.; Jneid, H.; Garcia, S.; Cass, A.; Thwin, S.S.; Connor, T.A.; Chertow, G.M.; Bhatt, D.L.; Shunk, K.; et al. Outcomes after angiography with sodium bicarbonate and acetylcysteine. N. Engl. J. Med. 2018, 378, 603–614. [Google Scholar] [CrossRef]
- Weisbord, S.D.; Palevsky, P.M. Strategies for the prevention of contrast-induced acute kidney injury. Curr. Opin. Nephrol. Hypertens. 2010, 19, 539–549. [Google Scholar] [CrossRef]
- Briguori, C.; Visconti, G.; Focaccio, A.; Airoldi, F.; Valgimigli, M.; Sangiorgi, G.M.; Golia, B.; Ricciardelli, B.; Condorelli, G.; REMEDIAL II Investigators. Renal insufficiency after contrast media administration trial ii (remedial ii): Renal guard system in high-risk patients for contrast-induced acute kidney injury. Circulation 2011, 124, 1260–1269. [Google Scholar] [CrossRef]
- Marenzi, G.; Ferrari, C.; Marana, I.; Assanelli, E.; De Metrio, M.; Teruzzi, G.; Veglia, F.; Fabbiocchi, F.; Montorsi, P.; Bartorelli, A.L. Prevention of contrast nephropathy with furosemide-induced diuresis and matched hydration—The MYTHOS trial. JACC Cardiovasc. Interv. 2012, 5, 90–97. [Google Scholar] [CrossRef]
- Briguori, C.; Visconti, G.; Donahue, M.; De Micco, F.; Focaccio, A.; Golia, B.; Signoriello, G.; Ciardiello, C.; Donnarumma, E.; Condorelli, G. Renal Guard system in high-risk patients for contrast-induced acute kidney injury. Am. Heart J. 2016, 173, 67–76. [Google Scholar] [CrossRef]
- Solomon, R. Forced diuresis with the Renal-Guard system: Impact on contrast induced acute kidney injury. J. Cardiol. 2014, 63, 9–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- ACT-Investigators. Acetylcysteine for prevention of renal outcomes in patients undergoing coronary and peripheral vascular angiography: Main results from the randomized Acetylcysteine for Contrast-induced nephropathy Trial (ACT). Circulation 2011, 124, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Sadat, U.; Usman, A.; Gillard, J.H.; Boyle, J.R. Does ascorbic acid protect against contrast-induced acute kidney injury in patients undergoing coronary angiography: A systematic review with meta-analysis of randomized, controlled trials. J. Am. Coll. Cardiol. 2013, 62, 2167–2175. [Google Scholar] [CrossRef] [PubMed]
- DiMari, J.; Megyesi, J.; Udvarbelyi, N.; Price, P.; Davis, R.; Safirstein, R. N-acetylcysteine ameliorates ischemic renal failure. Am. J. Physiol. 1997, 272, F292–F298. [Google Scholar]
- Sun, Z.; Fu, Q.; Cao, J.; Jin, W.; Cheng, L.; Li, Z. Intravenous N-acetylcysteine for prevention of contrast-induced nephropathy: A meta-analysis of randomized, controlled trials. PLoS ONE 2013, 8, e55124. [Google Scholar] [CrossRef]
- Xu, R.; Tao, A.; Bai, Y.; Deng, Y.; Chen, G. Effectiveness of n-acetylcysteine for the prevention of contrast-induced nephropathy: A systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2016, 5, e003968. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, J.; Lei, L.; Xue, Y.; Liu, L.; Huang, H.; Chen, S.; Liu, Y.; Lin, Y.; Tao, J.; et al. Effect of N-acetylcysteine on prevention of contrast-associated acute kidney injury in patients with STEMI undergoing primary percutaneous coronary intervention: A systematic review and meta-analysis of randomized controlled trials. BMJ Open 2020, 10, e039009. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Fu, L.; Mei, C.; Dai, B. Efficacy of short-term high-dose statin in preventing contrast-induced nephropathy: A meta-analysis of seven randomized controlled trials. PLoS ONE 2012, 7, e34450. [Google Scholar] [CrossRef]
- Leoncini, M.; Toso, A.; Maioli, M.; Tropeano, F.; Villani, S.; Bellandi, F. Early high-dose rosuvastatin for contrast-induced nephropathy prevention in acute coronary syndrome: Results from the PRATO-ACS Study (Protective Effect of Rosuvastatin and Antiplatelet Therapy On contrast-induced acute kidney injury and myocardial damage in patients with Acute Coronary Syndrome). J. Am. Coll. Cardiol. 2014, 63, 71–79. [Google Scholar]
- Han, Y.; Zhu, G.; Han, L.; Hou, F.; Huang, W.; Liu, H.; Gan, J.; Jiang, T.; Li, X.; Wang, W.; et al. Short-term rosuvastatin therapy for prevention of contrast induced acute kidney injury in patients with diabetes and chronic kidney disease. J. Am. Coll. Cardiol. 2014, 63, 62–70. [Google Scholar] [CrossRef]
- Crimi, G.; Ferlini, M.; Gallo, F.; Sormani, M.P.; Raineri, C.; Bramucci, E.; De Ferrari, G.M.; Pica, S.; Marinoni, B.; Repetto, A.; et al. Remote ischemic postconditioning as a strategy to reduce acute kidney injury during primary PCI_ a post-hoc analysis of a randomized trial. Int. J. Cardiol. 2014, 177, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Bei, W.J.; Duan, C.Y.; Chen, J.Y.; Wang, K.; Liu, Y.H.; Liu, Y.; Tan, N. Remote ischemic conditioning for preventing contrast-induced acute kidney injury in patients undergoing percutaneous coronary interventions/coronary angiography: A meta-analysis of randomized controlled trials. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Andò, G.; Cortese, B.; Russo, F.; Rothenbuhler, M.; Frigoli, E.; Gargiulo, G.; Briguori, C.; Vranckx, P.; Leonardi, S.; Guiducci, V.; et al. Acute kidney injury after Radial or Femoral Access for Invasive Acute Coronary Syndrome Management: AKI-MATRIX. J. Am. Coll. Cardiol. 2017, S0735–1097, 36897–36903. [Google Scholar] [CrossRef] [PubMed]
| Study | Population a | Time Period | N of Variables | Only Pre-Procedural Variables | CA-AKI Definition | C-Statistics |
|---|---|---|---|---|---|---|
| Meharan et al. JACC 2004 | 5571 patients undergoing PCI (only chronic CS) | - | 8 | No | Increase in SCr ≥ 25% or ≥0.5 mg/dL within 48 h | 0.69 |
| Marenzi et al. JACC 2004 | 208 patients undergoing PCI (only acute CS) | 2001–2003 | 5 | No | Increase in SCr ≥ 0.5 mg/dL within 72 h | - |
| Bartholomew et al. Am. J. Cardiol. 2004 | 10,481 (both acute and chronic CS) | 1993–1998 | 8 | No | Increase in SCr ≥ 1 mg/dL | 0.89 |
| Tziakas et al. Int. J. Cardiol. 2011 | 488 patients undergoing PCI (both acute and chronic CS) | 2008–2010 | 5 | No | Increase in SCr ≥ 25% or ≥0.5 mg/dL within 48 h | 0.759 |
| Gurm et al. JACC 2013 | 48,001 PCI procedures (both acute and chronic CS) | 2010–2012 | 15 | Yes | Increase in SCr ≥ 0.5 mg/dL within 7 days | 0.839 |
| Gao et al. Clin. Exp. Nephrol. 2014 | 2764 patients undergoing coronary angiography or PCI (both acute and chronic CS) | 2005–2010 | 7 | No | Increase in SCr ≥ 44.2 umol/L or ≥25% and >upper limit of normal value within 72 h | 0.76 |
| Tsai et al. JAHA 2014 | 662,504 patients undergoing PCI (both acute and chronic CS) | 2009–2011 | 11 | Yes | Increase in SCr ≥ 50% or ≥0.3 mg/dL | 0.71 |
| Meharan et al. Lancet 2021 | 14,616 patients undergoing PCI (both acute and chronic CS) | 2012–2017 | 8 | Yes | Increase in SCr ≥ 50% or ≥0.3 mg/dL within 48 h | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandurino-Mirizzi, A.; Munafò, A.; Crimi, G. Contrast-Associated Acute Kidney Injury. J. Clin. Med. 2022, 11, 2167. https://doi.org/10.3390/jcm11082167
Mandurino-Mirizzi A, Munafò A, Crimi G. Contrast-Associated Acute Kidney Injury. Journal of Clinical Medicine. 2022; 11(8):2167. https://doi.org/10.3390/jcm11082167
Chicago/Turabian StyleMandurino-Mirizzi, Alessandro, Andrea Munafò, and Gabriele Crimi. 2022. "Contrast-Associated Acute Kidney Injury" Journal of Clinical Medicine 11, no. 8: 2167. https://doi.org/10.3390/jcm11082167
APA StyleMandurino-Mirizzi, A., Munafò, A., & Crimi, G. (2022). Contrast-Associated Acute Kidney Injury. Journal of Clinical Medicine, 11(8), 2167. https://doi.org/10.3390/jcm11082167






