What Predicts Improvement of Dizziness after Multimodal and Interdisciplinary Day Care Treatment?
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Assessments
2.3. Statistics
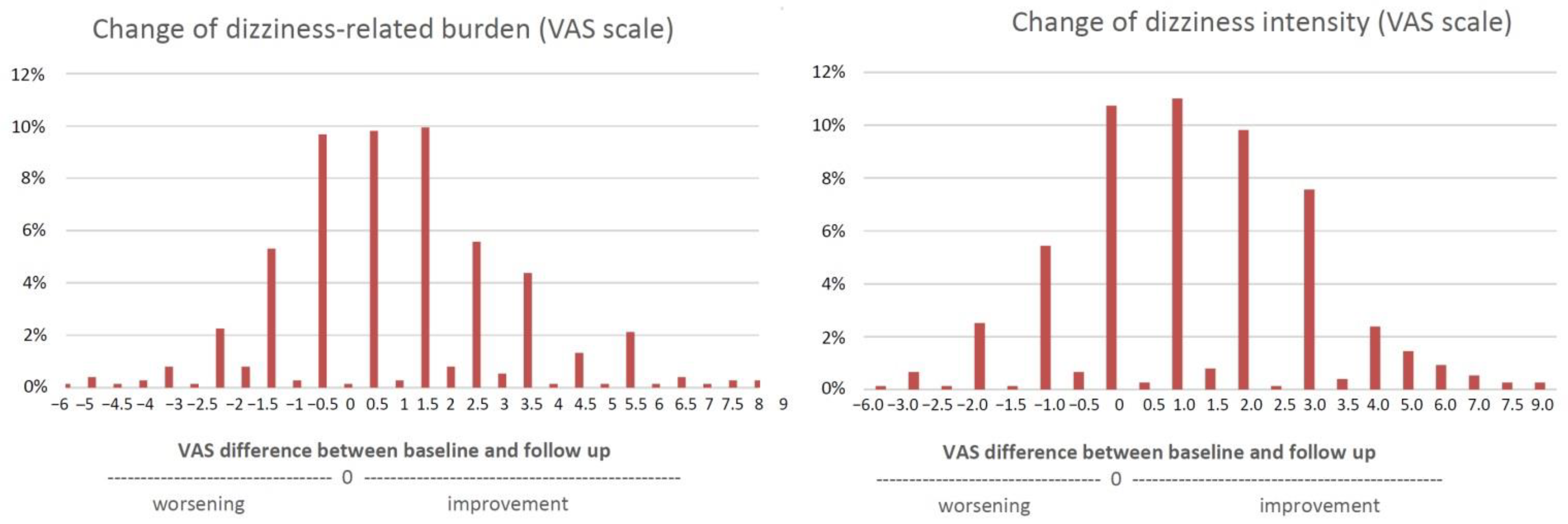
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hannaford, P.C.; Simpson, J.A.; Bisset, A.F.; Davis, A.; McKerrow, W.; Mills, R. The Prevalence of Ear, Nose and Throat Problems in the Community: Results from a National Cross-Sectional Postal Survey in Scotland. Fam. Pract. 2005, 22, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Neuhauser, H.K. The Epidemiology of Dizziness and Vertigo. Handb. Clin. Neurol. 2016, 137, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Yardley, L.; Masson, E.; Verschuur, C.; Haacke, N.; Luxon, L. Symptoms, Anxiety and Handicap in Dizzy Patients: Development of the Vertigo Symptom Scale. J. Psychosom. Res. 1992, 36, 731–741. [Google Scholar] [CrossRef]
- Axer, H.; Axer, M.; Sauer, H.; Witte, O.W.; Hagemann, G. Falls and Gait Disorders in Geriatric Neurology. Clin. Neurol. Neurosurg. 2010, 112, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Dietzek, M.; Finn, S.; Karvouniari, P.; Zeller, M.A.; Klingner, C.M.; Guntinas-Lichius, O.; Witte, O.W.; Axer, H. In Older Patients Treated for Dizziness and Vertigo in Multimodal Rehabilitation Somatic Deficits Prevail While Anxiety Plays a Minor Role Compared to Young and Middle Aged Patients. Front. Aging Neurosci. 2018, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, A.; Finn, S.; Axer, H. Age-Associated Characteristics of Patients With Chronic Dizziness and Vertigo. J. Geriatr. Psychiatry Neurol. 2021, 8919887211036185. [Google Scholar] [CrossRef] [PubMed]
- Prell, T.; Wassermann, A.; Zipprich, H.M.; Finn, S.; Axer, H. Impact of Common Dizziness Associated Symptoms on Dizziness Handicap in Older Adults. Front. Neurol. 2021, 12, 2364. [Google Scholar] [CrossRef] [PubMed]
- Raymond, M.J.; Vivas, E.X. Current and Emerging Medical Therapies for Dizziness. Otolaryngol. Clin. N. Am. 2021, 54, 1037–1056. [Google Scholar] [CrossRef]
- Zwergal, A.; Strupp, M.; Brandt, T. Advances in Pharmacotherapy of Vestibular and Ocular Motor Disorders. Expert Opin. Pharmacother. 2019, 20, 1267–1276. [Google Scholar] [CrossRef]
- Kundakci, B.; Sultana, A.; Taylor, A.J.; Alshehri, M.A. The Effectiveness of Exercise-Based Vestibular Rehabilitation in Adult Patients with Chronic Dizziness: A Systematic Review. F1000Research 2018, 7, 276. [Google Scholar] [CrossRef]
- McDonnell, M.N.; Hillier, S.L. Vestibular Rehabilitation for Unilateral Peripheral Vestibular Dysfunction. Cochrane Database Syst. Rev. 2015, 1, CD005397. [Google Scholar] [CrossRef] [PubMed]
- Edelman, S.; Mahoney, A.E.J.; Cremer, P.D. Cognitive Behavior Therapy for Chronic Subjective Dizziness: A Randomized, Controlled Trial. Am. J. Otolaryngol. 2012, 33, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Obermann, M.; Bock, E.; Sabev, N.; Lehmann, N.; Weber, R.; Gerwig, M.; Frings, M.; Arweiler-Harbeck, D.; Lang, S.; Diener, H.-C. Long-Term Outcome of Vertigo and Dizziness Associated Disorders Following Treatment in Specialized Tertiary Care: The Dizziness and Vertigo Registry (DiVeR) Study. J. Neurol. 2015, 262, 2083–2091. [Google Scholar] [CrossRef] [PubMed]
- Limburg, K.; Schmid-Mühlbauer, G.; Sattel, H.; Dinkel, A.; Radziej, K.; Gonzales, M.; Ronel, J.; Lahmann, C. Potential Effects of Multimodal Psychosomatic Inpatient Treatment for Patients with Functional Vertigo and Dizziness Symptoms—A Pilot Trial. Psychol. Psychother. 2019, 92, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Lin Se To, P.; Ajit Singh, D.K.; Whitney, S.L. Effects of Customized Vestibular Rehabilitation plus Canalith Repositioning Maneuver on Gait and Balance in Adults with Benign Paroxysmal Positional Vertigo: A Randomized Controlled Trial. J. Vestib. Res. 2022, 32, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Nada, E.H.; Ibraheem, O.A.; Hassaan, M.R. Vestibular Rehabilitation Therapy Outcomes in Patients With Persistent Postural-Perceptual Dizziness. Ann. Otol. Rhinol. Laryngol. 2019, 128, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, K.M.O.B.D.F.; Freitas, R.V.D.M.; Ferreira, L.M.D.B.M.; Deshpande, N.; Guerra, R.O. Effects of Balance Vestibular Rehabilitation Therapy in Elderly with Benign Paroxysmal Positional Vertigo: A Randomized Controlled Trial. Disabil. Rehabil. 2017, 39, 1198–1206. [Google Scholar] [CrossRef]
- Axer, H.; Finn, S.; Wassermann, A.; Guntinas-Lichius, O.; Klingner, C.M.; Witte, O.W. Multimodal Treatment of Persistent Postural-Perceptual Dizziness. Brain Behav. 2020, 10, e01864. [Google Scholar] [CrossRef]
- Kondo, M.; Kiyomizu, K.; Goto, F.; Kitahara, T.; Imai, T.; Hashimoto, M.; Shimogori, H.; Ikezono, T.; Nakayama, M.; Watanabe, N.; et al. Analysis of Vestibular-Balance Symptoms According to Symptom Duration: Dimensionality of the Vertigo Symptom Scale-Short Form. Health Qual. Life Outcomes 2015, 13, 4. [Google Scholar] [CrossRef]
- Chambless, D.L.; Caputo, G.C.; Bright, P.; Gallagher, R. Assessment of Fear of Fear in Agoraphobics: The Body Sensations Questionnaire and the Agoraphobic Cognitions Questionnaire. J. Consult. Clin. Psychol. 1984, 52, 1090–1097. [Google Scholar] [CrossRef]
- Chambless, D.L.; Caputo, G.C.; Jasin, S.E.; Gracely, E.J.; Williams, C. The Mobility Inventory for Agoraphobia. Behav. Res. Ther. 1985, 23, 35–44. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Herdman, D.; Norton, S.; Pavlou, M.; Murdin, L.; Moss-Morris, R. The Role of Prediagnosis Audiovestibular Dysfunction Versus Distress, Illness-Related Cognitions, and Behaviors in Predicted Ongoing Dizziness Handicap. Psychosom. Med. 2020, 82, 787–795. [Google Scholar] [CrossRef]
- Jeong, J.; Jung, J.; Lee, J.M.; Suh, M.J.; Kwak, S.H.; Kim, S.H. Effects of Saccular Function on Recovery of Subjective Dizziness After Vestibular Rehabilitation. Otol. Neurotol. 2017, 38, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Kim, J.-S.; Park, H.Y. Predictors of Treatment Response to Pharmacotherapy in Patients with Persistent Postural-Perceptual Dizziness. J. Neurol. 2021, 268, 2523–2532. [Google Scholar] [CrossRef] [PubMed]
- Balatsouras, D.G.; Aspris, A.; Ganelis, P.; Koukoutsis, G.; Moukos, A.; Fassolis, A.; Katotomichelakis, M. Duration of Benign Paroxysmal Positional Vertigo as a Predictor for Therapy. B-ENT 2015, 11, 199–203. [Google Scholar] [PubMed]
- Do, Y.-K.; Kim, J.; Park, C.Y.; Chung, M.-H.; Moon, I.S.; Yang, H.-S. The Effect of Early Canalith Repositioning on Benign Paroxysmal Positional Vertigo on Recurrence. Clin. Exp. Otorhinolaryngol. 2011, 4, 113–117. [Google Scholar] [CrossRef]
- Teggi, R.; Quaglieri, S.; Gatti, O.; Benazzo, M.; Bussi, M. Residual Dizziness after Successful Repositioning Maneuvers for Idiopathic Benign Paroxysmal Positional Vertigo. ORL J. Otorhinolaryngol. Relat. Spec. 2013, 75, 74–81. [Google Scholar] [CrossRef]
- Teggi, R.; Giordano, L.; Bondi, S.; Fabiano, B.; Bussi, M. Residual Dizziness after Successful Repositioning Maneuvers for Idiopathic Benign Paroxysmal Positional Vertigo in the Elderly. Eur. Arch. Otorhinolaryngol. 2011, 268, 507–511. [Google Scholar] [CrossRef]
- Bigelow, R.T.; Semenov, Y.R.; du Lac, S.; Hoffman, H.J.; Agrawal, Y. Vestibular Vertigo and Comorbid Cognitive and Psychiatric Impairment: The 2008 National Health Interview Survey. J. Neurol. Neurosurg. Psychiatr. 2016, 87, 367–372. [Google Scholar] [CrossRef]
- Lahmann, C.; Henningsen, P.; Brandt, T.; Strupp, M.; Jahn, K.; Dieterich, M.; Eckhardt-Henn, A.; Feuerecker, R.; Dinkel, A.; Schmid, G. Psychiatric Comorbidity and Psychosocial Impairment among Patients with Vertigo and Dizziness. J. Neurol. Neurosurg. Psychiatr. 2015, 86, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Kim, Y.B.; Park, I.-S.; Hong, S.J.; Kim, H.; Hong, S.M. Clinical Analysis of Dizzy Patients with High Levels of Depression and Anxiety. J. Audiol. Otol. 2016, 20, 174–178. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhai, F.; Wang, J.; Zhang, Y.; Dai, C.-F. Quantitative Analysis of Psychiatric Disorders in Intractable Peripheral Vertiginous Patients: A Prospective Study. Otol. Neurotol. 2016, 37, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Brandt, T.; Dieterich, M. “Excess Anxiety” and “Less Anxiety”: Both Depend on Vestibular Function. Curr. Opin. Neurol. 2020, 33, 136–141. [Google Scholar] [CrossRef]
- Patel, J.J.; Levy, D.A.; Nguyen, S.A.; Rizk, H.G.; Meyer, T.A. Depression in Ménière’s Disease: A Systematic Review and Meta-Analysis. J. Laryngol. Otol. 2020, 134, 293–301. [Google Scholar] [CrossRef]
- Tyrrell, J.; White, M.P.; Barrett, G.; Ronan, N.; Phoenix, C.; Whinney, D.J.; Osborne, N.J. Mental Health and Subjective Well-Being of Individuals With Ménière’s: Cross-Sectional Analysis in the UK Biobank. Otol. Neurotol. 2015, 36, 854–861. [Google Scholar] [CrossRef]
- Arroll, M.; Dancey, C.P.; Attree, E.A.; Smith, S.; James, T. People with Symptoms of Ménière’s Disease: The Relationship between Illness Intrusiveness, Illness Uncertainty, Dizziness Handicap, and Depression. Otol. Neurotol. 2012, 33, 816–823. [Google Scholar] [CrossRef]
- Yuan, Q.; Yu, L.; Shi, D.; Ke, X.; Zhang, H. Anxiety and Depression among Patients with Different Types of Vestibular Peripheral Vertigo. Medicine 2015, 94, e453. [Google Scholar] [CrossRef]
- Habs, M.; Strobl, R.; Grill, E.; Dieterich, M.; Becker-Bense, S. Primary or Secondary Chronic Functional Dizziness: Does It Make a Difference? A DizzyReg Study in 356 Patients. J. Neurol. 2020, 267, 212–222. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Tsai, S.-J.; Shen, C.-C.; Lu, T.; Hung, Y.-M.; Hu, L.-Y. Risk of Benign Paroxysmal Positional Vertigo in Patients with Depressive Disorders: A Nationwide Population-Based Cohort Study. BMJ Open 2019, 9, e026936. [Google Scholar] [CrossRef]
- Wei, W.; Sayyid, Z.N.; Ma, X.; Wang, T.; Dong, Y. Presence of Anxiety and Depression Symptoms Affects the First Time Treatment Efficacy and Recurrence of Benign Paroxysmal Positional Vertigo. Front. Neurol. 2018, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, M.; Kitahara, T.; Okayasu, T.; Yamashita, A.; Hasukawa, A.; Ota, I.; Yamanaka, T. Negative Prognostic Factors for Psychological Conditions in Patients with Audiovestibular Diseases. Auris Nasus Larynx 2016, 43, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Kurre, A.; Straumann, D.; van Gool, C.J.; Gloor-Juzi, T.; Bastiaenen, C.H. Gender Differences in Patients with Dizziness and Unsteadiness Regarding Self-Perceived Disability, Anxiety, Depression, and Its Associations. BMC Ear Nose Throat Disord. 2012, 12, 2. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Yano, E. Somatic Symptoms for Predicting Depression: One-Year Follow-Up Study in Annual Health Examinations. Psychiatry Clin. Neurosci. 2006, 60, 219–225. [Google Scholar] [CrossRef]
- Radziej, K.; Probst, T.; Limburg, K.; Dinkel, A.; Dieterich, M.; Lahmann, C. The Longitudinal Effect of Vertigo and Dizziness Symptoms on Psychological Distress: Symptom-Related Fears and Beliefs as Mediators. J. Nerv. Ment. Dis. 2018, 206, 277–285. [Google Scholar] [CrossRef]
- Probst, T.; Dinkel, A.; Schmid-Mühlbauer, G.; Radziej, K.; Limburg, K.; Pieh, C.; Lahmann, C. Psychological Distress Longitudinally Mediates the Effect of Vertigo Symptoms on Vertigo-Related Handicap. J. Psychosom. Res. 2017, 93, 62–68. [Google Scholar] [CrossRef]



| Categorical Variables | N | % | ||
|---|---|---|---|---|
| Sex | Female | 461 | 61.5 | |
| Male | 289 | 38.5 | ||
| Diagnosis category | Somatic | 350 | 53.8 | |
| non-somatic psychological | 254 | 39.1 | ||
| Non-somatic unspecific | 46 | 7.1 | ||
| Duration of symptoms | 3–6 months | 95 | 13.3 | |
| >6 months | 621 | 86.7 | ||
| Medical diagnosis | BPPV | 24 | 3.2 | |
| BV | 35 | 4.6 | ||
| CV | 43 | 5.7 | ||
| MD | 55 | 7.3 | ||
| MultD | 117 | 15.5 | ||
| PPPD | 351 | 46.6 | ||
| VM | 30 | 4.0 | ||
| VN | 73 | 9.7 | ||
| VP | 10 | 1.3 | ||
| VS | 16 | 2.1 | ||
| Continuous/permanent dizziness/vertigo | Yes | 399 | 56.4 | |
| No | 308 | 43.6 | ||
| Attack-like | Yes | 402 | 60.5 | |
| No | 263 | 39.5 | ||
| Metric Variables | Mean | SD | 95% Lower CI | 95% Upper CI |
| Age (years) | 57.67 | 14.98 | 56.59 | 58.74 |
| BSQ1 | 8.15 | 5.64 | 7.74 | 8.57 |
| VSS-V | 11.21 | 8.70 | 10.58 | 11.84 |
| VSS-A | 13.98 | 10.31 | 13.23 | 14.73 |
| VSS total score | 25.19 | 16.28 | 24.01 | 26.37 |
| HADS anxiety | 6.84 | 4.04 | 6.55 | 7.14 |
| HADS depression | 6.18 | 3.89 | 5.90 | 6.47 |
| ACQ | 19.63 | 5.84 | 19.13 | 20.14 |
| MI accompanied | 2.31 | 1.12 | 2.22 | 2.40 |
| MI alone | 1.92 | 0.96 | 1.84 | 2.01 |
| Mean | SD | SE | p | Effect Size | |
|---|---|---|---|---|---|
| BSQ1 | 7.751 | 5.178 | 0.266 | ||
| BSQ1 follow-up | 6.860 | 5.754 | 0.296 | <0.001 | 0.235 |
| VSS-V | 11.039 | 8.240 | 0.396 | ||
| VSS-V follow-up | 8.477 | 8.424 | 0.405 | <0.001 | 0.403 |
| VSS-A | 13.088 | 9.895 | 0.476 | ||
| VSS-A follow-up | 11.963 | 9.320 | 0.448 | 0.008 | 0.151 |
| VSS total score | 24.127 | 15.427 | 0.742 | ||
| VSS total follow-up | 20.440 | 15.688 | 0.755 | <0.001 | 0.320 |
| HADS-A | 6.633 | 3.908 | 0.190 | ||
| HADS-A follow-up | 6.111 | 4.002 | 0.195 | <0.001 | 0.204 |
| HADS-D | 5.969 | 3.893 | 0.190 | ||
| HADS-D follow-up | 5.668 | 3.779 | 0.184 | 0.110 | 0.098 |
| ACQ | 19.357 | 5.452 | 0.328 | ||
| ACQ follow-up | 18.715 | 5.145 | 0.309 | 0.070 | 0.133 |
| MI accompanied | 1.925 | 0.952 | 0.059 | ||
| MI accompanied follow-up | 1.977 | 0.914 | 0.056 | 0.182 | −0.097 |
| MI alone | 2.317 | 1.054 | 0.065 | ||
| MI alone follow-up | 1.647 | 0.812 | 0.050 | <0.001 | 0.720 |
| Variable | Coefficient | SE | p | Beta | |
|---|---|---|---|---|---|
| Model 1 (corrected R2 = 0.13) 1 | |||||
| Constant | 0.173 | 0.107 | 0.106 | ||
| Vertigo diagnoses (CV, MultD) # | −0.562 | 0.215 | 0.01 | 0.500 | |
| Vertigo diagnoses (MD, VM, VN, VP, VS) # | 0.317 | 0.138 | 0.022 | 0.500 | |
| Permanent vertigo present | −0.369 | 0.124 | 0.003 | 0.295 | |
| Disease duration < 6 m | 0.453 | 0.182 | 0.014 | 0.205 | |
| Model 2 (corrected R2 = 0.16) 2 | |||||
| Constant | 0.822 | 0.243 | 0.001 | ||
| Vertigo diagnoses (CV, MultD) # | −0.452 | 0.215 | 0.036 | 0.306 | |
| Vertigo diagnoses (MD, VM, VN, VP, VS) # | 0.321 | 0.135 | 0.019 | 0.306 | |
| Disease duration < 6 m | 0.565 | 0.183 | 0.002 | 0.232 | |
| Burden | −0.091 | 0.035 | 0.011 | 0.160 | |
| Permanent vertigo present | −0.285 | 0.128 | 0.027 | 0.121 | |
| MI alone | −0.142 | 0.064 | 0.027 | 0.120 | |
| VSS | 0.007 | 0.005 | 0.115 | 0.061 | |
| Model 3 (corrected R2 = 0.30) 3 | |||||
| Constant | 1.070 | 0.220 | <0.001 | ||
| HADS depression | −0.102 | 0.015 | <0.001 | 0.622 | |
| Vertigo diagnoses (CV, MultD) # | −0.295 | 0.197 | 0.136 | 0.099 | |
| Vertigo diagnoses (MD, VM, VN, VP, VS) # | 0.246 | 0.124 | 0.049 | 0.099 | |
| VSS | 0.009 | 0.004 | 0.023 | 0.069 | |
| Disease duration < 6 m | 0.360 | 0.166 | 0.031 | 0.062 | |
| Permanent vertigo present | −0.239 | 0.118 | 0.043 | 0.054 | |
| Intensity | −0.067 | 0.034 | 0.049 | 0.052 | |
| MI alone | −0.105 | 0.059 | 0.076 | 0.042 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prell, T.; Finn, S.; Zipprich, H.M.; Axer, H. What Predicts Improvement of Dizziness after Multimodal and Interdisciplinary Day Care Treatment? J. Clin. Med. 2022, 11, 2005. https://doi.org/10.3390/jcm11072005
Prell T, Finn S, Zipprich HM, Axer H. What Predicts Improvement of Dizziness after Multimodal and Interdisciplinary Day Care Treatment? Journal of Clinical Medicine. 2022; 11(7):2005. https://doi.org/10.3390/jcm11072005
Chicago/Turabian StylePrell, Tino, Sigrid Finn, Hannah M. Zipprich, and Hubertus Axer. 2022. "What Predicts Improvement of Dizziness after Multimodal and Interdisciplinary Day Care Treatment?" Journal of Clinical Medicine 11, no. 7: 2005. https://doi.org/10.3390/jcm11072005
APA StylePrell, T., Finn, S., Zipprich, H. M., & Axer, H. (2022). What Predicts Improvement of Dizziness after Multimodal and Interdisciplinary Day Care Treatment? Journal of Clinical Medicine, 11(7), 2005. https://doi.org/10.3390/jcm11072005








