Ocular Complications of Giant Cell Arteritis: An Acute Therapeutic Emergency
Abstract
1. Introduction
2. From a Benign Disease of the Temporal Arteries to an Acute Ophthalmological Emergency: A Brief Historical Overview
3. Ocular Manifestations of GCA
3.1. Histopathological Data
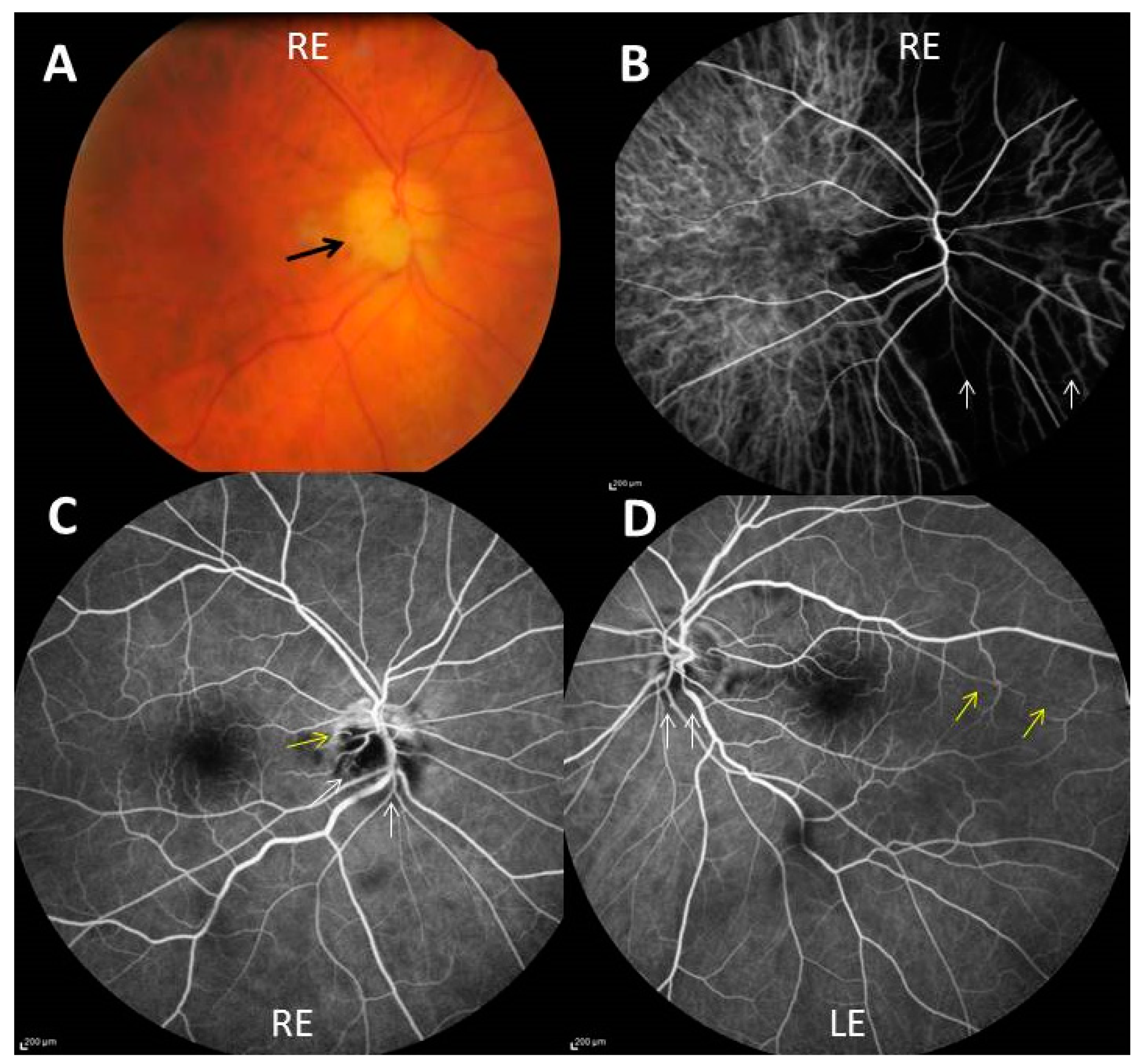
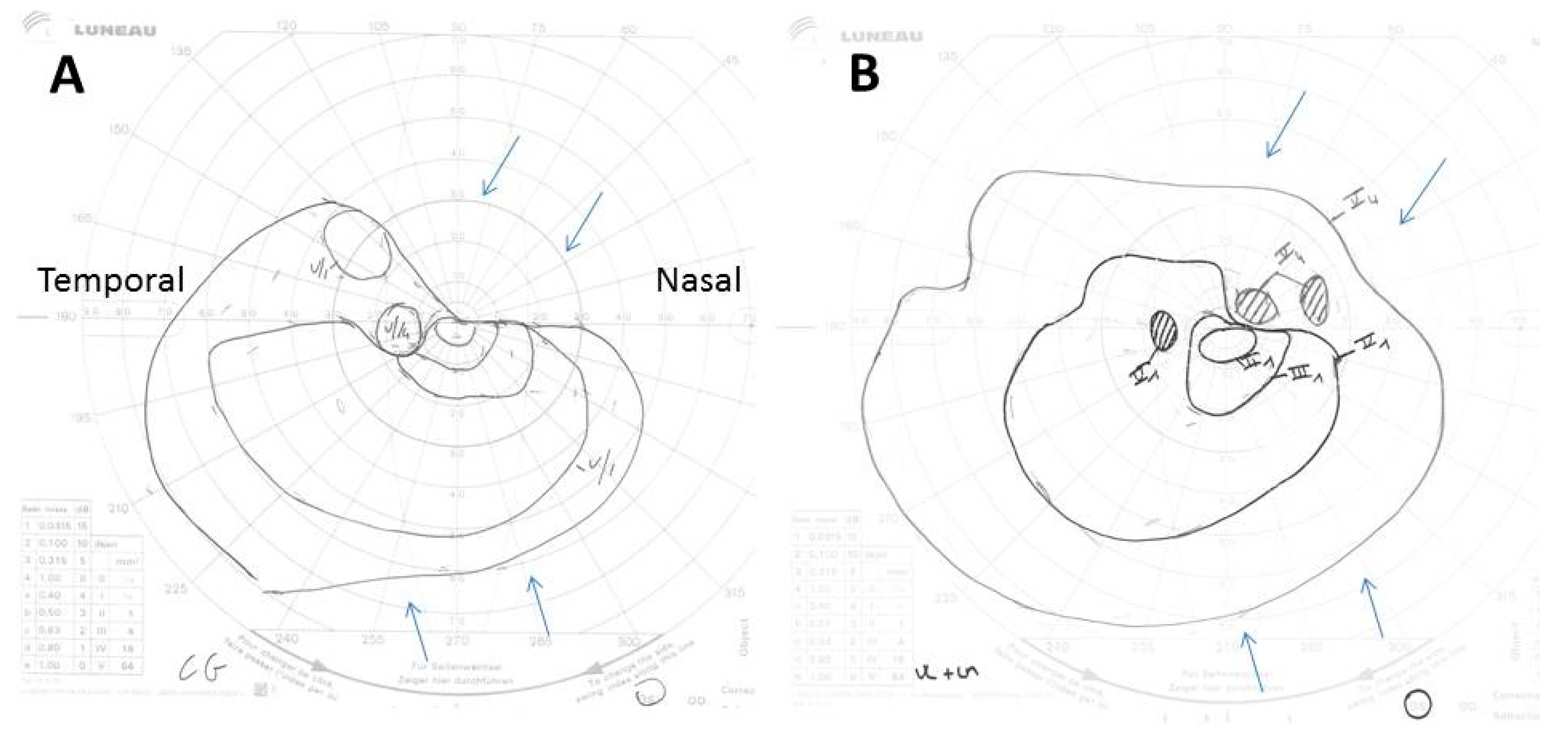
3.2. Causes of Permanent Visual Loss
3.3. Transient Visual Manifestations
3.4. Time Element of the Development of Ocular Manifestations
4. Frequency of Permanent Visual Loss
5. Diagnosis of GCA in the Presence of Ocular Ischemic Manifestations
6. Treatment of GCA Patients with Ocular Manifestations
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Horton, B.T.; Magath, T.B.; Brown, G.E. An undescribed form of arteritis of the temporal vessels. Proc. Staff Meet. Mayo Clinic 1932, 7, 700–701. [Google Scholar]
- Chasnoff, J.; Vorzimer, J.J. Temporal arteritis: A local manifestation of a systemic disease. Ann. Intern. Med. 1944, 20, 327. [Google Scholar] [CrossRef]
- Cooke, W.T.; Cloake, P.C.P.; Govan, A.D.T.; Colbeck, J.C. Temporal Arteritis: A Generalized Vascular Disease. QJM Int. J. Med. 1946, 15, 47–75. [Google Scholar] [CrossRef] [PubMed]
- Crosby, R.C.; Wadsworth, R.C. Temporal arteritis; review of the literature and report of five additional cases. Arch. Intern. Med. 1948, 81, 831–864. [Google Scholar] [CrossRef]
- Gilmour, J.R. Giant-cell chronic arteritis. J. Pathol. Bacteriol. 1941, 53, 263–277. [Google Scholar] [CrossRef]
- Jennings, G. Arteritis of the temporal vessels. Lancet 1938, 231, 424. [Google Scholar] [CrossRef]
- Johnson, R.H.; Harley, R.D.; Horton, B.T. Arteritis of the Temporal Vessels Associated with Loss of Vision. Am. J. Ophthalmol. 1943, 26, 147–151. [Google Scholar] [CrossRef]
- Wagener, H.P. Temporal arteritis and loss of vision. Am. J. Med. Sci. 1946, 212, 225–228. [Google Scholar] [CrossRef]
- Bruce, G.M. Temporal arteritis as a cause of blindness; review of literature and report of a case. Trans. Am. Ophthalmol. Soc. 1949, 47, 300–316. [Google Scholar]
- Chavany, J.A.; Taptas, J.N. A propos d’un cas d’artérite temporale (importance de l’artérite de Horton dans la céphalée des vieillards). Presse Med. 1948, 69, 835. [Google Scholar]
- Wagener, H.P.; Hollenhorst, R.W. The ocular lesions of temporal arteritis. Am. J. Ophthalmol. 1958, 45, 617–630. [Google Scholar] [CrossRef]
- Shick, R.M.; Baggenstoss, A.H.; Fuller, B.F.; Polley, H.F. Effects of cortisone and ACTH on periarteritis nodosa and cranial arteritis. Proc. Staff. Meet. Mayo Clin. 1950, 25, 492–494. [Google Scholar] [PubMed]
- Whitfield, A.; Cooke, W.T.; Jameson-Evans, P.; Rudd, C. Temporal arteritis and its treatment with cortisone and A.C.T.H. Lancet 1953, 261, 408–412. [Google Scholar] [CrossRef]
- Birkhead, N.C.; Wagener, H.P.; Shick, R.M. Treatment of Temporal Arteritis with Adrenal Corticosteroids. J. Am. Med. Assoc. 1957, 163, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, I.M.S.; Russel, R.W.R. Arteries of the head and neck in giant cell arteritis. Arch. Neurol. 1972, 27, 378–391. [Google Scholar] [CrossRef]
- Butt, Z.; Cullen, J.F.; Mutlukan, E. Pattern of arterial involvement of the head, neck, and eyes in giant cell arteritis: Three case reports. Br. J. Ophthalmol. 1991, 75, 368–371. [Google Scholar] [CrossRef][Green Version]
- Shigeyasu, M.; Sasaki, N.; Nishino, S.; Sakai, N. Giant cell arteritis with simultaneous onset of multiple intracranial vascular occlusions: A case report. Surg. Neurol. Int. 2022, 13, 21. [Google Scholar] [CrossRef]
- Hayreh, S.S.; Podhajsky, P.A.; Zimmerman, B. Ocular manifestations of giant cell arteritis. Am. J. Ophthalmol. 1998, 125, 509–520. [Google Scholar] [CrossRef]
- Aiello, P.D.; Trautmann, J.C.; McPhee, T.J.; Kunselman, A.R.; Hunder, G.G. Visual Prognosis in Giant Cell Arteritis. Ophthalmology 1993, 100, 550–555. [Google Scholar] [CrossRef]
- Liu, G.T.; Glaser, J.S.; Schatz, N.J.; Smith, J.L. Visual Morbidity in Giant Cell Arteritis. Ophthalmology 1994, 101, 1779–1785. [Google Scholar] [CrossRef]
- González-Gay, M.A.; García-Porrúa, C.; Llorca, J.; Hajeer, A.H.; Brañas, F.; Dababneh, A.; González-Louzao, C.; Rodriguez-Gil, E.; Rodríguez-Ledo, P.; Ollier, W.E.R. Visual Manifestations of Giant Cell Arteritis: Trends and Clinical Spectrum in 161 Patients. Medicine 2000, 79, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Salvarani, C.; Cimino, L.; Macchioni, P.; Consonni, D.; Cantini, F.; Bajocchi, G.; Catanoso, M.G.; Boiardi, L. Risk factors for visual loss in an Italian population-based cohort of patients with giant cell arteritis. Arthritis Care Res. 2005, 53, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Daneshmeyer, H.; Savino, P.; Gamble, G. Poor Prognosis of Visual Outcome after Visual Loss from Giant Cell Arteritis. Ophthalmology 2005, 112, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Whitefield, A.G.W.; Bateman, M.; Cooke, W.T. Temporal arteritis. Br. J. Ophthalmol. 1963, 47, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Hollenhorst, R.W.; Brown, J.R.; Wagener, H.P.; Shick, R.M. Neurologic aspects of temporal arteritis. Neurology 1960, 10, 490. [Google Scholar] [CrossRef] [PubMed]
- Liozon, E.; Ly, K.H.; Robert, P.Y. Ocular complications of giant cell arteritis. Rev. Med. Interne 2013, 34, 421–430. [Google Scholar] [CrossRef]
- Prior, J.A.; Ranjbar, H.; Belcher, J.; Mackie, S.L.; Helliwell, T.; Liddle, J.; Mallen, C.D. Diagnostic delay for giant cell arteritis—A systematic review and meta-analysis. BMC Med. 2017, 15, 120. [Google Scholar] [CrossRef]
- Cid, M.C.; Font, C.; Oristrell, J.; de la Sierra, A.; Coll-Vinent, B.; López-Soto, A.; Vilaseca, J.; Urbano-Márquez, A.; Grau, J.M. Association between strong inflammatory response and low risk of developing visual loss and other cranial ischemic complications in giant cell (temporal) arteritis. Arthritis Rheum. 1998, 41, 26–32. [Google Scholar] [CrossRef]
- Liozon, E.; Herrmann, F.; Ly, K.; Robert, P.-Y.; Loustaud, V.; Soria, P.; Vidal, E. Risk factors for visual loss in giant cell (temporal) arteritis: A prospective study of 174 patients. Am. J. Med. 2001, 111, 211–217. [Google Scholar] [CrossRef]
- Nesher, G.; Berkun, Y.; Mates, M.; Baras, M.; Nesher, R.; Rubinow, A.; Sonnenblick, M. Risk Factors for Cranial Ischemic Complications in Giant Cell Arteritis. Medicine 2004, 83, 114–122. [Google Scholar] [CrossRef]
- Czihal, M.; Tschaidse, J.; Bernau, C.; Lottspeich, C.; Köhler, A.; DeChant, C.; Schulze-Koops, H.; Hoffmann, U.; Mackert, M.J.; Thurau, S. Ocular ischaemic complications in giant cell arteritis: CHADS2-score predicts risk of permanent visual impairment. Clin. Exp. Rheumatol. 2019, 37, S61–S64. [Google Scholar]
- Parsons-Smith, G. SUDDEN BLINDNESS IN CRANIAL ARTERITIS. Br. J. Ophthalmol. 1959, 43, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.J.; Cogan, D.G. Occult Temporal Arteritis. Arch. Ophthalmol. 1962, 68, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S.; Podhajsky, P.A.; Zimmerman, B. Occult giant cell arteritis: Ocular manifestations. Am. J. Ophthalmol. 1998, 125, 521–526. [Google Scholar] [CrossRef]
- Chen, J.J.; Leavitt, J.A.; Fang, C.F.; Crowson, C.S.; Matteson, E.L.; Warrington, K.J. Evaluating the incidence of arteritic ischemic optic neuropathy and other causes of visual loss from giant cell arteritis. Ophthalmology 2016, 123, 1999–2003. [Google Scholar] [CrossRef]
- Saleh, M.; Turesson, C.; Englund, M.; Merkel, P.A.; Mohammad, A.J. Visual Complications in Patients with Biopsy-proven Giant Cell Arteritis: A Population-based Study. J. Rheumatol. 2016, 43, 1559–1565. [Google Scholar] [CrossRef]
- Bienvenu, B.; Ly, K.H.; Lambert, M.; Agard, C.; André, M.; Benhamou, Y.; Bonnotte, B.; de Boysson, H.; Espitia, O.; Fau, G.; et al. Management of giant cell arteritis: Recommendations of the French Study Group for Large Vessel Vasculitis (GEFA). Rev. Méd. Interne 2016, 37, 154–165. [Google Scholar] [CrossRef]
- Dejaco, C.; Ramiro, S.; Duftner, C.; Besson, F.L.; Bley, T.A.; Blockmans, D.; Brouwer, E.; Cimmino, M.A.; Clark, E.; Dasgupta, B.; et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann. Rheum. Dis. 2018, 77, 636–643. [Google Scholar] [CrossRef]
- Mackie, S.L.; Dejaco, C.; Appenzeller, S.; Camellino, D.; Duftner, C.; Gonzalez-Chiappe, S.; Mahr, A.; Mukhtyar, C.; Reynolds, G.; De Souza, A.W.S.; et al. British Society for Rheumatology guideline on diagnosis and treatment of giant cell arteritis: Executive summary. Rheumatology 2020, 59, 487–494. [Google Scholar] [CrossRef]
- Duftner, C.; Dejaco, C.; Sepriano, A.; Falzon, L.; Schmidt, W.A.; Ramiro, S. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: A systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open 2018, 4, e000612. [Google Scholar] [CrossRef]
- Prieto-Peña, D.; Castañeda, S.; Martínez-Rodríguez, I.; Atienza-Mateo, B.; Blanco, R.; González-Gay, M.A. Imaging Tests in the Early Diagnosis of Giant Cell Arteritis. J. Clin. Med. 2021, 10, 3704. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Williams, M.; Maw, W.W.; Achilleos, K.; Elsideeg, S.; Dejaco, C.; Borg, F.; Gupta, S.; Dasgupta, B. Fast track pathway reduces sight loss in giant cell arteritis: Results of a longitudinal observational cohort study. Clin. Exp. Rheumatol. 2015, 33, 103–106. [Google Scholar]
- Diamantopoulos, A.P.; Haugeberg, G.; Lindland, A.; Myklebust, G. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: Towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology 2016, 55, 66–70. [Google Scholar] [CrossRef]
- Hauenstein, C.; Reinhard, M.; Geiger, J.; Markl, M.; Hetzel, A.; Treszl, A.; Vaith, P.; Bley, T.A. Effects of early corticosteroid treatment on magnetic resonance imaging and ultrasound findings in giant cell arteritis. Rheumatology 2012, 51, 1999–2003. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.D.; Gormsen, L.C.; Hansen, I.T.; Keller, K.K.; Therkildsen, P.; Hauge, E.M. Three days high-dose glucocrticooid treatment attenuates large-vessel 18-FDG uptake in large-vessel giant cell arteritis but with a limited impact on diagnostic accuracy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Klink, T.; Geiger, J.; Both, M.; Ness, T.; Heinzelmann, S.; Reinhard, M.; Holl-Ulrich, K.; Duwendag, D.; Vaith, P.; Bley, T.A. Giant Cell Arteritis: Diagnostic Accuracy of MR Imaging of Superficial Cranial Arteries in Initial Diagnosis—Results from a Multicenter Trial. Radiology 2014, 273, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, E.; Maldini, C.; Gonzalez-Chiappe, S.; Chevret, S.; Mahr, A. Sensitivity of temporal artery biopsy in the diagnosis of giant cell arteritis: A systematic literature review and meta-analysis. Rheumatology 2020, 59, 1011–1020. [Google Scholar] [CrossRef]
- Achkar, A.A.; Lie, J.T.; Hunder, G.G.; O’Fallon, W.M.; Gabriel, S.E. How does previous corticosteroid treatment affect the biopsy findings in giant cell (temporal) arteritis? Ann. Intern. Med. 1994, 120, 987–992. [Google Scholar] [CrossRef]
- Narváez, J.; Bernad, B.; Roig-Vilaseca, D.; García-Gómez, C.; Gómez-Vaquero, C.; Juanola, X.; Rodriguez-Moreno, J.; Nolla, J.M.; Valverde, J. Influence of Previous Corticosteroid Therapy on Temporal Artery Biopsy Yield in Giant Cell Arteritis. Semin. Arthritis Rheum. 2007, 37, 13–19. [Google Scholar] [CrossRef]
- Maleszewski, J.J.; Younge, B.R.; Fritzlen, J.T.; Hunder, G.G.; Goronzy, J.J.; Warrington, K.; Weyand, C.M. Clinical and pathological evolution of giant cell arteritis: A prospective study of follow-up temporal artery biopsies in 40 treated patients. Mod. Pathol. 2017, 30, 788–796. [Google Scholar] [CrossRef]
- Hayreh, S.S.; Zimmerman, B.; Kardon, R.H. Visual improvement with corticosteroid therapy in giant cell arteritis. Report of a large study and review of literature. Acta Ophthalmol. Scand. 2002, 80, 355–367. [Google Scholar] [CrossRef] [PubMed]
- González-Gay, M.A.; Blanco, R.; Rodríguez-Valverde, V.; Martínez-Taboada, V.M.; Delgado-Rodriguez, M.; Figueroa, M.; Uriarte, E. Permanent visual loss and cerebrovascular accidents in giant cell arteritis: Predictors and response to treatment. Arthritis Care Res. 1998, 41, 1497–1504. [Google Scholar] [CrossRef]
- Hayreh, S.S.; Zimmerman, B. Visual deterioration while on high-doses of corticosteroid therapy. Ophthalmology 2003, 110, 1204–1215. [Google Scholar] [CrossRef]
- Fraser, J.A.; Weyand, C.M.; Newman, N.J.; Biousse, V. The treatment of giant cell arteritis. Rev. Neurol. Dis. 2008, 5, 140–152. [Google Scholar]
- Hellmich, B.; Agueda, A.; Monti, S.; Buttgereit, F.; De Boysson, H.; Brouwer, E.; Cassie, R.; Cid, M.C.; Dasgupta, B.; Dejaco, C.; et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann. Rheum. Dis. 2020, 79, 19–30. [Google Scholar] [CrossRef]
- Gonzalez-Gay, M.A.; Vazquez-Rodriguez, T.R.; Gomez-Acebo, I.; Pego-Reigosa, R.; Lopez-Diaz, M.J.; Vazquez-Triñanes, M.C.; Miranda-Filloy, J.A.; Blanco, R.; Dierssen, T.; Gonzalez-Juanatey, C.; et al. Strokes at Time of Disease Diagnosis in a Series of 287 Patients With Biopsy-Proven Giant Cell Arteritis. Medicine 2009, 88, 227–235. [Google Scholar] [CrossRef]
- Stone, J.H.; Tuckwell, K.; Dimonaco, S.; Klearman, M.; Aringer, M.; Blockmans, D.E.; Brouwer, E.; Cid, M.C.; Dasgupta, B.; Rech, J.; et al. Trial of Tocilizumab in Giant-Cell Arteritis. N. Engl. J. Med. 2017, 377, 317–328. [Google Scholar] [CrossRef]
- Amsler, J.; Kysela, I.; Tappeiner, C.; Seitz, L.; Christ, L.; Scholz, G.; Stalder, O.; Kollert, F.; Reichenbach, S.; Villiger, P.M. Vision loss in patients with giant cell arteritis treated with tocilizumab. Arthritis Res. Ther. 2021, 23, 92. [Google Scholar] [CrossRef]
| Anatomical Site | Main Vascular Lesions | Visual Symptoms | Clinical Examination | * Frequency |
|---|---|---|---|---|
| Orbit | ||||
| Eye muscles | Branches of the ophtalmic artery | † Diplopia (all types), ophtalmoplegia | Paresis off one or more extraocular muscles | 5–10% |
| Orbital tissues | Branches of the ophtalmic artery | Diplopia, pain, conjunctival edema | Eye motility disorders, proptosis, chemosis | Rare (≤1%) |
| Ocular apparatus (from front to back) | ||||
| Anterior segment (often generalized ocular ischemia) | Anterior ciliary arteries (ophtalmic artery) | ‡ Visual loss | Hypotonia, pseudo-uveitis, pupillary abnormalities | Rare (≤1%) |
| Retina | Central retinal artery | ‡ Visual loss | Pale edematous retina, cherry red macula, ±cotton wool spots | 10% |
| ϕ Cilioretinal A. occlusion | 22% | |||
| Choroid | Posterior ciliary arteries | ‡ Visual loss | Areas of choroidal infarction | 10% |
| Optic nerve: | ||||
| Anterior | Posterior ciliary arteries | ‡ Visual loss | Shalky white optic disc edema ± peripapillary hemorrhages. Optic atrophy after 4–6 weeks | 80–90% |
| Posterior | Nutrient arteries of optic nerve trunk | ‡ Visual loss | Normal fundus. Optic atrophy after 4–6 weeks | 5% |
| Occipital brain | Vertebral arteries | ‡ Visual loss | Homonymous lateral hemianopsia (occipital stroke) | Rare (≤1%) |
| First Author, Year of Publication [Ref] * | Study Population | Patients with any Degree of Permanent Vision Loss in at Least One Eye | Patients with Complete Blindness in at Least One Eye | Total Number of Completely Blind Eyes | ||
|---|---|---|---|---|---|---|
| Total | Bilateral | Total | Bilateral | |||
| N | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Bruce, 1949 [9] | 84 | 34 (40) | NA | 22 (26) | 13 (15) | 35 (21) |
| Birkhead, 1957 [14] | † 55 | 21 (38) | 7 (13) | 13 (24) | 5 (9) | 18 (16) |
| ‡ 53 | 23 (43) | 16 (30) | 15 (28) | 9 (17) | 24 (23) | |
| ϕ 250 | 121 (48) | 92 (37) | 67 (27) | 54 (22) | 121 (24) | |
| Parsons-Smith, 1959 [32] | 50 | 23 (46) | NA | NA | NA | 33 (33) |
| Aiello, 1993 [19] | 245 | 34 (14) | 8 (3) | 12 (5) | 1 (0.4) | 14 (6) |
| Gonzalez-Gay, 2000 [21] | 161 | 24 (15) | 8 (5) | NA | NA | NA |
| Liozon, 2001 [29] | 174 | 23 (13) | NA | NA | 3 (14) | NA |
| Nesher, 2004 [30] | 166 | 32 (19) | NA | NA | NA | NA |
| Salvarini, 2016 [22] | 136 | 26 (19) | 7 (5) | NA | NA | NA |
| Chen, 2016 [35] | 245 | 20 (8) | 6 (2) | 4 (2) | 2 (1) | 6 (2) |
| Saleh, 2016 [36] | 840 | 85 (10) | 13 (2) | 18 (2) | 2 (2) | 20 (2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Héron, E.; Sedira, N.; Dahia, O.; Jamart, C. Ocular Complications of Giant Cell Arteritis: An Acute Therapeutic Emergency. J. Clin. Med. 2022, 11, 1997. https://doi.org/10.3390/jcm11071997
Héron E, Sedira N, Dahia O, Jamart C. Ocular Complications of Giant Cell Arteritis: An Acute Therapeutic Emergency. Journal of Clinical Medicine. 2022; 11(7):1997. https://doi.org/10.3390/jcm11071997
Chicago/Turabian StyleHéron, Emmanuel, Neila Sedira, Ouassila Dahia, and Céline Jamart. 2022. "Ocular Complications of Giant Cell Arteritis: An Acute Therapeutic Emergency" Journal of Clinical Medicine 11, no. 7: 1997. https://doi.org/10.3390/jcm11071997
APA StyleHéron, E., Sedira, N., Dahia, O., & Jamart, C. (2022). Ocular Complications of Giant Cell Arteritis: An Acute Therapeutic Emergency. Journal of Clinical Medicine, 11(7), 1997. https://doi.org/10.3390/jcm11071997





