Predictors Associated with Adverse Pregnancy Outcomes in a Cohort of Women with Systematic Lupus Erythematosus from Romania—An Observational Study (Stage 2)
Abstract
:1. Introduction
2. Materials and Methods
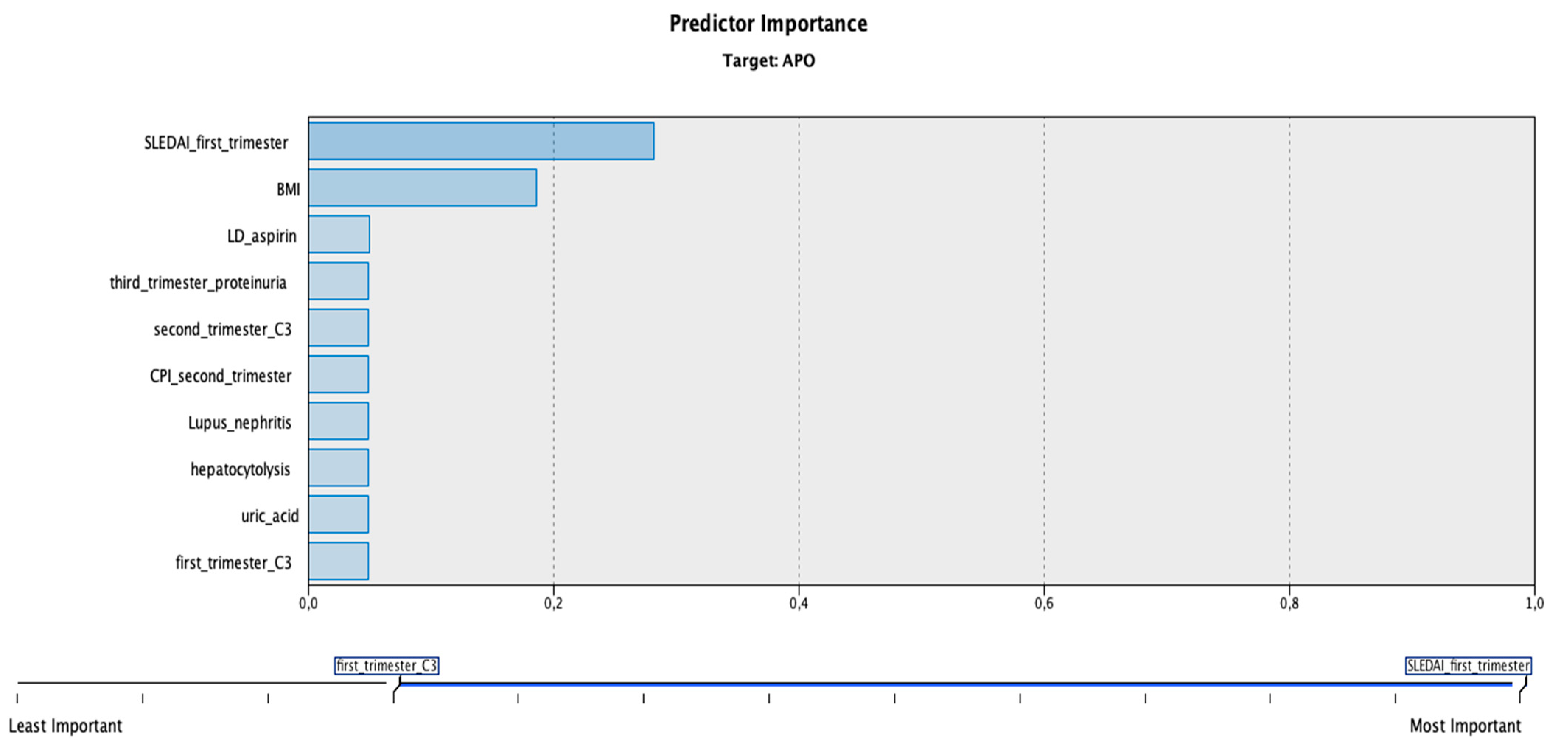
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clowse, M.E.; Jamison, M.; Myers, E.; James, A.H. A national study of the complications of lupus in pregnancy. Am. J. Obstet. Gynecol. 2008, 199, 127.e1–127.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyth, A.; Oliveira, G.H.; Lahr, B.D.; Bailey, K.R.; Norby, S.M.; Garovic, V.D. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin. J. Am. Soc. Nephrol. 2010, 5, 2060–2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajendran, A.; Eudy, A.M.; Balevic, S.J.; Clowse, M.E.B. The importance of pregnancy planning in lupus pregnancies. Lupus 2021, 30, 741–751. [Google Scholar] [CrossRef]
- Njagu, R.; Criscione-Schreiber, L.G.; Eudy, A.; Snyderman, A.; Clowse, M.E.B. Impact of a Multifaceted Educational Program to Improve Provider Skills for Lupus Pregnancy Planning and Management: A Mixed-Methods Approach. ACR Open Rheumatol. 2020, 2, 378–387. [Google Scholar] [CrossRef]
- Gergianaki, I.; Fanouriakis, A.; Repa, A.; Tzanakakis, M.; Adamichou, C.; Pompieri, A.; Spirou, G.; Bertsias, A.; Kabouraki, E.; Tzanakis, I.; et al. Epidemiology and burden of systemic lupus erythematosus in a Southern European population: Data from the community-based lupus registry of Crete, Greece. Ann. Rheum. Dis. 2017, 76, 1992–2000. [Google Scholar] [CrossRef]
- Ramanujan, S.A.; Cravens, E.N.; Krishfield, S.M.; Kyttaris, V.C.; Moulton, V.R. Estrogen-Induced hsa-miR-10b-5p Is Elevated in T Cells From Patients with Systemic Lupus Erythematosus and Down-Regulates Serine/Arginine-Rich Splicing Factor 1. Arthritis Rheumatol. 2021, 73, 2052–2058. [Google Scholar] [CrossRef] [PubMed]
- Nusbaum, J.S.; Mirza, I.; Shum, J.; Freilich, R.W.; Cohen, R.E.; Pillinger, M.H.; Izmirly, P.M.; Buyon, J.P. Sex Differences in Systemic Lupus Erythematosus: Epidemiology, Clinical Considerations, and Disease Pathogenesis. Mayo Clin. Proc. 2020, 95, 384–394. [Google Scholar] [CrossRef]
- Hochberg, M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef]
- Petri, M.; Orbai, A.-M.; Alarcón, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019, 71, 1400–1412. [Google Scholar] [CrossRef] [Green Version]
- Adamichou, C.; Nikolopoulos, D.; Genitsaridi, I.; Bortoluzzi, A.; Fanouriakis, A.; Papastefanakis, E.; Kalogiannaki, E.; Gergianaki, I.; Sidiropoulos, P.; Boumpas, D.T.; et al. In an early SLE cohort the ACR-1997, SLICC-2012 and EULAR/ACR-2019 criteria classify non-overlapping groups of patients: Use of all three criteria ensures optimal capture for clinical studies while their modification earlier classification and treatment. Ann. Rheum. Dis. 2020, 79, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Buyon, J.P.; Kim, M.Y.; Guerra, M.M.; Laskin, C.A.; Petri, M.; Lockshin, M.D.; Sammaritano, L.; Branch, D.W.; Porter, T.F.; Sawitzke, A.; et al. Predictors of Pregnancy Outcomes in Patients with Lupus: A Cohort Study. Ann. Intern. Med. 2015, 163, 153–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bramham, K.; Hunt, B.J.; Bewley, S.; Germain, S.; Calatayud, I.; Khamashta, M.A.; Nelson-Piercy, C. Pregnancy outcomes in systemic lupus erythematosus with and without previous nephritis. J. Rheumatol. 2011, 38, 1906–1913. [Google Scholar] [CrossRef]
- Kim, M.Y.; Guerra, M.M.; Kaplowitz, E.; Laskin, C.A.; Petri, M.; Branch, D.W.; Lockshin, M.D.; Sammaritano, L.R.; Merrill, J.T.; Porter, T.F.; et al. Complement activation predicts adverse pregnancy outcome in patients with systemic lupus erythematosus and/or antiphospholipid antibodies. Ann. Rheum. Dis. 2018, 77, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef] [Green Version]
- Lubchenco, L.O.; Hansman, C.; Dressler, M.; Boyd, E. Intrauterine Growth as Estimated from Liveborn Birth-Weight Data at 24 to 42 Weeks of Gestation. Pediatrics 1963, 32, 793–800. [Google Scholar] [CrossRef]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef] [Green Version]
- Howson, C.; Kinney, M.; Lawn, J. PMNCH, Save the Children, Who. Born too Soon: The Global Action Report on Preterm Birth; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.-B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef] [Green Version]
- Gladman, D.D.; Ibañez, D.; Urowitz, M.B. Systemic lupus erythematosus disease activity index 2000. J. Rheumatol. 2002, 29, 288–291. [Google Scholar]
- Petri, M.; Genovese, M.; Engle, E.; Hochberg, M. Definition, incidence, and clinical description of flare in systemic lupus erythematosus. A prospective cohort study. Arthritis Rheum. 1991, 34, 937–944. [Google Scholar] [CrossRef]
- Zamani, B.; Shayestehpour, M.; Esfahanian, F.; Akbari, H. The study of factors associated with pregnancy outcomes in patients with systemic lupus erythematosus. BMC Res. Notes 2020, 13, 185. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Jung, J.Y.; Kim, H.A.; Yang, J.I.; Kwak, D.W.; Suh, C.H. Lupus Low Disease Activity State Achievement Is Important for Reducing Adverse Outcomes in Pregnant Patients with Systemic Lupus Erythematosus. J. Rheumatol. 2021, 48, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Larosa, M.; Le Guern, V.; Guettrot-Imbert, G.; Morel, N.; Abisror, N.; Morati-Hafsaoui, C.; Orquevaux, P.; Diot, E.; Doria, A.; Reynauld, F.; et al. Evaluation of lupus anticoagulant, damage, and remission as predictors of pregnancy complications in lupus women: The French, G.R.2 study. Rheumatology 2022. [Google Scholar] [CrossRef]
- Pardos-Gea, J.; Marques-Soares, J.R.; Buján, S.; Ordi-Ros, J.; Alijotas-Reig, J. Persistent thrombocytopenia predicts poor long-term survival in patients with antiphospholipid syndrome: A 38-year follow-up study. Rheumatology 2021, 61, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.G.; Ding, H.J. Predictors of adverse pregnancy outcome in a cohort of women with systemic lupus erythematosus in Malaysia. Med. J. Malays. 2021, 76, 466–473. [Google Scholar]
- Zhan, Z.; Zhan, Y.; Lao, M.; Yang, X.; Wang, X.; Chen, D. Role of foetal umbilical artery Doppler on prediction of adverse pregnancy outcomes in patients with systemic lupus erythematosus. Clin. Exp. Rheumatol. 2018, 36, 871–878. [Google Scholar]
- López-Farfán, J.A.; Martínez-Marín, D.G.; Van Der Heyden-Pardo, T. Uterine artery score in patients with systemic lupus erythematous as a predictor of intrauterine growth restriction. Ginecol. Obstet. Mex. 2011, 79, 137–142. [Google Scholar]
- Cavallasca, J.A.; Laborde, H.A.; Ruda-Vega, H.; Nasswetter, G.G. Maternal and fetal outcomes of 72 pregnancies in Argentine patients with systemic lupus erythematosus (SLE). Clin. Rheumatol. 2008, 27, 41–46. [Google Scholar] [CrossRef]
- Clowse, M.E.; Magder, L.S.; Witter, F.; Petri, M. The impact of increased lupus activity on obstetric outcomes. Arthritis Rheum. 2005, 52, 514–521. [Google Scholar] [CrossRef]
- Kalok, A.; Abdul Cader, R.; Indirayani, I.; Abdul Karim, A.K.; Shah, S.A.; Mohamed Ismail, N.A.; Omar, M.H.; Shafiee, M.N. Pregnancy outcomes in systemic lupus erythematosus (SLE) women. Horm. Mol. Biol. Clin. Investig. 2019, 40. [Google Scholar] [CrossRef]
- Anuwutnavin, S.; Chuenchitkultavorn, V.; Nitiyarom, R.; Rekhawasin, T.; Kanjanauthai, S.; Sompagdee, N. Prenatal predisposing factors associated with neonatal lupus erythematosus. Lupus 2022, 31, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Costa Cascais, F.; Fraga, S.; Sousa, S.; Pinto, M. Neonatal lupus: A clinical challenge. BMJ Case Rep. 2021, 14, e246590. [Google Scholar] [CrossRef] [PubMed]
- Limaye, M.A.; Buyon, J.P.; Cuneo, B.F.; Mehta-Lee, S.S. A review of fetal and neonatal consequences of maternal systemic lupus erythematosus. Prenat. Diagn. 2020, 40, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- De Carolis, S.; Garufi, C.; Garufi, E.; De Carolis, M.P.; Botta, A.; Tabacco, S.; Salvi, S. Autoimmune Congenital Heart Block: A Review of Biomarkers and Management of Pregnancy. Front. Pediatrics 2020, 8, 607515. [Google Scholar] [CrossRef] [PubMed]
- Lazzaroni, M.G.; Fredi, M.; Andreoli, L.; Chighizola, C.B.; Del Ross, T.; Gerosa, M.; Kuzenko, A.; Raimondo, M.-G.; Lojacono, A.; Ramazzotto, F.; et al. Triple Antiphospholipid (aPL) Antibodies Positivity Is Associated with Pregnancy Complications in aPL Carriers: A Multicenter Study on 62 Pregnancies. Front. Immunol. 2019, 10, 1948. [Google Scholar] [CrossRef] [Green Version]
- Buyon, J.P.; Kim, M.Y.; Guerra, M.M.; Lu, S.; Reeves, E.; Petri, M.; Laskin, C.A.; Lockshin, M.D.; Sammaritano, L.R.; Branch, D.W.; et al. Kidney Outcomes and Risk Factors for Nephritis (Flare/De Novo) in a Multiethnic Cohort of Pregnant Patients with Lupus. Clin. J. Am. Soc. Nephrol. 2017, 12, 940–946. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, L.; Alkenaris, H.; Zervou, M.I.; Vlachakis, D.; Matalliotakis, I.; Spandidos, D.A.; Bertsias, G.; Goulielmos, G.N.; Eliopoulos, E. Epione application: An integrated web-toolkit of clinical genomics and personalized medicine in systemic lupus erythematosus. Int. J. Mol. Med. 2022, 49, 8. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, E.M.; Park, J.K.; Jeon, H.S.; Oh, S.; Hong, S.; Jung, Y.M.; Kim, B.J.; Kim, S.M.; Norwitz, E.R.; et al. Metabolic Biomarkers in Midtrimester Maternal Plasma Can Accurately Predict Adverse Pregnancy Outcome in Patients with SLE. Sci. Rep. 2019, 9, 15169. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.S.; Lee, S.M.; Jung, Y.M.; Oh, S.; Park, J.K.; Lee, E.B.; Park, C.-W.; Park, J.S.; Han, D.; Jun, J.K. Proteomic biomarkers in mid-trimester amniotic fluid associated with adverse pregnancy outcomes in patients with systemic lupus erythematosus. PLoS ONE 2020, 15, e0235838. [Google Scholar] [CrossRef]
- Sugiura-Ogasawara, M.; Omae, Y.; Kawashima, M.; Toyo-Oka, L.; Khor, S.S.; Sawai, H.; Horita, T.; Atsumi, T.; Murashima, A.; Fujita, D.; et al. The first genome-wide association study identifying new susceptibility loci for obstetric antiphospholipid syndrome. J. Hum. Genet. 2017, 62, 831–838. [Google Scholar] [CrossRef]
| Patient Data | No APOs | At Least One APO | p Value | |
|---|---|---|---|---|
| Demographics | Age (years, mean ± SD) | 30.53 ± 4.29 | 26.9 ± 6.6 | 0.054 |
| Medium n (%) | Rural = 8 (61.5%) Urban = 7 (58.3%) | Rural = 5 (38.5%) Urban = 5 (41.7%) | 0.87 | |
| BMI (kg/m2, mean ± SD) | 21.87 ± 2.47 | 26.4 ± 1.075 | <0.001 | |
| Patient history | Venous thrombosis n (%) | Yes = 1 (50%) No = 14 (60.9%) | Yes = 1 (50%) No = 9 (39.1%) | 0.763 |
| Recurrent pregnancy loss n (%) | Yes = 2 (50%) No = 13 (61.9%) | Yes = 2 (50%) No = 8 (38.1%) | 0.656 | |
| Lupus nephritis n (%) | Yes = 1 (20%) No = 14 (70%) | Yes = 4 (80%) No = 6 (30%) | 0.041 | |
| Maternal diabetes n (%) | Yes = 2 (40%) No = 13 (59.1%) | Yes = 3 (60%) No = 7 (40.9%) | 0.307 | |
| Thyroid disorder n (%) | Yes = 2 (66.7%) No = 13 (86.7%) | Yes = 1 (33.3%) No = 9 (90%) | 0.802 | |
| Chronic hypertension n (%) | Yes = 0 (0%) No = 15 (71.4%) | Yes = 4 (100%) No = 6 (28.6%) | 0.008 | |
| Clinical and paraclinical data | Uterine artery PI (mean ± SD) | 1.15 ± 0.2 | 1.58 ± 0.6 | <0.001 |
| Thrombocytopenia n (%) | Yes = 4 (40%) | Yes = 6 (60%) | 0.096 | |
| No = 11 (73.3%) | No = 4 (26.7%) | |||
| Proteinuria n (%) | Yes = 2 (22.2%) | Yes = 7 (77.8%) | 0.004 | |
| No = 13 (81.3%) | No = 3 (18.8%) | |||
| Anti-dsDNA n (%) | Yes = 10 (62.5%) | Yes = 6 (37.5%) | 0.734 | |
| No = 5 (55.6%) | No = 4 (44.4%) | |||
| C3 n (%) | Normal = 13 (81.3%) | Normal = 3 (18.8%) | 0.004 | |
| Low = 2 (22.2%) | Low = 7 (77.8%) | |||
| C4 n (%) | Normal = 13 (65%) | Normal = 7 (35%) | 0.307 | |
| Low = 2 (40%) | Low = 3 (60%) | |||
| LAC n (%) | Negative = 11 (64.7%) | Negative = 6 (35.3%) | 0.484 | |
| Positive = 4 (50%) | Positive = 4 (50%) | |||
| aCL n (%) | Negative = 14 (60.8%) | Negative = 9 (39.1%) | 0.763 | |
| Positive = 1 (50%) | Positive = 1 (50%) | |||
| Anti-β2 glycoprotein-I antibodies n (%) | Negative = 13 (59.09%) | Negative = 9 (40.9%) | 0.801 | |
| Positive = 2 (66.6%) | Positive = 1 (33.3%) | |||
| Anti-Ro n (%) | Negative = 12 (57.1%) | Negative = 9 (42.9%) | 0.504 | |
| Positive = 3 (75%) | Positive = 1 (25%) | |||
| Anti-La n (%) | Negative = 14 (60.9%) | Negative = 9 (39.1%) | 0.763 | |
| Positive = 1 (50%) | Positive = 1 (50%) | |||
| SLEDAI-2k n (%) | ≥4 = 3 (27.3%) | ≥4 = 8 (72.7%) | 0.003 | |
| <4 = 12 (85.7%) | <4 = 2 (14.3%) | |||
| PGA n (%) | ≥1 = 2 (20%) | ≥1 = 8 (80%) | <0.001 | |
| <1 = 13 (86.7%) | <1 = 2 (13.3%) | |||
| Patient Data | No APOs | At Least One APO | p Value | |
|---|---|---|---|---|
| Clinical data | Uterine artery PI (mean ± SD) | 1.21 ± 0.12 | 1.66 ± 0.59 | <0.001 |
| Cerebroplacental ratio n (%) | <1 = 0 (0%) | <1 = 3 (100%) | 0.024 | |
| >1 = 15 (68.2%) | >1 = 7 (31.8%) | |||
| Thrombocytopenia n (%) | Yes = 4 (44.4%) | Yes = 5 (55.6%) | 0.234 | |
| No = 11 (68.8%) | No = 5 (31.3%) | |||
| Proteinuria n (%) | Yes = 4 (36.4%) | Yes = 7 (63.6%) | 0.032 | |
| No = 11 (78.6%) | No = 3 (21.4%) | |||
| Anti-dsDNA n (%) | Yes = 9 (64.3%) | Yes = 5 (35.7%) | 0.622 | |
| No = 6 (54.5%) | No = 5 (45.5%) | |||
| C3 n (%) | Normal = 12 (92.3%) | Normal = 1 (7.7%) | <0.001 | |
| Low = 3 (25%) | Low = 9 (75%) | |||
| C4 n (%) | Normal = 12 (70.6%) | Normal = 5 (29.4%) | 0.11 | |
| Low = 3 (37.5%) | Low = 5 (62.5%) | |||
| SLEDAI-2k n (%) | ≥4 = 2 (18.2%) | ≥4 = 9 (81.8%) | <0.001 | |
| <4 = 13 (92.9%) | <4 = 1 (7.1%) | |||
| PGA n (%) | ≥1 = 1 (12.5%) | ≥1 = 7 (87.5%) | <0.001 | |
| <1 = 14 (82.4%) | <1 = 3 (17.6%) | |||
| Patient Data | No APOs | At Least One APO | p Value | |
|---|---|---|---|---|
| Clinical data | Cerebroplacental ratio n (%) | <1 = 0 (0%) | <1 = 3 (100%) | 0.024 |
| >1 = 15 (68.2%) | >1 = 7 (31.8%) | |||
| Venous duct n (%) | Abnormal = 0 (0%) | Abnormal = 2 (100%) | 0.071 | |
| Normal = 15 (65.2%) | Normal = 8 (34.8%) | |||
| Fetal abdominal circumference (<10th percentile) n (%) | Normal = 15 (93.8%) | Normal = 1 (6.3%) | <0.001 | |
| Low = 0 (0%) | Low = 9 (100%) | |||
| Anemia n (%) | Yes = 5 (71.4%) | Yes = 2 (28.6%) | 0.467 | |
| No = 10 (55.6%) | No = 8 (44.4%) | |||
| Leukopenia n (%) | Yes = 1 (50%) | Yes = 1 (50%) | 0.763 | |
| No = 14 (60.9%) | No = 9 (39.1%) | |||
| Thrombocytopenia n (%) | Yes = 4 (44.4%) | Yes = 5 (55.6%) | 0.234 | |
| No = 11 (68.8%) | No = 5 (31.3%) | |||
| Hepatic cytolysis n (%) | Yes = 0 (0%) | Yes = 3 (100%) | 0.024 | |
| No = 15 (68.2%) | No = 7 (31.8%) | |||
| Proteinuria n (%) | Yes = 6 (40%) | Yes = 9 (60%) | 0.012 | |
| No = 9 (90%) | No = 1 (10%) | |||
| Active urinary cast n (%) | Yes = 1 (20%) | Yes = 4 (80%) | 0.041 | |
| No = 14 (70%) | No = 6 (30%) | |||
| Uric acid n (%) | High = 1 (12.5%) | High = 7 (87.5%) | <0.001 | |
| Normal = 14 (82.4%) | Normal = 3 (17.6%) | |||
| Anti-dsDNA n (%) | Yes = 7 (58.3%) | Yes = 5 (41.7%) | 0.87 | |
| No = 8 (61.5%) | No = 5 (38.5%) | |||
| C3 n (%) | Normal = 13 (86.7%) | Normal = 2 (13.3%) | <0.001 | |
| Low = 2 (20%) | Low = 8 (80%) | |||
| C4 n (%) | Normal = 11 (73.3%) | Normal = 4 (26.7%) | 0.096 | |
| Low = 4 (40%) | Low = 6 (60%) | |||
| SLEDAI-2k n (%) | ≥4 = 13 (76.5%) | ≥4 = 4 (23.5%) | 0.014 | |
| <4 = 2 (25%) | <4 = 6 (75%) | |||
| PGA n (%) | ≥1 = 4 (36.4%) | ≥1 = 7 (63.6%) | 0.032 | |
| <1 = 11 (78.6%) | <1 = 3 (21.4%) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicoveanu, P.; Vasilache, I.-A.; Nemescu, D.; Carauleanu, A.; Scripcariu, I.-S.; Rudisteanu, D.; Burlui, A.; Rezus, E.; Socolov, D. Predictors Associated with Adverse Pregnancy Outcomes in a Cohort of Women with Systematic Lupus Erythematosus from Romania—An Observational Study (Stage 2). J. Clin. Med. 2022, 11, 1964. https://doi.org/10.3390/jcm11071964
Vicoveanu P, Vasilache I-A, Nemescu D, Carauleanu A, Scripcariu I-S, Rudisteanu D, Burlui A, Rezus E, Socolov D. Predictors Associated with Adverse Pregnancy Outcomes in a Cohort of Women with Systematic Lupus Erythematosus from Romania—An Observational Study (Stage 2). Journal of Clinical Medicine. 2022; 11(7):1964. https://doi.org/10.3390/jcm11071964
Chicago/Turabian StyleVicoveanu, Petronela, Ingrid-Andrada Vasilache, Dragos Nemescu, Alexandru Carauleanu, Ioana-Sadiye Scripcariu, Dorina Rudisteanu, Alexandra Burlui, Elena Rezus, and Demetra Socolov. 2022. "Predictors Associated with Adverse Pregnancy Outcomes in a Cohort of Women with Systematic Lupus Erythematosus from Romania—An Observational Study (Stage 2)" Journal of Clinical Medicine 11, no. 7: 1964. https://doi.org/10.3390/jcm11071964
APA StyleVicoveanu, P., Vasilache, I.-A., Nemescu, D., Carauleanu, A., Scripcariu, I.-S., Rudisteanu, D., Burlui, A., Rezus, E., & Socolov, D. (2022). Predictors Associated with Adverse Pregnancy Outcomes in a Cohort of Women with Systematic Lupus Erythematosus from Romania—An Observational Study (Stage 2). Journal of Clinical Medicine, 11(7), 1964. https://doi.org/10.3390/jcm11071964







