Abstract
Prosthesis–patient mismatch (PPM) is associated with worse outcomes following surgical aortic valve replacement (SAVR). PPM has been identified in a significant proportion of TAVR, particularly in patients with small aortic annuli. Our objective was to evaluate the hemodynamic performances of balloon-expandable (BE) (Sapiens 3TM) versus two different self-expandable (SE) (Evolut ProTM, Accurate NeoTM) TAVR devices in patients with small aortic annulus defined by a computed tomography aortic annulus area (AAA) between 330 and 440 mm2. We enrolled 131 consecutive patients corresponding to 76 Sapiens 3 23 mm (58.0%), 26 Evolut Pro (19.9%) and 29 Accurate Neo (22.1%). Mean age was 82.5 ± 7.06 years, 22.9% of patients were male and mean Euroscore was 4.0%. Mean AAA was 374 ± 27 mm2 for Sapiens 3, 383 ± 29 mm2 for Corevalve Evolut Pro and 389 ± 25 mm2 for Accurate Neo. BE devices were associated with significantly higher rates of PPM (39.5%) as compared to SE devices (15.4% for Corevalve Evolut Pro and 6.9% for Accurate Neo) (p < 0.0001). Paravalvular leaks ≥ 2/4 were more often observed in SE devices (15.4% for Corevalve Evolut Pro and 17.2% for Accurate Neo) than in BE devices (2.6%) (p = 0.007). In conclusion, SE TAVR devices did achieve better hemodynamic results despite higher rates of paravalvular leaks. Therefore, SE TAVI devices could be considered as first choice in small aortic anatomy.
1. Introduction
Continuous development has improved the results of transcatheter aortic valve replacement (TAVR). Eligible surgical risk classes and volume of TAVR have expanded over years, and this technique has exceeded surgical aortic valve replacement (SAVR) in the US and European countries [1]. While prosthesis–patient mismatch (PPM) has been associated with impairment of long term survival following SAVR [2,3,4,5,6,7], particular attention is required for TAVR, considering that this technique became the first line aortic valve replacement strategy. In large cohorts, despite a lower incidence than in SAVR, PPM has been identified in a significant proportion of TAVR and particularly in patients with small aortic annuli [8,9]. The balloon-expandable (BE) valve and the self-expandable (SE) valve have been proven to be effective in the management of severe symptomatic aortic stenosis with some difference in hemodynamic results [10,11,12]. In this population of patients with small aortic annuli, valvular anatomic characteristics should perhaps dictate the type of valve to be used.
Therefore, our objective was to evaluate the hemodynamic performances of balloon-expandable (BE) versus two different self-expandable (SE) TAVR devices in patients with small aortic annulus defined by a computed tomography (CT) aortic annulus area between 330 and 440 mm2.
2. Materials and Methods
2.1. Study Population and Design
In La Timone Hospital, from June 2019 to April 2021, we prospectively enrolled all patients with a small aortic annulus between 330 and 440 mm2 on cardiac CT undergoing TAVI for severe symptomatic aortic stenosis. TAVR was performed using either a 23-mm Sapiens 3TM (Edwards Lifesciences, Irvine, CA, USA) balloon-expandable (BE) valve, an Accurate NeoTM S or M (Boston Scientific, Marlborough, MA, USA) or a 26- or 29-mm Evolut ProTM (Medtronic, Dublin, Ireland) self-expanding (SE) valve, according to physician preference. This study complied with the provisions of the Declaration of Helsinki and all patients provided written consent. All patients were over 18. The exclusion criteria were patients who did not receive a complete post-procedure echocardiography evaluation because of intraoperative or early postoperative death; incomplete ultrasound data not allowing calculation of the effective orifice area (EOA) of the prosthesis; and missing echocardiography data at 1 month of follow-up. As the patients were treated with commercially available devices (validated in those indications) we did not require an ethical committee for implantation of BE in patients with small aortic annuli. Of note, this study complied with the provisions of the Declaration of Helsinki and all patients provided written consent.
2.2. Procedure
Each patient underwent a multidisciplinary preoperative heart team evaluation combined with a CT scan to validate the indication for the procedure. Transfemoral access was used almost exclusively.
2.3. PPM Definition
According to the Valve Academic Research Consortium (VARC)-3 criteria [13], we defined the severity of PPM according to the indexed EOA (iEOA) of the prosthetic valve and classified PPM severity as: None or mild, >0.85 cm2/m2; moderate, between 0.85 and 0.65 cm2/m2; and severe, <0.65 cm2/m2.
2.4. Paravalvular Leak Definition
Aortic regurgitation (paravalvular leak) was assessed by using colourflow Doppler signal and graded in 5 groups: None or trivial (=0/4), mild (=1/4), mild-to-moderate (=2/4), moderate-to-severe (=3/4), or severe (=4/4).
2.5. Small Aortic Annulus Definition
We took as definition for small annuli the patients who could benefit from the smallest Sapiens 3 valve available in France (i.e., Sapiens 3 23 mm valve) according to the manufacturers’ labeling ranking (330–440 mm2).
2.6. Follow Up
Follow up was done at one month with clinical consultation and echocardiography. During clinical consultation, vital status and NYHA status were checked. We evaluated left ventricular ejection fraction (LVEF), effective orifice area (EOA), indexed EOA, PPM and grade of paravalvular leak.
2.7. Endpoints
The primary study endpoint was the occurrence of moderate or severe PPM at one month. The secondary endpoints were the occurrence of paravalvular leak ≥ 2 at one month and pacemaker implantation during the thirty-first days.
2.8. Statistical Analysis
Statistical analysis was performed using PASW Statistics version 18.0. Continuous variables were reported as means and standard deviation or as medians and range (according to their distribution), and categorical variables were reported as count and percentages. Standard two-sided tests were used to compare continuous characteristics (Student t or Mann–Whitney U tests) or categorical characteristics (chi-square or Fisher exact tests) among patient groups. For all tests, statistical significance was defined as p < 0.05.
3. Results
A total of 143 consecutive patients were included prospectively from between June 2019 and April 2021. Twelve patients were excluded (Figure 1). We finally studied 131 patients corresponding to 76 Sapiens 3TM 23 mm (58.0%), 26 Evolut ProTM (19.9%) and 29 Accurate NeoTM (22.1%). Mean age was 82.5 ± 7.06 years, 22.9% of patients were male and mean Euroscore was 3.9%. Transfemoral TAVR was performed in 99.2% of cases. There was no difference in baseline characteristics between groups (Table 1, Table 2 and Table 3). Mean AAA was non significantly different within the three groups (374 ± 27 mm2 for Sapiens 3383 ± 29 mm2 for Corevalve Evolut Pro and 389 ± 25 mm2 for Accurate Neo; p = 0.06). Postdilatation was performed in seven patients (zero Sapiens 3TM, four Accurate NeoTM and three Evolut ProTM).
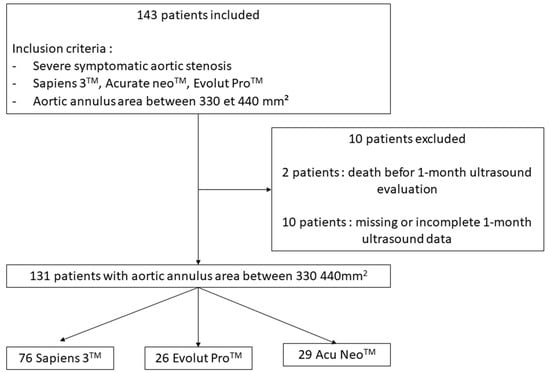
Figure 1.
Flow chart.

Table 1.
Patients’ characteristics at the baseline.

Table 2.
Echocardiography data at the baseline and access data.

Table 3.
Echocardiography data at one month.
3.1. Occurrence of Moderate or Severe PPM at One Month
Primary endpoint occurred for 30/76 patients (39.5%) in the Sapiens 3TM 23 mm group, 4/26 patients (15.4%) in the Evolut ProTM and 2/29 patients (6.9%) in the Accurate NeoTM group with a significant difference between the three groups (p < 0.0001). Interestingly, BE devices were associated with higher rates of moderate PPM (26.3%) with a significant difference (p = 0.008) and severe PPM (13.2%) without significant difference as compared to SE (Figure 2 and Figure 3).
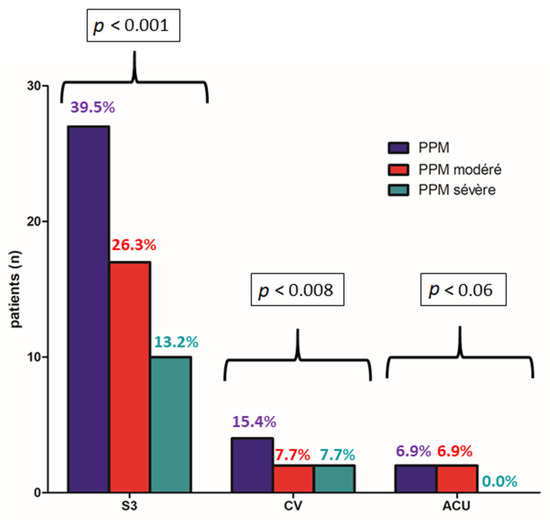
Figure 2.
Occurrence of moderate or severe PPM at one month according to balloon-expandable (Sapiens 3TM) vs. self-expandable (Evolut ProTM, Accurate NeoTM) TAVR devices.
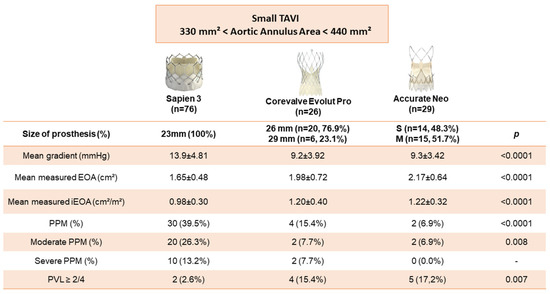
Figure 3.
Hemodynamic characteristics at 1 month after TAVR according to balloon-expandable (Sapiens 3TM) vs. self-expandable (Evolut ProTM, Accurate NeoTM) TAVR devices.
Furthermore, mean gradients were higher in the Sapiens 3TM 23 mm group (13.9 ± 4.81 mmHg) than in the Evolut ProTM group (9.2 ± 3.92 mmHg) and in the Accurate NeoTM group (9.3 ± 3.42 mmHg) with a significant difference (p < 0.0001).
3.2. Occurrence of Paravalvular Leak at One Month and Pacemaker Implantation during the Thirty-First Days
Paravalvular leaks ≥ 2/4 were more often observed in the Evolut ProTM group (4/26; 15.4%) and in the Accurate NeoTM group (5/17; 17.2%) than in the Sapiens 3TM 23 mm group (2/76; 2.6%) with a significant difference between the three groups (p < 0.007) (Figure 2).
Pacemaker implantation during the thirty-first days was the same in the three group (Sapiens 3TM 23 mm 18.4%, Evolut ProTM 15.4%, Accurate NeoTM 17.2%) (p = 0.93) (Table 4).

Table 4.
Post-procedural complication.
4. Discussion
The main findings of this study are:
- (1)
- PPM was more often observed with BE TAVR than with SE TAVR and mean gradients were higher with BE TAVR
- (2)
- The majority of paravalvular leaks ≥ 2/4 occurred with SE TAVR compared to BE TAVR
PPM remains an under-explored complication of TAVR, which may be associated with worse clinical outcomes and accelerated structural valve deterioration [14]. Small aortic annuli are at particular risk of PPM, mostly when treated with intra-annular and bulkier devices, due to higher risk of overcrowding of the left ventricle outflow tract [15]. When considering BE TAVR, 23 mm size of the Sapiens 3 valve has been associated with significant incidence of PPM compared to larger sizes [8]. We confirmed that supra-annular TAVR design achieved larger iEOA and better hemodynamics in small aortic annuli, although our data are in favour of overall low risk of PPM. Our results corroborate those from the CHOICE randomized trial, with a more accurate definition of small aortic annulus based on CT and addition of a second SE TAVR device [16]. Hase et al. compared Sapiens 3 and Corevalve and showed that SE TAVR had better hemodynamic performance in small annuli [17]. Recently, studies have questioned the prognostic impact of mismatch in the global population of TAVR. Concerning SE valves, Tang et al. showed that the presence of a severe mismatch did not impact one-year mortality [18]. Conversely, for this type of valve, Leone et al. demonstrated that severe PPM had an impact on the prognosis of patients after TAVR (higher rates of all cause mortality) [19]. However, in light of our results, patients with a small aortic annulus seem to benefit from extensive screening. It will probably participate in the reduction of the occurrence of a mismatch and structural valve deterioration.
Another important finding of our study is the presence of a higher rate of paravalvular leaks with SE valves compared to the two BE TAVR. This finding is supported by the SOLVE TAVI study [12]. In these small anatomies, careful CT analysis could identify high-risk situations for PVL (aortic annuli with important calcifications).
Strength and Limitation
There were several limitations to this study, the most important one being its single center nature with a small study population.
Then, the echocardiography measurements, particularly the LVOT diameter and LVOT VTI assessment, are particularly challenging and may overestimate the incidence of PPM [20]. The use of predicted iEOA could allow to get rid of these echocardiography variabilities [21].
Moreover, our assessment of paravalvular leak in four grades may increase their severity compared to a definition based on five grades [13] which would explain the higher rates of paravalvular leaks in our study.
5. Conclusions
In small aortic annuli, SE TAVR devices did achieve better hemodynamic results despite higher rates of paravalvular leaks. Our work suggests that, in this population, SE TAVR design may be favoured. Indeed, there is a compromise to make in small aortic annuli between PVL and PPM and a special interest should be given to the choice of valve.
Author Contributions
Conceptualization, J.F., A.T., T.C. and P.D.; Data curation, J.F.; Investigation, J.F. and P.L.; Validation, A.P., P.M., P.L., N.J., V.G. and F.C.; Visualization, A.P., P.M., N.J., V.G. and F.C.; Writing—original draft, J.F. and P.D.; Writing—review & editing, A.T., T.C. and P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethics committee of the our institution approved the study protocol and publication of data.
Informed Consent Statement
The patients provided informed written consent for the publication.
Conflicts of Interest
TC reports consulting or speaker activities for Edwards, Boston Scientific and Med-tronic. All other authors declare no competing interests.
Abbreviations
| TAVR | transcatheter aortic valve replacement |
| SAVR | surgical aortic valve replacement |
| PPM | prosthesis–patient mismatch |
| BE | balloon-expandable |
| SE | self-expandable |
| CT | computed tomography |
| EOA | effective orifice area |
| LVEF | left ventricular ejection fraction |
| VARC | Valve Academic Research Consortium |
| iEOA | indexed EOA |
| LVOT | left ventricular outflow tract obstruction |
| LVOT VTI | left ventricular outflow tract obstruction velocity time integral |
References
- Carroll, J.D.; Mack, M.J.; Vemulapalli, S.; Herrmann, H.C.; Gleason, T.G.; Hanzel, G.; Deeb, G.M.; Thourani, V.H.; Cohen, D.J.; Desai, N.; et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2020, 76, 2492–2516. [Google Scholar] [CrossRef] [PubMed]
- Blais, C.; Dumesnil, J.G.; Baillot, R.; Simard, S.; Doyle, D.; Pibarot, P. Impact of valve prosthesis-patient mismatch on short-term mortality after aortic valve replacement. Circulation 2003, 108, 983–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pibarot, P.; Dumesnil, J.G. Prosthesis-patient mismatch: Definition, clinical impact, and prevention. Heart 2006, 92, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Tasca, G.; Mhagna, Z.; Perotti, S.; Centurini, P.B.; Sabatini, T.; Amaducci, A.; Brunelli, F.; Cirillo, M.; Tomba, M.D.; Quiani, E.; et al. Impact of prosthesis-patient mismatch on cardiac events and midterm mortality after aortic valve replacement in patients with pure aortic stenosis. Circulation 2006, 113, 570–576. [Google Scholar] [CrossRef] [Green Version]
- Mohty, D.; Dumesnil, J.G.; Echahidi, N.; Mathieu, P.; Dagenais, F.; Voisine, P.; Pibarot, P. Impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: Influence of age, obesity, and left ventricular dysfunction. J. Am. Coll. Cardiol. 2009, 53, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Head, S.J.; Mokhles, M.M.; Osnabrugge, R.L.; Pibarot, P.; Mack, M.J.; Takkenberg, J.; Bogers, A.J.; Kappetein, A.P. The impact of prosthesis-patient mismatch on long-term survival after aortic valve replacement: A systematic review and meta-analysis of 34 observational studies comprising 27,186 patients with 133,141 patient-years. Eur. Heart J. 2012, 33, 1518–1529. [Google Scholar] [CrossRef] [Green Version]
- Pibarot, P.; Weissman, N.J.; Stewart, W.J.; Hahn, R.; Lindman, B.; McAndrew, T.; Kodali, S.K.; Mack, M.J.; Thourani, V.H.; Miller, D.C.; et al. Incidence and sequelae of prosthesis-patient mismatch in transcatheter versus surgical valve replacement in high-risk patients with severe aortic stenosis: A PARTNER trial cohort—A analysis. J. Am. Coll. Cardiol. 2014, 64, 1323–1334. [Google Scholar] [CrossRef] [Green Version]
- Miyasaka, M.; Tada, N.; Taguri, M.; Kato, S.; Enta, Y.; Otomo, T.; Hata, M.; Watanabe, Y.; Naganuma, T.; Araki, M.; et al. Incidence, Predictors, and Clinical Impact of Prosthesis-Patient Mismatch Following Transcatheter Aortic Valve Replacement in Asian Patients: The OCEAN-TAVI Registry. JACC Cardiovasc. Interv. 2018, 11, 771–780. [Google Scholar] [CrossRef]
- Herrmann, H.C.; Daneshvar, S.A.; Fonarow, G.C.; Stebbins, A.; Vemulapalli, S.; Desai, N.D.; Malenka, D.J.; Thourani, V.H.; Rymer, J.; Kosinski, A.S. Prosthesis-Patient Mismatch in Patients Undergoing Transcatheter Aortic Valve Replacement: From the STS/ACC TVT Registry. J. Am. Coll. Cardiol. 2018, 72, 2701–2711. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Thiele, H.; Kurz, T.; Feistritzer, H.-J.; Stachel, G.; Hartung, P.; Eitel, I.; Marquetand, C.; Nef, H.; Doerr, O.; Lauten, A.; et al. Comparison of newer generation self-expandable vs. balloon-expandable valves in transcatheter aortic valve implantation: The randomized SOLVE-TAVI trial. Eur. Heart J. 2020, 41, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- VARC-3 Writing Committee; Généreux, P.; Piazza, N.; Alu, M.C.; Nazif, T.; Hahn, R.T.; Pibarot, P.; Bax, J.J.; Leipsic, J.A.; Blanke, P.; et al. Valve Academic Research Consortium 3, updated endpoint definitions for aortic valve clinical research. Eur. Heart J. 2021, 42, 1825–1857. [Google Scholar] [CrossRef] [PubMed]
- Pibarot, P.; Magne, J.; Leipsic, J.; Côté, N.; Blanke, P.; Thourani, V.H.; Hahn, R. Imaging for Predicting and Assessing Prosthesis-Patient Mismatch After Aortic Valve Replacement. JACC Cardiovasc. Imaging 2019, 12, 149–162. [Google Scholar] [CrossRef]
- Theron, A.; Pinto, J.; Grisoli, D.; Griffiths, K.; Salaun, E.; Jaussaud, N.; Ravis, E.; Lambert, M.; Messous, L.; Amanatiou, C.; et al. Patient-prosthesis mismatch in new generation trans-catheter heart valves: A propensity score analysis. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Abdelghani, M.; Mankerious, N.; Allali, A.; Landt, M.; Kaur, J.; Sulimov, D.S.; Merten, C.; Sachse, S.; Mehilli, J.; Neumann, F.-J.; et al. Bioprosthetic Valve Performance After Transcatheter Aortic Valve Replacement with Self-Expanding Versus Balloon-Expandable Valves in Large Versus Small Aortic Valve Annuli: Insights from the CHOICE Trial and the CHOICE-Extend Registry. JACC Cardiovasc. Interv. 2018, 11, 2507–2518. [Google Scholar] [CrossRef]
- Hase, H.; Yoshijima, N.; Yanagisawa, R.; Tanaka, M.; Tsuruta, H.; Shimizu, H.; Fukuda, K.; Naganuma, T.; Mizutani, K.; Yamawaki, M.; et al. Transcatheter aortic valve replacement with Evolut R versus Sapien 3 in Japanese patients with a small aortic annulus: The OCEAN-TAVI registry. Catheter. Cardiovasc. Interv. 2021, 97, E875–E886. [Google Scholar] [CrossRef]
- Tang, G.H.; Sengupta, A.; Alexis, S.L.; Bapat, V.N.; Adams, D.H.; Sharma, S.K.; Kini, A.S.; Kodali, S.K.; Ramlawi, B.; Gada, H.; et al. Outcomes of Prosthesis-Patient Mismatch Following Supra-Annular Transcatheter Aortic Valve Replacement: From the STS/ACC TVT Registry. JACC Cardiovasc. Interv. 2021, 14, 964–976. [Google Scholar] [CrossRef]
- Leone, P.P.; Regazzoli, D.; Pagnesi, M.; Sanz-Sanchez, J.; Chiarito, M.; Cannata, F.; Van Mieghem, N.M.; Barbanti, M.; Tamburino, C.; Teles, R.; et al. Predictors and Clinical Impact of Prosthesis-Patient Mismatch After Self-Exapndable TAVR in Small Annuli. JACC Cardiovasc. Interv. 2021, 14, 1218–1228. [Google Scholar] [CrossRef]
- Clavel, M.A.; Rodés-Cabau, J.; Dumont, É.; Bagur, R.; Bergeron, S.; De Larochellière, R.; Doyle, D.; Larose, É.; Dumesnil, J.G.; Pibarot, P. Validation and characterization of transcatheter aortic valve effective orifice area measured by Doppler echocardiography. JACC Cardiovasc. Imaging 2011, 4, 1053–1062. [Google Scholar] [CrossRef] [Green Version]
- Ternacle, J.; Guimaraes, L.; Vincent, F.; Côté, N.; Côté, M.; Lachance, D.; Clavel, M.-A.; Abbas, A.E.; Pibarot, P.; Rodés-Cabau, J. Reclassification of prosthesis-patient mismatch after transcatheter aortic valve replacement using predicted vs. measured indexed effective orifice area. Eur. Heart J. Cardiovasc. Imaging 2020, 22, 11–20. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).