Impact of Activity-Oriented Propioceptive Antiedema Therapy on the Health-Related Quality of Life of Women with Upper-Limb Lymphedema Secondary to Breast Cancer—A Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
- -
- Activities with neurodynamic slip patterns;
- -
- Proprioceptive neuromuscular facilitation activities;
- -
- Proprioceptive cohesive anti-edema bandage, with a technique similar to the Coban-type bandage, but without compression and with a high cotton content. The patient and/or caregiver is instructed on its use and placement and modifications or adaptations are recommended for optimal performance in their ADLs.
2.2. Study Participants and Recruitment
2.3. Sample Size
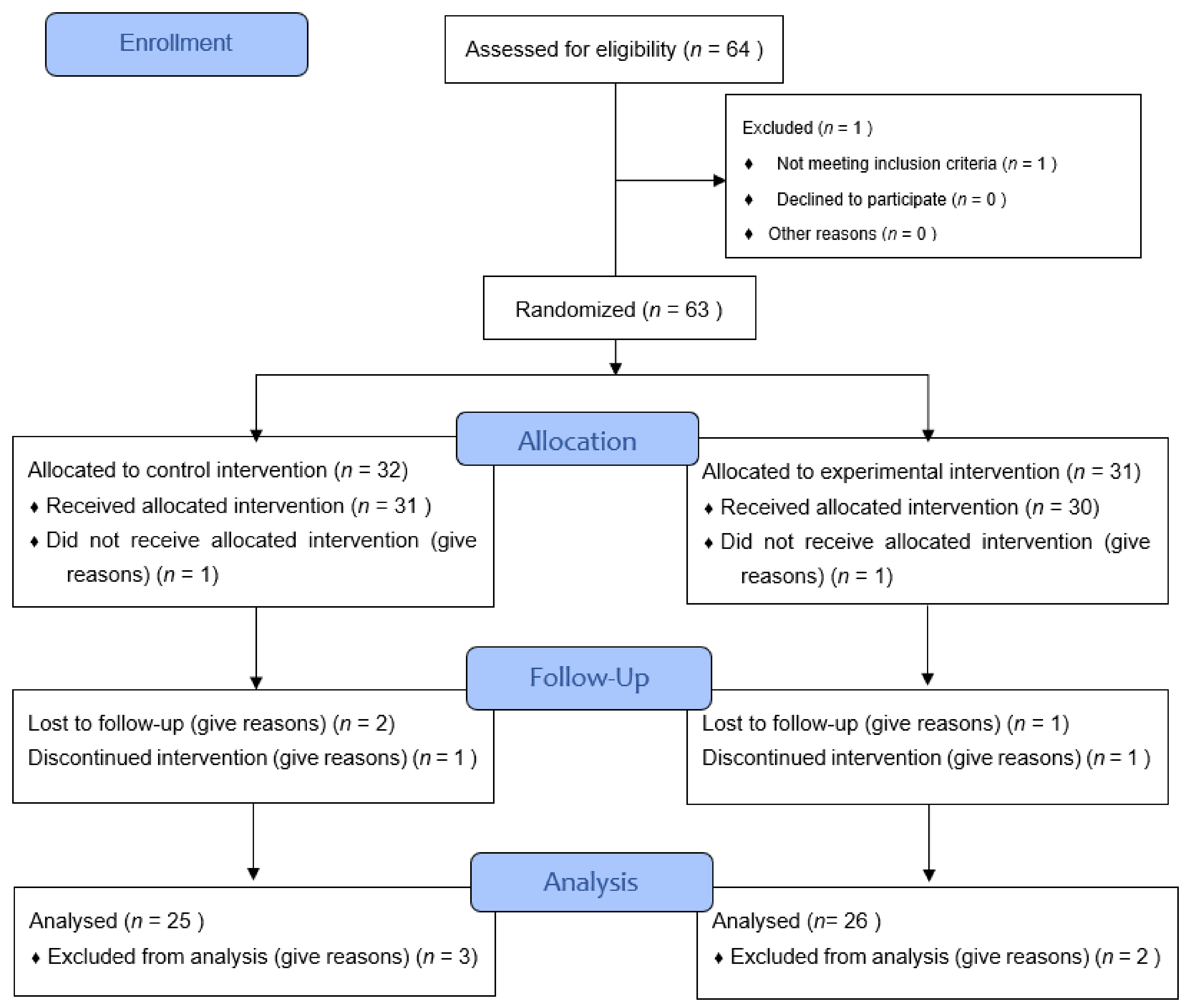
2.4. Procedure and Randomisation
2.5. Main Outcomes
2.6. Statistical Analysis
3. Results
3.1. Main Characteristics of the Participants
3.2. Quality of Life: Pain, Heaviness, Tightness and Performance
3.3. HRQoL: Differences between Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kassamani, Y.W.; Brunelle, C.L.; Gillespie, T.C.; Bernstein, M.C.; Bucci, L.K.; Nassif, T.; Taghian, A.G. Diagnostic Criteria for Breast Cancer-Related Lymphedema of the Upper Extremity: The Need for Universal Agreement. Ann. Surg. Oncol. 2021, 29, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Viehoff, P.B.; Gielink, P.D.C.; Damstra, R.J.; Heerkens, Y.F.; Van Ravensberg, D.C.; Neumann, M.H.A. Functioning in lymphedema from the patients’ perspective using the International Classification of Functioning, Disability and health (ICF) as a reference. Acta Oncol. 2015, 54, 411–421. [Google Scholar] [CrossRef] [PubMed]
- De Britto, L.R.P.B.; de Souza, E.C.; Ribeiro, L.T.; Schwingel, P.A.; de Melo, A.R.A.; do Nascimento, P.L.; Portela, G.J.S. The impact of sensory alterations on upper limb function after a mastectomy. Mastology 2017, 27, 287–292. [Google Scholar] [CrossRef]
- Eaton, L.H.; Narkthong, N.; Hulett, J.M. Psychosocial Issues Associated with Breast Cancer-Related Lymphedema: A Literature Review. Curr. Breast Cancer Rep. 2020, 12, 216–224. [Google Scholar] [CrossRef]
- Marchica, P.; D’Arpa, S.; Magno, S.; Rossi, C.; Forcina, L.; Capizzi, V.; Oieni, S.; Amato, C.; Piazza, D.; Gebbia, V. Integrated Treatment of Breast Cancer-related Lymphedema: A Descriptive Review of the State of the Art. Anticancer Res. 2021, 41, 3233–3246. [Google Scholar] [CrossRef]
- Davies, C.C.; Levenhagen, K.; Ryans, K.; Perdomo, M.; Gilchrist, L. An Executive Summary of the APTA Academy for Oncologic Physical Therapy Clinical Practice Guideline: Interventions for Breast Cancer–Related Lymphedema. Rehabilitation Oncol. 2020, 38, 103–109. [Google Scholar] [CrossRef]
- Fish, M.L.; Grover, R.; Schwarz, G.S. Quality-of-life outcomes in surgical vs nonsurgical treatment of breast cancer-related lymphedema: A systematic review. JAMA Surg. 2020, 155, 513–519. [Google Scholar] [CrossRef]
- Tang, N.S.J.; Ramakrishnan, A.; Shayan, R. Quality-of-life outcomes after operative management of primary and secondary lymphoedema: A systematic review. ANZ J. Surg. 2021, 91, 2624–2636. [Google Scholar] [CrossRef]
- Bergmann, A.; Baiocchi, J.M.T.; de Andrade, M.F.C. Conservative treatment of lymphedema: The state of the art. J. Vasc. Bras. 2021, 20, 1–10. [Google Scholar] [CrossRef]
- Congress, I. The diagnosis and treatment of peripheral lymphedema: 2020 consensus document of the international society of lymphology. Lymphology 2020, 53, 3–19. [Google Scholar]
- Pappalardo, M.; Starnoni, M.; Franceschini, G.; Baccarani, A.; De Santis, G. Personalized Medicine Breast Cancer-Related Lymphedema: Recent Updates on Diagnosis, Severity and Available Treatments. J. Pers. Med. 2021, 11, 402. [Google Scholar] [CrossRef]
- Longhurst, E.; Dylke, E.S.; Kilbreath, S.L. Use of compression garments by women with lymphoedema secondary to breast cancer treatment. Support. Care Cancer 2018, 26, 2625–2632. [Google Scholar] [CrossRef]
- Hasenoehrl, T.; Palma, S.; Ramazanova, D.; Kölbl, H.; Dorner, T.E.; Keilani, M.; Crevenna, R. Resistance exercise and breast cancer–related lymphedema—a systematic review update and meta-analysis. Support. Care Cancer 2020, 28, 3593–3603. [Google Scholar] [CrossRef]
- Hayes, S.; Singh, B.; Bloomquist, K.; Johansson, K. Do Women with Breast Cancer–related Lymphoedema Need to Wear Compression While Exercising?: Results from a Systematic Review and Meta-analysis. Curr. Breast Cancer Rep. 2020, 12, 193–201. [Google Scholar] [CrossRef]
- Rabe, E.; Partsch, H.; Morrison, N.; Meissner, M.H.; Mosti, G.; Lattimer, C.R.; Carpentier, P.H.; Gaillard, S.; Jünger, M.; Urbanek, T.; et al. Risks and contraindications of medical compression treatment—A critical reappraisal. An international consensus statement. Phlebology 2020, 35, 447–460. [Google Scholar] [CrossRef]
- Ezzo, J.; Manheimer, E.; Mcneely, M.L.; Howell, D.M.; Weiss, R.; Johansson, K.I.; Bao, T.; Bily, L.; Tuppo, C.M.; Williams, A.F.; et al. Manual lymphatic drainage for lymphedema following breast cancer treatment. Cochrane Database Syst. Rev. 2015, 5, CD003475. [Google Scholar] [CrossRef]
- Beelen, L.M.; van Dishoeck, A.M.; Tsangaris, E.; Coriddi, M.; Dayan, J.H.; Pusic, A.L.; Klassen, A.; Vasilic, D. Patient-Reported Outcome Measures in Lymphedema: A Systematic Review and COSMIN Analysis. Ann. Surg. Oncol. 2021, 28, 1656–1668. [Google Scholar] [CrossRef]
- Tánori-Tapia, J.M.; Romero-Pérez, E.M.; Camberos, N.A.; Horta-Gim, M.A.; Núñez-Othón, G.; Medina-Pérez, C.; de Paz, J.A. Determination of the minimum detectable change in the total and segmental volumes of the upper limb, evaluated by perimeter measurements. Healthcare 2020, 8, 285. [Google Scholar] [CrossRef]
- Muñoz-Alcaraz, M.N.; Pérula-de-Torres, L.Á.; Serrano-Merino, J.; Jiménez-Vílchez, A.J.; Olmo-Carmona, M.V.; Muñoz-García, M.T.; Bartolomé-Moreno, C.; Oliván-Blázquez, B.; Magallón-Botaya, R. Efficacy and efficiency of a new therapeutic approach based on activity-oriented proprioceptive antiedema therapy (AAPT) for edema reduction and improved occupational performance in the rehabilitation of breast cancer-related arm lymphedema in women: A controlled, randomized clinical trial. BMC Cancer 2020, 20, 1074. [Google Scholar] [CrossRef]
- Kizil, R.; Dilek, B.; Şahin, E.; Engin, O.; Soylu, A.C.; Akalin, E.; Alper, S. Is Continuous Passive Motion Effective in Patients with Lymphedema? A Randomized Controlled Trial. Lymphat. Res. Biol. 2018, 16, 263–269. [Google Scholar] [CrossRef]
- Martín, M.L.; Hernández, M.A.; Avendaño, C.; Rodríguez, F.; Martínez, H. Manual lymphatic drainage therapy in patients with breast cancer related lymphoedema. BMC Cancer 2011, 11, 94. [Google Scholar] [CrossRef]
- Alonso Álvarez, B. ULL-27 Quality of Life Questionnaire: A Specific Instrument for Patients with Upper Limb Lymphedema after Breast Cancer. Cross-Cultural Adaptation and Validation of Its Spanish Version. Ph.D. Thesis, Complutense University of Madrid, Madrid, Spain, 2016. Available online: https://eprints.ucm.es/id/eprint/38892/ (accessed on 23 March 2022).
- Herdman, M.; Badia, X.; Berra, S. El EuroQol-5D: Una alternativa sencilla para la medición de la calidad de vida relacionada con la salud en atención primaria. Atención Primaria 2001, 28, 425–429. [Google Scholar] [CrossRef]
- Cormie, P.; Galvão, D.A.; Spry, N.; Newton, R.U. Neither heavy nor light load resistance exercise acutely exacerbates lymphedema in breast cancer survivor. Integr. Cancer Ther. 2013, 12, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Hervás, M.T.; Collado, M.J.N.; Peiró, S.; Pérez, J.L.R.; Matéu, P.L.; Tello, I.M. Spanish version of the DASH questionnaire. Cross-cultural adaptation, reliability, validity and sensitivity to changes. Clin. Med. 2006, 127, 441–447. [Google Scholar] [CrossRef]
- Ferguson, C.J. Una cartilla de tamaño de efecto: Una guía para médicos e investigadores. Prof. Psicol. Res. Pr. 2009, 40, 532–538. [Google Scholar] [CrossRef]
- Sousa, H.; Castro, S.; Abreu, J.; Pereira, M.G. A systematic review of factors affecting quality of life after postmastectomy breast reconstruction in women with breast cancer. Psychooncology 2019, 28, 2107–2118. [Google Scholar] [CrossRef]
- Togawa, K.; Ma, H.; Smith, A.W.; Neuhouser, M.L.; George, S.M.; Baumgartner, K.B.; McTiernan, A.; Baumgartner, R.; Ballard, R.M.; Bernstein, L. Self-reported symptoms of arm lymphedema and health-related quality of life among female breast cancer survivors. Sci. Rep. 2021, 11, 10701. [Google Scholar] [CrossRef]
- Bojinović-Rodić, D.; Popović-Petrović, S.; Tomić, S.; Markez, S.; Živanić, D. Funkcija ruke i kvalitet života kod bolesnica sa limfedemom nakon lečenja karcinoma dojke. Vojnosanit. Pregl. 2016, 73, 825–830. [Google Scholar] [CrossRef]
- Jiménez Buñuales, M.T.; González Diego, P.; Martín Moreno, J.M. La Clasificación Internacional del Funcionamiento de la Discapacidad y de la Salud (CIF) 2001. Rev. Esp. Salud Publica 2002, 76, 271–279. [Google Scholar]
- Nascimento, P.F.; Mello, M.J.G.; Correia, N.D.B.; Lucena, N.C.; Albuquerque, R.C.; De Matos, R.M.A.; Bergmann, A. Women’s occupational performance and quality of life during breast cancer treatment. BMJ Support Palliative Care 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Al Onazi, M.; Dolgoy, N.; Parkinson, J.; McNeely, M.L. Exploring adherence to daytime compression in women with breast cancer related lymphedema: A multi-methods study. Plast. Aesthetic Res. 2020, 2020, 23. [Google Scholar] [CrossRef]
- Baumann, F.T.; Reike, A.; Reimer, V.; Schumann, M.; Hallek, M.; Taaffe, D.R.; Newton, R.U.; Galvao, D.A. Effects of physical exercise on breast cancer-related secondary lymphedema: A systematic review. Breast Cancer Res. Treat. 2018, 170, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Pekarek, L.; Guijarro, L.G.; Castellanos, A.J.; Sanchez-Trujillo, L.; García-Honduvilla, N.; Álvarez-Mon, M.; Buján, J.; et al. Physical activity as an imperative support in breast cancer management. Cancers 2021, 13, 55. [Google Scholar] [CrossRef] [PubMed]
- Adraskela, K.; Veisaki, E.; Koutsilieris, M.; Philippou, A. Physical Exercise Positively Influences Breast Cancer Evolution. Clin. Breast Cancer 2017, 17, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Stout, N.L.; Santa Mina, D.; Lyons, K.D.; Robb, K.; Silver, J.K. A systematic review of rehabilitation and exercise recommendations in oncology guidelines. CA. Cancer J. Clin. 2021, 71, 149–175. [Google Scholar] [CrossRef]
- Mokhatri-Hesari, P.; Montazeri, A. Health-related quality of life in breast cancer patients: Review of reviews from 2008 to 2018. Health Qual. Life Outcomes 2020, 18, 338. [Google Scholar] [CrossRef]
- Salgarello, M.; Mangialardi, M.L.; Pino, V.; Gentileschi, S.; Visconti, G. A Prospective Evaluation of Health-Related Quality of Life following Lymphaticovenular Anastomosis for Upper and Lower Extremities Lymphedema. J. Reconstr. Microsurg. 2018, 34, 701–707. [Google Scholar] [CrossRef]
- Stuiver, M.M.; ten Tusscher, M.R.; Agasi-Idenburg, C.S.; Lucas, C.; Aaronson, N.K.; Bossuyt, P.M. Conservative interventions for preventing clinically detectable upper-limb lymphoedema in patients who are at risk of developing lymphoedema after breast cancer therapy. Cochrane Database Syst. Rev. 2015, CD009765. [Google Scholar] [CrossRef]
| Variables | Total (n = 51) | CG (n = 25) | EG (n = 26) | p |
|---|---|---|---|---|
| Age (mean ± SD) | 59.24 (±9.55) | 61.80 (±9.83) | 56.77 (±8.76) | |
| Occupation | ||||
| Active | 26 (51%) | 8 (32%) | 18 (69%) | |
| Unemployed | 4 (8%) | 3 (12%) | 1 (4%) | |
| Retired | 19 (37%) | 12 (48%) | 7 (27%) | |
| Other | 2 (4%) | 2 (8%) | 0 (0%) | |
| Type of treatment received | ||||
| Conservative | 2 (4%) | 1 (4%) | 1 (4%) | |
| Mastectomy | 49 (96%) | 24 (96%) | 25 (96%) | |
| Complications | ||||
| No | 43 (84%) | 21 (84%) | 22 (84%) | |
| Infection | 3 (6%) | 2 (8%) | 1 (4%) | |
| Seroma | 4 (8%) | 1 (4%) | 3 (12%) | |
| Keloid scar | 1 (2%) | 1 (4%) | 0 (0%) | |
| HRQoL | 65.40 | 66.54 | 0.822 | |
| QuickDASH | 46.88 | 37.92 | 0.162 | |
| ULL-27 physical | 48.54 | 41.75 | 0.250 | |
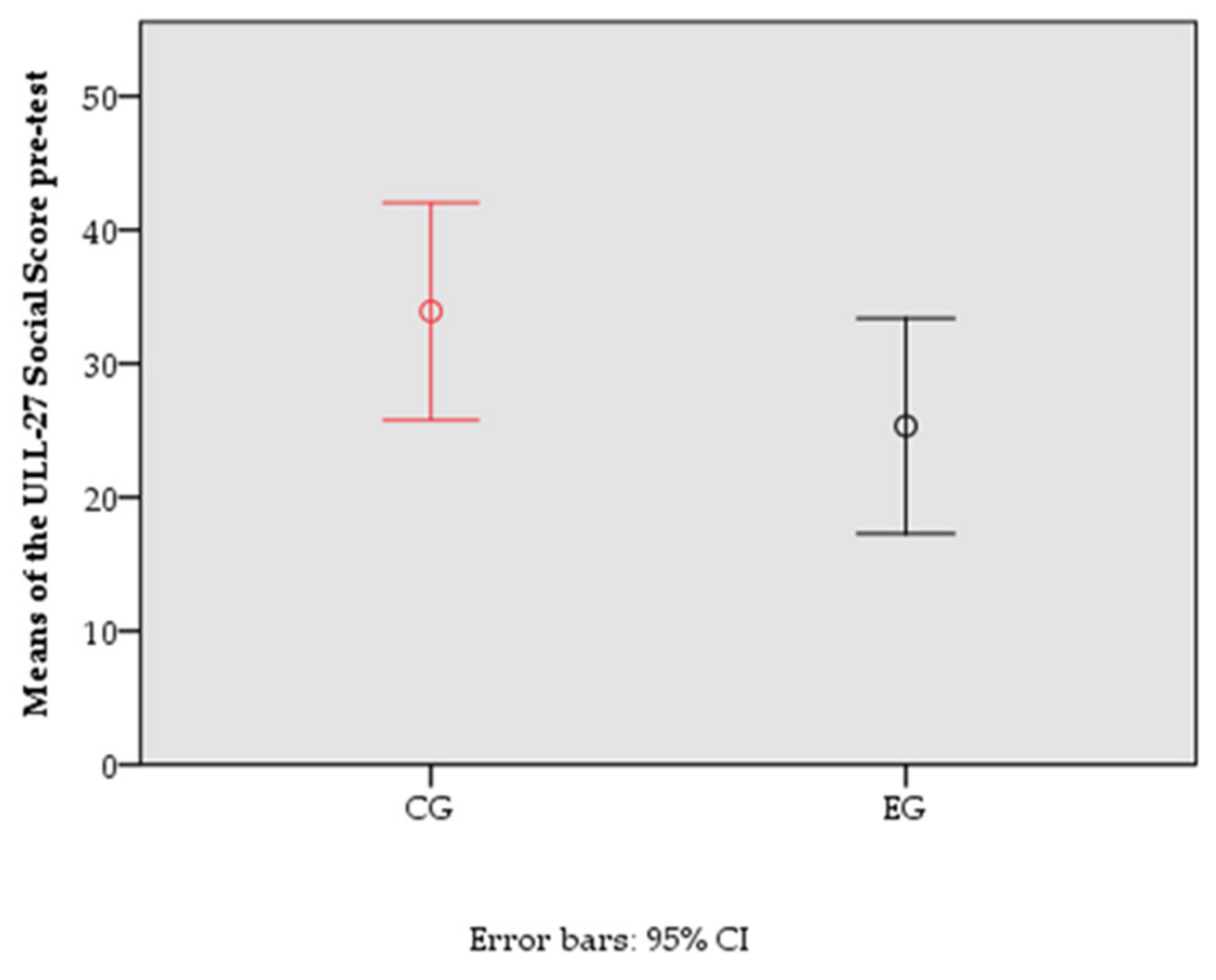
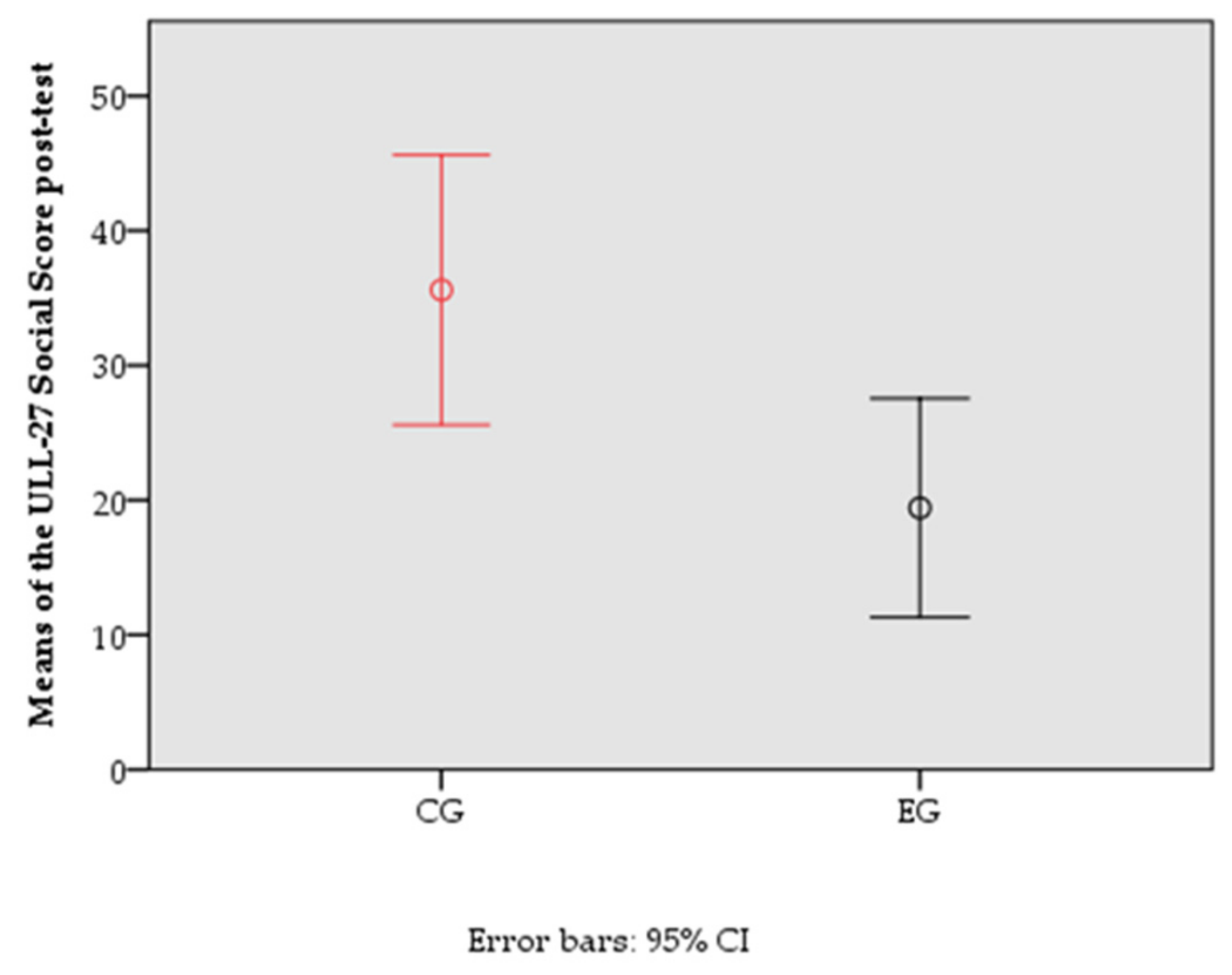
| ULL-27 social | 23.09 | 24.04 | 0.137 | |
| ULL-27 psychological | 48.76 | 45.89 | 0.504 | |
| VAS | 4.04 | 2.65 | 0.063 |
| Differential ULL-27-Ph Score | Differential ULL-27-S Score | Differential ULL-27-Ps Score | Differential EQol-5D Score | |||||
|---|---|---|---|---|---|---|---|---|
| r | p-Value | r | p-Value | r | p-Value | r | p-Value | |
| Differential VAS-P score | 0.135 | 0.346 | 0.252 | 0.074 | 0.173 | 0.225 | 0.279 | 0.048 |
| Differential VAS-H score | 0.049 | 0.733 | 0.247 | 0.052 | 0.037 | 0.794 | 0.247 | 0.080 |
| Differential VAS-T score | 0.318 | 0.023 | 0.256 | 0.070 | 0.185 | 0.193 | 0.154 | 0.282 |
| Differential Q-DASH score | 0.021 | 0.886 | 0.293 | 0.037 | 0.266 | 0.059 | 0.393 | 0.004 |
| Variable | Source | Type III Sum of Square | df | MS | F | p-Value | η2 |
|---|---|---|---|---|---|---|---|
| Differential ULL-27-Ph score | ULL-27-Ph pre-test | 1,802,313,482 | 1 | 1,802,313,482.445 | 3,406,908.005 | <0.001 | 1 |
| CG/EG | 1573.655 | 1 | 1573.655 | 2.974 | 0.091 | 0.058 | |
| Error | 25,392.833 | 48 | 529.017 | ||||
| Differential ULL-27-S score | ULL-27-S pre-test | 1119.004 | 1 | 1119.004 | 4.928 | 0.031 | 0.093 |
| CG/EG | 1021.240 | 1 | 1021.240 | 4.497 | 0.039 | 0.085 | |
| Error | 10,898.700 | 48 | 227.056 | ||||
| Differential ULL-27-Ps score | ULL-27-Ps pre-test | 970.213 | 1 | 970.213 | 10.145 | 0.002 | 0.174 |
| CG/EG | 64.764 | 1 | 64.764 | 0.677 | 0.414 | 0.013 | |
| Error | 4590.119 | 48 | 95.627 | ||||
| Differential EQol-5D score | EQ-5D-5L pre-test | 628.345 | 1 | 628.345 | 3.920 | 0.053 | 0.075 |
| CG/EG | 122.982 | 1 | 122.982 | 0.767 | 0.385 | 0.015 | |
| Error | 7692.455 | 48 | 160.259 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Alcaraz, M.N.; Pérula-de Torres, L.A.; Jiménez-Vílchez, A.J.; Rodríguez-Fernández, P.; Olmo-Carmona, M.V.; Muñoz-García, M.T.; Jorge-Gutiérrez, P.; Serrano-Merino, J.; Romero-Rodríguez, E.; Rodríguez-Elena, L.; et al. Impact of Activity-Oriented Propioceptive Antiedema Therapy on the Health-Related Quality of Life of Women with Upper-Limb Lymphedema Secondary to Breast Cancer—A Randomized Clinical Trial. J. Clin. Med. 2022, 11, 1884. https://doi.org/10.3390/jcm11071884
Muñoz-Alcaraz MN, Pérula-de Torres LA, Jiménez-Vílchez AJ, Rodríguez-Fernández P, Olmo-Carmona MV, Muñoz-García MT, Jorge-Gutiérrez P, Serrano-Merino J, Romero-Rodríguez E, Rodríguez-Elena L, et al. Impact of Activity-Oriented Propioceptive Antiedema Therapy on the Health-Related Quality of Life of Women with Upper-Limb Lymphedema Secondary to Breast Cancer—A Randomized Clinical Trial. Journal of Clinical Medicine. 2022; 11(7):1884. https://doi.org/10.3390/jcm11071884
Chicago/Turabian StyleMuñoz-Alcaraz, María Nieves, Luis A. Pérula-de Torres, Antonio José Jiménez-Vílchez, Paula Rodríguez-Fernández, María Victoria Olmo-Carmona, María Teresa Muñoz-García, Presentación Jorge-Gutiérrez, Jesús Serrano-Merino, Esperanza Romero-Rodríguez, Lorena Rodríguez-Elena, and et al. 2022. "Impact of Activity-Oriented Propioceptive Antiedema Therapy on the Health-Related Quality of Life of Women with Upper-Limb Lymphedema Secondary to Breast Cancer—A Randomized Clinical Trial" Journal of Clinical Medicine 11, no. 7: 1884. https://doi.org/10.3390/jcm11071884
APA StyleMuñoz-Alcaraz, M. N., Pérula-de Torres, L. A., Jiménez-Vílchez, A. J., Rodríguez-Fernández, P., Olmo-Carmona, M. V., Muñoz-García, M. T., Jorge-Gutiérrez, P., Serrano-Merino, J., Romero-Rodríguez, E., Rodríguez-Elena, L., Refusta-Ainaga, R., Lahoz-Sánchez, M. P., Miró-Palacios, B., Medrano-Cid, M., Magallón-Botaya, R., Santamaría-Peláez, M., Mínguez-Mínguez, L. A., & González-Bernal, J. J. (2022). Impact of Activity-Oriented Propioceptive Antiedema Therapy on the Health-Related Quality of Life of Women with Upper-Limb Lymphedema Secondary to Breast Cancer—A Randomized Clinical Trial. Journal of Clinical Medicine, 11(7), 1884. https://doi.org/10.3390/jcm11071884










