Management of Acute Coronary Syndrome in Cancer Patients: It’s High Time We Dealt with It
Abstract
:1. Introduction
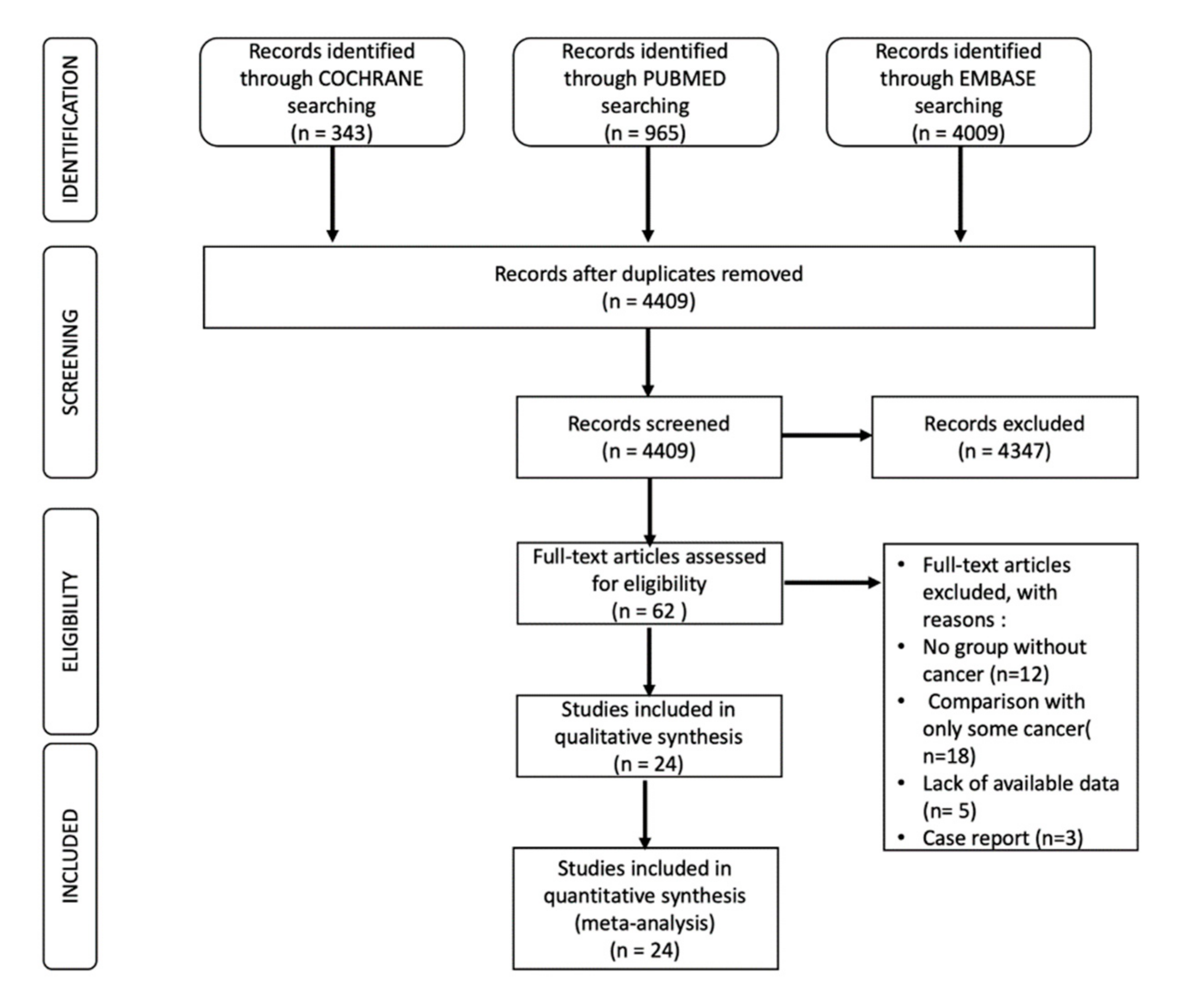
2. Search Strategy
3. Selection Process
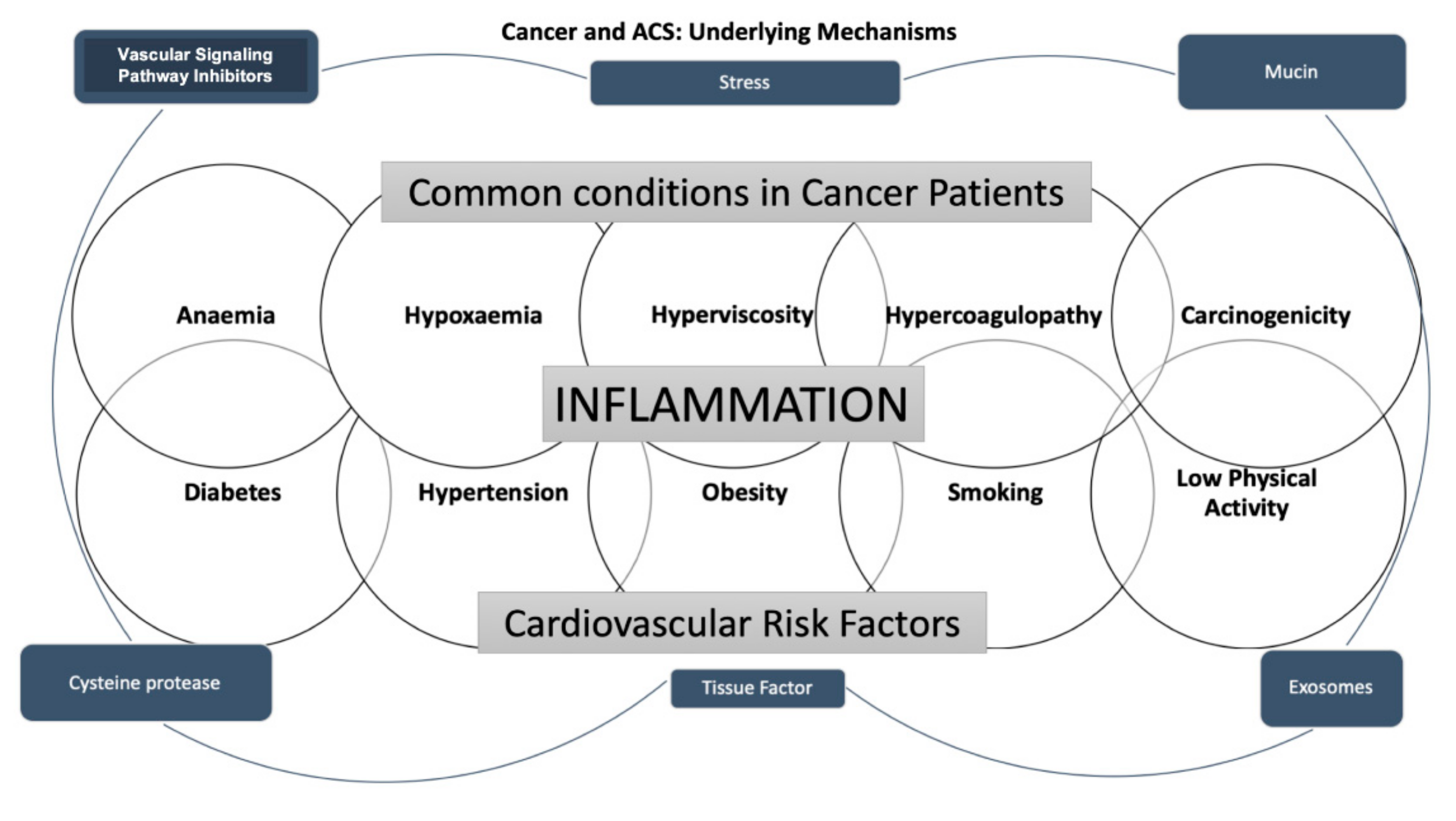
4. The Pathophysiologic Mechanism of Coronary Artery Disease in Cancer Treatment
5. Epidemiology
6. Chemotherapy and ACS Risk
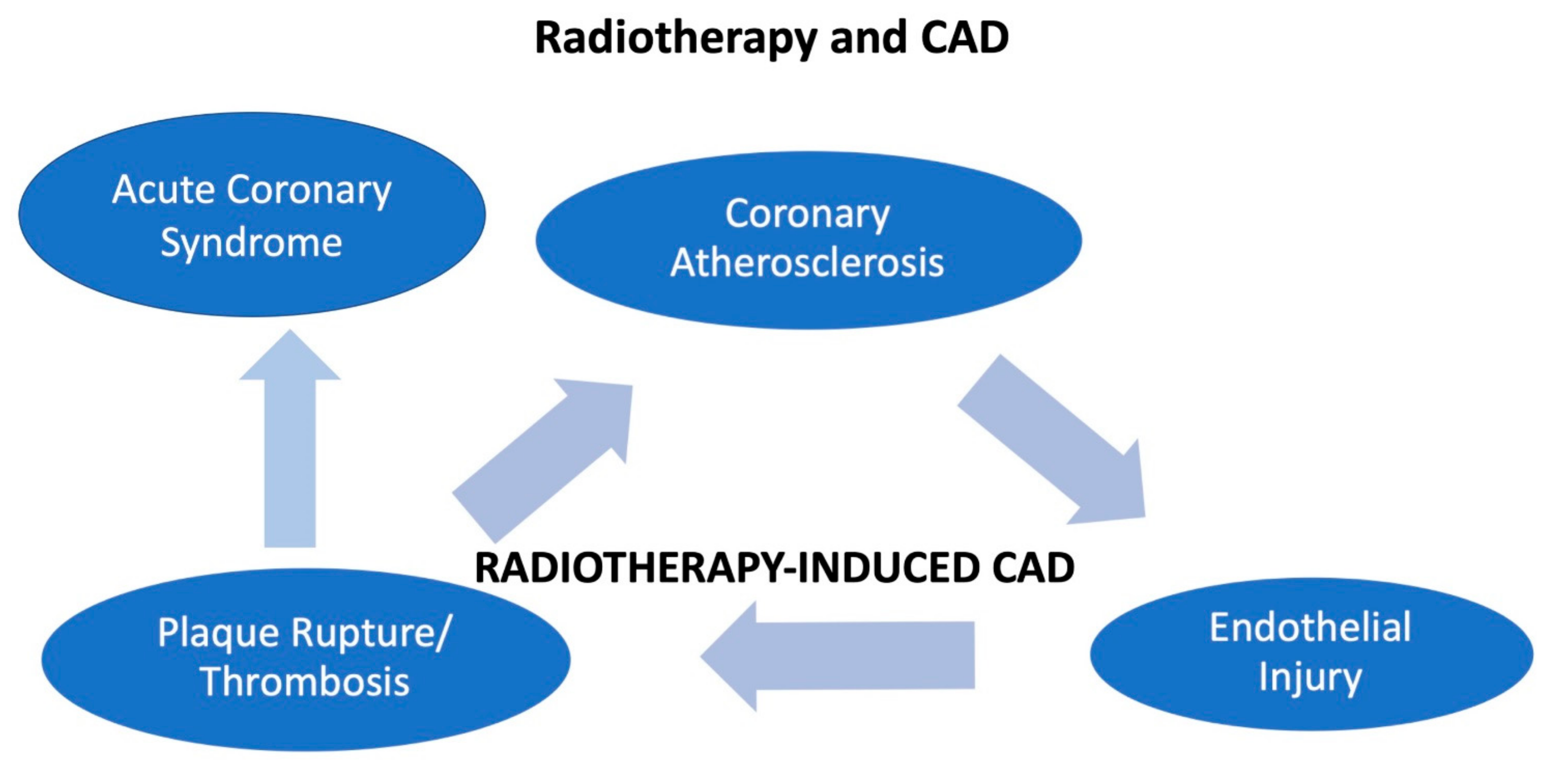
7. Radiotherapy and ACS
8. Clinical Presentation
9. Management
9.1. Cancer and Percutaneous Coronary Intervention
9.2. Pharmacological Treatment of ACS in Cancer Patients
9.3. Platelet Concentration Thresholds for Individual Elements of the Therapy
10. Discussion
11. Limitations
12. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Mertens, A.C.; Liu, Q.; Neglia, J.P.; Wasilewski, K.; Leisenring, W.; Armstrong, G.T.; Robison, L.L.; Yasui, Y. Cause-Specific Late Mortality Among 5-Year Survivors of Childhood Cancer: The Childhood Cancer Survivor Study. J. Natl. Cancer Inst. 2008, 100, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Witassek, F.; Erne, P.; Rickli, H.; Radovanovic, D. Treatment of patients with myocardial infarction depends on history of cancer. Eur. Heart J. Acute Cardiovasc. Care 2017, 7, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Caine, G.J.; Stonelake, P.S.; Lip, G.Y.H.; Kehoe, S.T. The Hypercoagulable State of Malignancy: Pathogenesis and Current Debate. Neoplasia 2002, 4, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gulati, R.; Lennon, R.J.; Lewis, B.R.; Park, J.; Sandhu, G.S.; Wright, R.S.; Lerman, A.; Herrmann, J. Cancer History Portends Worse Acute and Long-term Noncardiac (but Not Cardiac) Mortality after Primary Percutaneous Coronary Intervention for Acute ST-Segment Elevation Myocardial Infarction. Mayo Clin. Proc. 2016, 91, 1680–1692. [Google Scholar] [CrossRef]
- Gevaert, S.A.; Halvorsen, S.; Sinnaeve, P.R.; Sambola, A.; Gulati, G.; Lancellotti, P.; Van Der Meer, P.; Lyon, A.R.; Farmakis, D.; Lee, G.; et al. Evaluation and management of cancer patients presenting with acute cardiovascular disease: A Consensus Document of the Acute CardioVascular Care (ACVC) association and the ESC council of Cardio-Oncology—Part 1: Acute coronary syndromes and acute pericardial diseases. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, M.; D’Ascenzo, F.; Vadalà, P.; Wilton, S.B.; Noussan, P.; Colombo, F.; Roubín, S.R.; Abu Assi, E.; González-Juanatey, J.R.; Henriques, J.P.S.; et al. Prevalence and outcome of patients with cancer and acute coronary syndrome undergoing percutaneous coronary intervention: A BleeMACS substudy. Eur. Heart J. Acute Cardiovasc. Care 2017, 7, 631–638. [Google Scholar] [CrossRef]
- Edmondson, D.; von Känel, R. Post-traumatic stress disorder and cardiovascular disease. Lancet Psychiatry 2017, 4, 320–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariza-Solé, A.; Guerrero, C.; Formiga, F.; Aboal, J.; Abu-Assi, E.; Marín, F.; Bueno, H.; Alegre, O.; López-Palop, R.; Vidán, M.T.; et al. Global Geriatric Assessment and In-Hospital Bleeding Risk in Elderly Patients with Acute Coronary Syndromes: Insights from the LONGEVO-SCA Registry. Thromb. Haemost. 2018, 118, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Manzano-Fernández, S.; Sánchez-Martínez, M.; Flores-Blanco, P.J.; López-Cuenca, Á.; Gómez-Molina, M.; Pastor-Pérez, F.J.; Sánchez-Galian, M.J.; Cambronero-Sanchez, F.; Pérez, E.G.; García-Narbón, A.; et al. Comparison of the Global Registry of Acute Coronary Events Risk Score Versus the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse outcomes with Early Implementation of the ACC/AHA Guidelines Risk Score to Predict In-Hospital Mortality and Major Bleeding in Acute Coronary Syndromes. Am. J. Cardiol. 2016, 117, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, C.; Garay, A.; Ariza-Solé, A.; Formiga, F.; Raposeiras-Roubín, S.; Abu-Assi, E.; D’Ascenzo, F.; Kinnaird, T.; Manzano-Fernández, S.; Alegre, O.; et al. Anemia in patients with acute coronary syndromes treated with prasugrel or ticagrelor: Insights from the RENAMI registry. Thromb. Res. 2018, 167, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Marechal, P.; Donis, N.; Oury, C. Inflammation, cardiovascular disease, and cancer: A common link with far-reaching implications. Eur. Heart J. 2019, 40, 3910–3912. [Google Scholar] [CrossRef]
- Das, D.; Asher, A.; Ghosh, A.K. Cancer and Coronary Artery Disease: Common Associations, Diagnosis and Management Challenges. Curr. Treat. Options Oncol. 2019, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Bisceglia, I.; Canale, M.L.; Lestuzzi, C.; Parrini, I.; Russo, G.; Colivicchi, F.; Gabrielli, D.; Gulizia, M.M.; Iliescu, C.A. Acute coronary syndromes in cancer patients. J. Cardiovasc. Med. 2020, 21, 944–952. [Google Scholar] [CrossRef]
- Radmilovic, J.; Di Vilio, A.; D’Andrea, A.; Pastore, F.; Forni, A.; Desiderio, A.; Ragni, M.; Quaranta, G.; Cimmino, G.; Russo, V.; et al. The Pharmacological Approach to Oncologic Patients with Acute Coronary Syndrome. J. Clin. Med. 2020, 9, 3926. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenneman, C.G.; Sawyer, D.B. Cardio-Oncology: An Update on Cardiotoxicity of Cancer-Related Treatment. Circ. Res. 2016, 118, 1008–1020. [Google Scholar] [CrossRef] [Green Version]
- Canale, M.L.; Bisceglia, I.; Lestuzzi, C.; Parrini, I. Arterial Thrombosis in Cancer: Spotlight on the Neglected Vessels. Anticancer Res. 2019, 39, 4619–4625. [Google Scholar] [CrossRef]
- Libby, P.; Kobold, S. Inflammation: A common contributor to cancer, aging, and cardiovascular diseases—Expanding the concept of cardio-oncology. Cardiovasc. Res. 2019, 115, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Bick, R.L. Cancer-Associated Thrombosis. N. Engl. J. Med. 2003, 349, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Rak, J.; Milsom, C.; May, L.; Klement, P.; Yu, J. Tissue Factor in Cancer and Angiogenesis: The Molecular Link between Genetic Tumor Progression, Tumor Neovascularization, and Cancer Coagulopathy. Semin. Thromb. Hemost. 2006, 32, 54–70. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.T.H.; Chang, H.-M. Cancer and Clot: Between a Rock and a Hard Place. J. Am. Coll. Cardiol. 2017, 70, 939–941. [Google Scholar] [CrossRef]
- Mitrugno, A.; Tormoen, G.W.; Kuhn, P.; McCarty, O.J. The prothrombotic activity of cancer cells in the circulation. Blood Rev. 2015, 30, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giza, D.E.; Marmagkiolis, K.; Mouhayar, E.; Durand, J.-B.; Iliescu, C. Management of CAD in Patients with Active Cancer: The Interventional Cardiologists’ Perspective. Curr. Cardiol. Rep. 2017, 19, 56. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, A.; Potts, J.; Mohamed, M.O.; Parwani, P.; Swamy, P.; Lopez-Mattei, J.C.; Rashid, M.; Kwok, C.S.; Fischman, D.L.; Vassiliou, V.S.; et al. Acute myocardial infarction treatments and outcomes in 6.5 million patients with a current or historical diagnosis of cancer in the USA. Eur. Heart J. 2020, 41, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Navi, B.B.; Reiner, A.S.; Kamel, H.; Iadecola, C.; Okin, P.M.; Elkind, M.S.V.; Panageas, K.S.; DeAngelis, L.M. Risk of Arterial Thromboembolism in Patients with Cancer. J. Am. Coll. Cardiol. 2017, 70, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Navi, B.B.; Reiner, A.S.; Kamel, H.; Iadecola, C.; Okin, P.M.; Tagawa, S.T.; Panageas, K.S.; DeAngelis, L.M. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood 2019, 133, 781–789. [Google Scholar] [CrossRef]
- Park, J.Y.; Guo, W.; Al-Hijji, M.; El Sabbagh, A.; Begna, K.H.; Habermann, T.M.; Witzig, T.E.; Lewis, B.R.; Lerman, A.; Herrmann, J. Acute coronary syndromes in patients with active hematologic malignancies—Incidence, management, and outcomes. Int. J. Cardiol. 2019, 275, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Nanda, A.; Chen, M.-H.; Braccioforte, M.H.; Moran, B.J.; D’Amico, A.V. Hormonal Therapy Use for Prostate Cancer and Mortality in Men with Coronary Artery Disease–Induced Congestive Heart Failure or Myocardial Infarction. JAMA 2009, 302, 866–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sara, J.D.; Kaur, J.; Khodadadi, R.; Rehman, M.; Lobo, R.; Chakrabarti, S.; Herrmann, J.; Lerman, A.; Grothey, A. 5-fluorouracil and cardiotoxicity: A review. Ther. Adv. Med. Oncol. 2018, 10, 1758835918780140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Layoun, M.E.; Wickramasinghe, C.D.; Peralta, M.V.; Yang, E.H. Fluoropyrimidine-Induced Cardiotoxicity: Manifestations, Mechanisms, and Management. Curr. Oncol. Rep. 2016, 18, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Forni, M.; Malet-Martino, M.C.; Jaillais, P.; Shubinski, R.E.; Bachaud, J.M.; Lemaire, L.; Canal, P.; Chevreau, C.; Carrie, D.; Soulié, P. Cardiotoxicity of high-dose continuous infusion fluorouracil: A prospective clinical study. J. Clin. Oncol. 1992, 10, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Colombo, N. Acute coronary syndrome induced by oral capecitabine. Can. J. Cardiol. 2006, 22, 251–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanduri, J.; More, L.A.; Godishala, A.; Asnani, A. Fluoropyrimidine-Associated Cardiotoxicity. Cardiol. Clin. 2019, 37, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Adel, N.; Riedel, E.; Bhutani, M.; Feldman, D.R.; Tabbara, N.E.; Soff, G.; Parameswaran, R.; Hassoun, H. High Incidence of Thromboembolic Events in Patients Treated with Cisplatin-Based Chemotherapy: A Large Retrospective Analysis. J. Clin. Oncol. 2011, 29, 3466–3473. [Google Scholar] [CrossRef]
- Touyz, R.M.; Herrmann, J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. npj Precis. Oncol. 2018, 2, 13. [Google Scholar] [CrossRef] [Green Version]
- Pouwer, M.G.; Pieterman, E.J.; Verschuren, L.; Caspers, M.P.M.; Kluft, C.; Garcia, R.A.; Aman, J.; Jukema, J.W.; Princen, H.M.G. The BCR-ABL1 Inhibitors Imatinib and Ponatinib Decrease Plasma Cholesterol and Atherosclerosis, and Nilotinib and Ponatinib Activate Coagulation in a Translational Mouse Model. Front. Cardiovasc. Med. 2018, 5, 55. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, J.; Yang, E.H.; Iliescu, C.A.; Cilingiroglu, M.; Charitakis, K.; Hakeem, A.; Toutouzas, K.; Leesar, M.A.; Grines, C.L.; Marmagkiolis, K. Vascular Toxicities of Cancer Therapies: The Old and the New—An Evolving Avenue. Circulation 2016, 133, 1272–1289. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.R.; Yousaf, N.; Battisti, N.M.L.; Moslehi, J.; Larkin, J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018, 19, e447–e458. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cornell, R.F.; Lenihan, D.; Slosky, D.; Jagasia, M.; Piazza, G.; Moslehi, J. Cardiovascular Complications of Novel Multiple Myeloma Treatments. Circulation 2016, 133, 908–912. [Google Scholar] [CrossRef]
- Lee, D.H.; Fradley, M.G. Cardiovascular Complications of Multiple Myeloma Treatment: Evaluation, Management, and Prevention. Curr. Treat. Options Cardiovasc. Med. 2018, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Drobni, Z.D.; Alvi, R.M.; Taron, J.; Zafar, A.; Murphy, S.P.; Rambarat, P.K.; Mosarla, R.C.; Lee, C.; Zlotoff, D.A.; Raghu, V.K.; et al. Association Between Immune Checkpoint Inhibitors with Cardiovascular Events and Atherosclerotic Plaque. Circulation 2020, 142, 2299–2311. [Google Scholar] [CrossRef] [PubMed]
- Inno, A.; Chiampan, A.; Lanzoni, L.; Verzè, M.; Molon, G.; Gori, S. Immune Checkpoint Inhibitors and Atherosclerotic Vascular Events in Cancer Patients. Front. Cardiovasc. Med. 2021, 8, 652186. [Google Scholar] [CrossRef]
- Pasquier, E.; Honore, S.; Pourroy, B.; Jordan, M.A.; Lehmann, M.; Briand, C.; Braguer, D. Antiangiogenic Concentrations of Paclitaxel Induce an Increase in Microtubule Dynamics in Endothelial Cells but Not in Cancer Cells. Cancer Res. 2005, 65, 2433–2440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waxman, A.J.; Clasen, S.; Hwang, W.-T.; Garfall, A.; Vogl, D.T.; Carver, J.; O’Quinn, R.; Cohen, A.D.; Stadtmauer, E.A.; Ky, B.; et al. Carfilzomib-Associated Cardiovascular Adverse Events: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, e174519. [Google Scholar] [CrossRef]
- Jakubowiak, A.J.; DeCara, J.M.; Mezzi, K. Cardiovascular events during carfilzomib therapy for relapsed myeloma: Practical management aspects from two case studies. Hematology 2017, 22, 585–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khosrow-Khavar, F.; Filion, K.B.; Bouganim, N.; Suissa, S.; Azoulay, L. Aromatase Inhibitors and the Risk of Cardiovascular Outcomes in Women with Breast Cancer: A Population-Based Cohort Study. Circulation 2020, 141, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Fontanella, C.; Aita, M.; Cinausero, M.; Aprile, G.; Baldin, M.G.; Dusi, V.; Lestuzzi, C.; Fasola, G.; Puglisi, F. Capecitabine-induced cardiotoxicity: More evidence or clinical approaches to protect the patients’ heart? OncoTargets Ther. 2014, 7, 1783–1791. [Google Scholar] [CrossRef] [Green Version]
- Hilmi, M.; Ederhy, S.; Waintraub, X.; Funck-Brentano, C.; Cohen, A.; Vozy, A.; Lebrun-Vignes, B.; Moslehi, J.; Nguyen, L.S.; Salem, J.-E. Cardiotoxicity Associated with Gemcitabine: Literature Review and a Pharmacovigilance Study. Pharmaceuticals 2020, 13, 325. [Google Scholar] [CrossRef]
- Aghel, N.; Lipton, J.H.; Atenafu, E.G.; Kim, D.D.H.; Delgado, D.H. Cardiovascular Events after Exposure to Nilotinib in Chronic Myeloid Leukemia: Long-term Follow-up. Clin. Lymphoma Myeloma Leuk. 2017, 17, 870–878.e871. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Accurso, V.; Mancuso, S.; Contrino, A.D.; Sardo, M.; Novo, G.; Di Piazza, F.; Perez, A.; Russo, A.; Siragusa, S. Management of Ponatinib in Patients with Chronic Myeloid Leukemia with Cardiovascular Risk Factors. Chemotherapy 2019, 64, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Totzeck, M.; Mincu, R.I.; Rassaf, T. Cardiovascular Adverse Events in Patients with Cancer Treated with Bevacizumab: A Meta-Analysis of More Than 20,000 Patients. J. Am. Heart Assoc. 2017, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Duran, J.M.; Makarewich, C.A.; Trappanese, D.; Gross, P.; Husain, S.; Dunn, J.; Lal, H.; Sharp, T.E.; Starosta, T.; Vagnozzi, R.J.; et al. Sorafenib Cardiotoxicity Increases Mortality after Myocardial Infarction. Circ. Res. 2014, 114, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, G.; Autorino, R.; Bruni, G.; Cartenì, G.; Ricevuto, E.; Tudini, M.; Ficorella, C.; Romano, C.; Aieta, M.; Giordano, A.; et al. Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: A multicenter analysis. Ann. Oncol. 2009, 20, 1535–1542. [Google Scholar] [CrossRef]
- Justice, C.N.; Derbala, M.H.; Baich, T.M.; Kempton, A.N.; Guo, A.S.; Ho, T.H.; Smith, S.A. The Impact of Pazopanib on the Cardiovascular System. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 387–398. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J. Risk of regorafenib-induced cardiovascular events in patients with solid tumors: A systematic review and meta-analysis. Medicine 2018, 97, e12705. [Google Scholar] [CrossRef] [PubMed]
- Tanriverdi, O.; Ates, S.; Sandal, K.K.; Uylas, S.; Bosna, I.C.; Alkan, A. Left ventricular dysfunction associated with axitinib and nivolumab experience in an advanced renal cell carcinoma. J. Oncol. Pharm. Pract. 2020, 26, 1765–1768. [Google Scholar] [CrossRef]
- Grabowski, J.; Glode, A. Ramucirumab: A vascular endothelial growth factor receptor-2 inhibitor with activity in several malignancies. Am. J. Health-Syst. Pharm. 2016, 73, 957–968. [Google Scholar] [CrossRef]
- Sharma, T.; Dhingra, R.; Singh, S.; Sharma, S.; Tomar, P.; Malhotra, M.; Bhardwaj, T.R. Aflibercept: A Novel VEGF Targeted Agent to Explore the Future Perspectives of Anti-Angiogenic Therapy for the Treatment of Multiple Tumors. Mini-Rev. Med. Chem. 2013, 13, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Bikiewicz, A.; Banach, M.; von Haehling, S.; Maciejewski, M.; Bielecka-Dabrowa, A. Adjuvant breast cancer treatments cardiotoxicity and modern methods of detection and prevention of cardiac complications. ESC Heart Fail. 2021, 8, 2397–2418. [Google Scholar] [CrossRef] [PubMed]
- Gür, F.; Cengiz, M.; Kutlu, H.M.; Cengiz, B.P.; Ayhancı, A. Molecular docking analyses of Escin as regards cyclophosphamide-induced cardiotoxicity: In vivo and in Silico studies. Toxicol. Appl. Pharmacol. 2020, 411, 115386. [Google Scholar] [CrossRef] [PubMed]
- Herradón, E.; González, C.; González, A.; Uranga, J.A.; López-Miranda, V. Cardiovascular Toxicity Induced by Chronic Vincristine Treatment. Front. Pharmacol. 2021, 12, 692970. [Google Scholar] [CrossRef] [PubMed]
- Herradón, E.; González, C.; Uranga, J.A.; Abalo, R.; Martín, M.I.; López-Miranda, V. Characterization of Cardiovascular Alterations Induced by Different Chronic Cisplatin Treatments. Front. Pharmacol. 2017, 8, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gozhenko, A.; Bestanchuk, O.; Kaschenko, O.; Narbutova, T. Cumulative cardiotoxic effect of bleomycin in experiment. J. Educ. Health Sport 2021, 11, 301–308. [Google Scholar] [CrossRef]
- Desai, M.Y.; Jellis, C.L.; Kotecha, R.; Johnston, D.R.; Griffin, B.P. Radiation-Associated Cardiac Disease: A Practical Approach to Diagnosis and Management. JACC Cardiovasc. Imaging 2018, 11, 1132–1149. [Google Scholar] [CrossRef] [PubMed]
- Belzile-Dugas, E.; Eisenberg, M.J. Radiation-Induced Cardiovascular Disease: Review of an Underrecognized Pathology. J. Am. Heart Assoc. 2021, 10, e021686. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; Cutter, D.J.; Boerma, M.; Constine, L.S.; Fajardo, L.F.; Kodama, K.; Mabuchi, K.; Marks, L.B.; Mettler, F.A.; Pierce, L.J.; et al. Radiation-Related Heart Disease: Current Knowledge and Future Prospects. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 656–665. [Google Scholar] [CrossRef] [Green Version]
- Hendry, J.H. Threshold doses and circulatory disease risks. Ann. ICRP 2015, 44, 69–75. [Google Scholar] [CrossRef]
- Stewart, F.A. Mechanisms and dose-response relationships for radiation-induced cardiovascular disease. Ann. ICRP 2012, 41, 72–79. [Google Scholar] [CrossRef]
- Guddati, A.K.; Joy, P.S.; Kumar, G. Analysis of outcomes of percutaneous coronary intervention in metastatic cancer patients with acute coronary syndrome over a 10-year period. J. Cancer Res. Clin. Oncol. 2015, 142, 471–479. [Google Scholar] [CrossRef]
- Munoz, E.; Iliescu, G.; Vejpongsa, P.; Charitakis, K.; Karimzad, K.; Lopez-Mattei, J.; Yusuf, S.W.; Marmagkiolis, K.; Iliescu, C. Takotsubo Stress Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 68, 1143–1144. [Google Scholar] [CrossRef] [PubMed]
- Al-Hawwas, M.; Tsitlakidou, D.; Gupta, N.; Iliescu, C.; Cilingiroglu, M.; Marmagkiolis, K. Acute Coronary Syndrome Management in Cancer Patients: “Good News” in Cancer Patients? Curr. Oncol. Rep. 2018, 20, 78. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [Green Version]
- Potts, J.E.; Iliescu, C.A.; Lopez-Mattei, J.; Martinez, S.C.; Holmvang, L.; Ludman, P.; De Belder, M.A.; Kwok, C.S.; Rashid, M.; Fischman, D.L.; et al. Percutaneous coronary intervention in cancer patients: A report of the prevalence and outcomes in the United States. Eur. Heart J. 2018, 40, 1790–1800. [Google Scholar] [CrossRef] [Green Version]
- Borovac, J.A.; Kwok, C.S.; Iliescu, C.; Lee, H.J.; Kim, P.Y.; Palaskas, N.L.; Zaman, A.; Butler, R.; Lopez-Mattei, J.C.; Mamas, M.A. Percutaneous Coronary Intervention and Outcomes in Patients with Lymphoma in the United States (Nationwide Inpatient Sample [NIS] Analysis). Am. J. Cardiol. 2019, 124, 1190–1197. [Google Scholar] [CrossRef]
- Potts, J.; Mohamed, M.O.; Mattei, J.C.L.; Iliescu, C.A.; Konopleva, M.; Rashid, M.; Bagur, R.; Mamas, M.A. Percutaneous coronary intervention and in-hospital outcomes in patients with leukemia: A nationwide analysis. Catheter. Cardiovasc. Interv. 2019, 96, 53–63. [Google Scholar] [CrossRef]
- Mohamed, M.O.; Van Spall, H.G.C.; Kontopantelis, E.; Alkhouli, M.; Barac, A.; Elgendy, I.Y.; Khan, S.U.; Kwok, C.S.; Shoaib, A.; Bhatt, D.L.; et al. Effect of primary percutaneous coronary intervention on in-hospital outcomes among active cancer patients presenting with ST-elevation myocardial infarction: A propensity score matching analysis. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 829–839. [Google Scholar] [CrossRef]
- Mohamed, M.O.; Lopez-Mattei, J.C.; Parwani, P.; Iliescu, C.A.; Bharadwaj, A.; Kim, P.Y.; Palaskas, N.L.; Rashid, M.; Potts, J.; Kwok, C.S.; et al. Management strategies and clinical outcomes of acute myocardial infarction in leukaemia patients: Nationwide insights from United States hospitalisations. Int. J. Clin. Pract. 2020, 74, e13476. [Google Scholar] [CrossRef] [Green Version]
- Styczkiewicz, K.; Styczkiewicz, M.; Myćka, M.; Mędrek, S.; Kondraciuk, T.; Czerkies-Bieleń, A.; Wiśniewski, A.; Szmit, S.; Jankowski, P. Clinical presentation and treatment of acute coronary syndrome as well as 1-year survival of patients hospitalized due to cancer: A 7-year experience of a nonacademic center. Medicine 2020, 99, e18972. [Google Scholar] [CrossRef] [PubMed]
- Nabiałek-Trojanowska, I.; Dąbrowska-Kugacka, A.; Lewicka-Potocka, Z.; Abdulaziz, Y.; Szerszyńska, A.; Raczak, G.; Lewicka, E. Acute coronary syndrome in patients undergoing anticancer therapies: A single-center, controlled case study. Adv. Clin. Exp. Med. 2019, 28, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Balanescu, D.V.; Donisan, T.; Deswal, A.; Palaskas, N.; Song, J.; Lopez-Mattei, J.; Kim, P.Y.; Durand, J.-B.; Doundoua, D.; Marmagkiolis, K.; et al. Acute myocardial infarction in a high-risk cancer population: Outcomes following conservative versus invasive management. Int. J. Cardiol. 2020, 313, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Monlezun, D.J.; Lawless, S.; Palaskas, N.; Peerbhai, S.; Charitakis, K.; Marmagkiolis, K.; Lopez-Mattei, J.; Mamas, M.; Iliescu, C. Machine Learning-Augmented Propensity Score Analysis of Percutaneous Coronary Intervention in Over 30 Million Cancer and Non-cancer Patients. Front. Cardiovasc. Med. 2021, 8, 620857. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, C.A.; Grines, C.L.; Herrmann, J.; Yang, E.H.; Cilingiroglu, M.; Charitakis, K.; Hakeem, A.; Toutouzas, K.P.; Leesar, M.A.; Marmagkiolis, K. SCAI Expert consensus statement: Evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the cardiological society of india, and sociedad Latino Americana de Cardiologıa intervencionista). Catheter. Cardiovasc. Interv. 2016, 87, E202–E223. [Google Scholar] [CrossRef]
- Ueki, Y.; Vögeli, B.; Karagiannis, A.; Zanchin, T.; Zanchin, C.; Rhyner, D.; Otsuka, T.; Praz, F.; Siontis, G.C.M.; Moro, C.; et al. Ischemia and Bleeding in Cancer Patients Undergoing Percutaneous Coronary Intervention. JACC CardioOncol. 2019, 1, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Blann, A.D.; Dunmore, S. Arterial and Venous Thrombosis in Cancer Patients. Cardiol. Res. Pract. 2011, 2011, 394740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Werkum, J.W.; Heestermans, A.A.; Zomer, A.C.; Kelder, J.C.; Suttorp, M.-J.; Rensing, B.J.; Koolen, J.J.; Brueren, B.G.; Dambrink, J.-H.E.; Hautvast, R.W.; et al. Predictors of Coronary Stent Thrombosis: The Dutch Stent Thrombosis Registry. J. Am. Coll. Cardiol. 2009, 53, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Tabata, N.; Sueta, D.; Yamamoto, E.; Takashio, S.; Arima, Y.; Araki, S.; Yamanaga, K.; Ishii, M.; Sakamoto, K.; Kanazawa, H.; et al. Outcome of current and history of cancer on the risk of cardiovascular events following percutaneous coronary intervention: A Kumamoto University Malignancy and Atherosclerosis (KUMA) study. Eur. Heart J. Qual. Care Clin. Outcomes 2017, 4, 290–300. [Google Scholar] [CrossRef]
- Nakatsuma, K.; Shiomi, H.; Morimoto, T.; Watanabe, H.; Nakagawa, Y.; Furukawa, Y.; Kadota, K.; Ando, K.; Ono, K.; Shizuta, S.; et al. Influence of a history of cancer on long-term cardiovascular outcomes after coronary stent implantation (an Observation from Coronary Revascularization Demonstrating Outcome Study-Kyoto Registry Cohort-2). Eur. Heart J. Qual. Care Clin. Outcomes 2018, 4, 200–207. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.P.; Steg, G.; Bhatt, D.L. The management of antiplatelet therapy in acute coronary syndrome patients with thrombocytopenia: A clinical conundrum. Eur. Heart J. 2017, 38, 3488–3492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharadwaj, A.S.; Swamy, P.M.; Mamas, M.A. Outcomes of percutaneous coronary interventions in cancer patients. Expert Rev. Cardiovasc. Ther. 2020, 18, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Velders, M.A.; Hagström, E.; James, S.K. Temporal Trends in the Prevalence of Cancer and Its Impact on Outcome in Patients with First Myocardial Infarction: A Nationwide Study. J. Am. Heart Assoc. 2020, 9, e014383. [Google Scholar] [CrossRef]
- Heller, S.J.; Tokar, J.L.; Nguyen, M.T.; Haluszka, O.; Weinberg, D.S. Management of bleeding GI tumors. Gastrointest. Endosc. 2010, 72, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Wong, C.W.; Kontopantelis, E.; Barac, A.; Brown, S.-A.; Velagapudi, P.; Hilliard, A.A.; Bharadwaj, A.S.; Alraies, M.C.; Mohamed, M.; et al. Percutaneous coronary intervention in patients with cancer and readmissions within 90 days for acute myocardial infarction and bleeding in the USA. Eur. Heart J. 2021, 42, 1019–1034. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Morishima, T.; Fujii, Y.; Okawa, S.; Otsuka, T.; Kamada, R.; Yasui, T.; Shioyama, W.; Oka, T.; Tabuchi, T.; et al. Prognostic Impact of Cancer Activity on Clinically Relevant Bleeding Events after Percutaneous Coronary Intervention. J. Med. Investig. 2021, 68, 29–37. [Google Scholar] [CrossRef]
- Zaleska, M.; Mozenska, O.; Bil, J. Statins use and cancer: An update. Futur. Oncol. 2018, 14, 1497–1509. [Google Scholar] [CrossRef]
- Yusuf, S.W.; Daraban, N.; Abbasi, N.; Lei, X.; Durand, J.-B.; Daher, I.N. Treatment and Outcomes of Acute Coronary Syndrome in the Cancer Population. Clin. Cardiol. 2012, 35, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Sarkiss, M.G.; Yusuf, S.W.; Warneke, C.L.; Botz, G.; Lakkis, N.; Hirch-Ginsburg, C.; Champion, J.C.; Swafford, J.; Shaw, A.D.S.; Lenihan, D.J.; et al. Impact of aspirin therapy in cancer patients with thrombocytopenia and acute coronary syndromes. Cancer 2006, 109, 621–627. [Google Scholar] [CrossRef]
- Iliescu, C.; Balanescu, D.V.; Donisan, T.; Giza, D.E.; Gonzalez, E.D.M.; Cilingiroglu, M.; Song, J.; Mukerji, S.S.; Lopez-Mattei, J.C.; Kim, P.Y.; et al. Safety of Diagnostic and Therapeutic Cardiac Catheterization in Cancer Patients with Acute Coronary Syndrome and Chronic Thrombocytopenia. Am. J. Cardiol. 2018, 122, 1465–1470. [Google Scholar] [CrossRef]
- Alam, N.; Wright, A.K.; Ashcroft, D.M.; Renehan, A.G. Cancer and cardiovascular disease. Lancet 2020, 395, 1903–1904. [Google Scholar] [PubMed]
- Winther, J.F.; Bhatia, S.; Cederkvist, L.; Gudmundsdottir, T.; Madanat-Harjuoja, L.; Msc, L.T.; Wesenberg, F.; Hasle, H.; Holmqvist, A.S.; for the ALiCCS Study Group. Risk of cardiovascular disease among Nordic childhood cancer survivors with diabetes mellitus: A report from adult life after childhood cancer in Scandinavia. Cancer 2018, 124, 4393–4400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krone, R.J. Managing Coronary Artery Disease in the Cancer Patient. Prog. Cardiovasc. Dis. 2010, 53, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Guha, A.; Armanious, M.; Fradley, M.G. Update on cardio-oncology: Novel cancer therapeutics and associated cardiotoxicities. Trends Cardiovasc. Med. 2019, 29, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Moran, T.B.; Plana, J.C. Management of Patients with Acute Coronary Syndrome and Cancer. Curr. Cardiol. Rep. 2020, 22, 1–8. [Google Scholar] [CrossRef]
- Fox, K.A.A.; Dabbous, O.H.; Goldberg, R.J.; Pieper, K.S.; Eagle, K.A.; Van de Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; Anderson, F.A.; et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: Prospective multinational observational study (GRACE). BMJ 2006, 333, 1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subherwal, S.; Bach, R.G.; Chen, A.Y.; Gage, B.F.; Rao, S.V.; Newby, L.K.; Wang, T.Y.; Gibler, W.B.; Ohman, E.M.; Roe, M.T.; et al. Baseline Risk of Major Bleeding in Non–ST-Segment–Elevation Myocardial Infarction: The CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation 2009, 119, 1873–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, J.M.; Curtis, J.P.; Dai, D.; Fitzgerald, S.; Khandelwal, A.K.; Spertus, J.A.; Rao, S.V.; Singh, M.; Shaw, R.E.; Ho, K.; et al. Enhanced Mortality Risk Prediction with a Focus on High-Risk Percutaneous Coronary Intervention: Results From 1,208,137 Procedures in the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc. Interv. 2013, 6, 790–799. [Google Scholar] [CrossRef] [Green Version]
- Farooq, V.; Vergouwe, Y.; Räber, L.; Vranckx, P.; Garcia-Garcia, H.; Diletti, R.; Kappetein, A.P.; Morel, M.A.; De Vries, T.; Swart, M.; et al. Combined anatomical and clinical factors for the long-term risk stratification of patients undergoing percutaneous coronary intervention: The Logistic Clinical SYNTAX score. Eur. Heart J. 2012, 33, 3098–3104. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, S.; Ivanov, J.; Mackie, K.; Seidelin, P.H.; Džavík, V. The Toronto score for in-hospital mortality after percutaneous coronary interventions. Am. Heart J. 2009, 157, 156–163. [Google Scholar] [CrossRef] [PubMed]
- McAllister, K.; Ludman, P.F.; Hulme, W.; de Belder, M.A.; Stables, R.; Chowdhary, S.; Mamas, M.; Sperrin, M.; Buchan, I. A contemporary risk model for predicting 30-day mortality following percutaneous coronary intervention in England and Wales. Int. J. Cardiol. 2016, 210, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milazzo, V.; Cosentino, N.; Campodonico, J.; Lucci, C.; Cardinale, D.; Cipolla, C.M.; Marenzi, G. Characteristics, Management, and Outcomes of Acute Coronary Syndrome Patients with Cancer. J. Clin. Med. 2020, 9, 3642. [Google Scholar] [CrossRef]
- Kurisu, S.; Iwasaki, T.; Ishibashi, K.; Mitsuba, N.; Dohi, Y.; Kihara, Y. Comparison of treatment and outcome of acute myocardial infarction between cancer patients and non-cancer patients. Int. J. Cardiol. 2013, 167, 2335–2337. [Google Scholar] [CrossRef] [PubMed]
- Velders, M.A.; Boden, H.; Hofma, S.H.; Osanto, S.; van der Hoeven, B.L.; Heestermans, A.A.; Cannegieter, S.C.; Jukema, J.W.; Umans, V.A.; Schalij, M.J.; et al. Outcome after ST Elevation Myocardial Infarction in Patients with Cancer Treated with Primary Percutaneous Coronary Intervention. Am. J. Cardiol. 2013, 112, 1867–1872. [Google Scholar] [CrossRef]
- Landes, U.; Kornowski, R.; Bental, T.; Assali, A.; Vaknin-Assa, H.; Lev, E.; Iakobishvili, Z. Long-term outcomes after percutaneous coronary interventions in cancer survivors. Coron. Artery Dis. 2017, 28, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Valgimigli, M.; Bueno, H.; Byrne, R.; Collet, J.-P.; Costa, F.; Jeppsson, A.; Jüni, P.; Kastrati, A.; Kolh, P.; Mauri, L.; et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur. J. Cardio-Thorac. Surg. 2017, 53, 34–78. [Google Scholar] [CrossRef] [Green Version]
- Leiva, O.; AbdelHameid, D.; Connors, J.M.; Cannon, C.P.; Bhatt, D.L. Common Pathophysiology in Cancer, Atrial Fibrillation, Atherosclerosis, and Thrombosis. JACC CardioOncol. 2021, 3, 619–634. [Google Scholar] [CrossRef] [PubMed]
- Ueki, Y.; Bär, S.; Losdat, S.; Otsuka, T.; Zanchin, C.; Zanchin, T.; Gragnano, F.; Gargiulo, G.; Siontis, G.C.M.; Praz, F.; et al. Validation of the Academic Research Consortium for High Bleeding Risk (ARC-HBR) criteria in patients undergoing percutaneous coronary intervention and comparison with contemporary bleeding risk scores. EuroIntervention 2020, 16, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Sutton, N.; Seth, M.; Ruwende, C.; Gurm, H.S. Outcomes of Patients with Atrial Fibrillation Undergoing Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2016, 68, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Vranckx, P.; Angiolillo, D.J.; Valgimigli, M. Patients with Atrial Fibrillation and PCI or ACS: Does Predicted Risk Matter? J. Am. Coll. Cardiol. 2022, 79, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, G.; Wu, C.; Senthilselvan, A.; McMurtry, M.S. Secular Trends in Incidence and Mortality of Acute Venous Thromboembolism: The AB-VTE Population-Based Study. Am. J. Med. 2016, 129, e819–e825. [Google Scholar] [CrossRef]
- Lopes, R.D.; Heizer, G.; Aronson, R.; Vora, A.N.; Massaro, T.; Mehran, R.; Goodman, S.G.; Windecker, S.; Darius, H.; Li, J.; et al. Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation. N. Engl. J. Med. 2019, 380, 1509–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumbhani, D.J.; Cannon, C.P.; Beavers, C.J.; Bhatt, D.L.; Cuker, A.; Gluckman, T.J.; Marine, J.E.; Mehran, R.; Messe, S.R.; Patel, N.S. 2020 ACC Expert Consensus Decision Pathway for anticoagulant and antiplatelet therapy in patients with atrial fibrillation or venous thromboembolism undergoing percutaneous coronary intervention or with atherosclerotic cardiovascular disease: A report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 77, 629–658. [Google Scholar] [PubMed]
- Van Rein, N.; Heide-Jørgensen, U.; Lijfering, W.M.; Dekkers, O.M.; Sørensen, H.T.; Cannegieter, S.C. Major Bleeding Rates in Atrial Fibrillation Patients on Single, Dual, or Triple Antithrombotic Therapy. Circulation 2019, 139, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.L.; Sørensen, R.; Clausen, M.T.; Fog-Petersen, M.L.; Raunsø, J.; Gadsbøll, N.; Gislason, G.; Folke, F.; Andersen, S.S.; Schramm, T.K.; et al. Risk of Bleeding with Single, Dual, or Triple Therapy with Warfarin, Aspirin, and Clopidogrel in Patients with Atrial Fibrillation. Arch. Intern. Med. 2010, 170, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Vranckx, P.; Valgimigli, M.; Eckardt, L.; Tijssen, J.; Lewalter, T.; Gargiulo, G.; Batushkin, V.; Campo, G.; Lysak, Z.; Vakaliuk, I.; et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): A randomised, open-label, phase 3b trial. Lancet 2019, 394, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Bhatt, D.L.; Oldgren, J.; Lip, G.Y.; Ellis, S.G.; Kimura, T.; Maeng, M.; Merkely, B.; Zeymer, U.; Gropper, S.; et al. Dual Antithrombotic Therapy with Dabigatran after PCI in Atrial Fibrillation. N. Engl. J. Med. 2017, 377, 1513–1524. [Google Scholar] [CrossRef]
- Gibson, C.M.; Mehran, R.; Bode, C.; Halperin, J.; Verheugt, F.W.; Wildgoose, P.; Birmingham, M.; Ianus, J.; Burton, P.; van Eickels, M.; et al. Prevention of Bleeding in Patients with Atrial Fibrillation Undergoing PCI. N. Engl. J. Med. 2016, 375, 2423–2434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppens, M.; Eikelboom, J.W.; Hart, R.G.; Yusuf, S.; Lip, G.Y.; Dorian, P.; Shestakovska, O.; Connolly, S.J. The CHA2DS2-VASc score identifies those patients with atrial fibrillation and a CHADS2 score of 1 who are unlikely to benefit from oral anticoagulant therapy. Eur. Heart J. 2012, 34, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Lip, G.Y.; Frison, L.; Halperin, J.L.; Lane, D.A. Comparative Validation of a Novel Risk Score for Predicting Bleeding Risk in Anticoagulated Patients with Atrial Fibrillation: The HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) Score. J. Am. Coll. Cardiol. 2011, 57, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Hijazi, Z.; Oldgren, J.; Lindbäck, J.; Alexander, J.H.; Connolly, S.J.; Eikelboom, J.W.; Ezekowitz, M.D.; Held, C.; Hylek, E.M.; Lopes, R.D.; et al. The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: A derivation and validation study. Lancet 2016, 387, 2302–2311. [Google Scholar] [CrossRef]
- Urban, P.; Meredith, I.T.; Abizaid, A.; Pocock, S.J.; Carrie, D.; Naber, C.; Lipiecki, J.; Richardt, G.; Iñiguez, A.; Brunel, P.; et al. Polymer-free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N. Engl. J. Med. 2015, 373, 2038–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varenne, O.; Cook, S.; Sideris, G.; Kedev, S.; Cuisset, T.; Carrie, D.; Hovasse, T.; Garot, P.; El Mahmoud, R.; Spaulding, C.; et al. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): A randomised single-blind trial. Lancet 2018, 391, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Domei, T.; Morimoto, T.; Natsuaki, M.; Shiomi, H.; Toyota, T.; Ohya, M.; Suwa, S.; Takagi, K.; Nanasato, M.; et al. Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI: The STOPDAPT-2 Randomized Clinical Trial. JAMA J. Am. Med Assoc. 2019, 321, 2414–2427. [Google Scholar] [CrossRef] [Green Version]
- Windecker, S.; Latib, A.; Kedhi, E.; Kirtane, A.J.; Kandzari, D.E.; Mehran, R.; Price, M.J.; Abizaid, A.; Simon, D.I.; Worthley, S.G.; et al. Polymer-based or Polymer-free Stents in Patients at High Bleeding Risk. N. Engl. J. Med. 2020, 382, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
| Class | Agents | Cardiotoxic Effects |
|---|---|---|
| Immunomodulatory | Lenalidomide [40] Pomalidomide [41] Immune Checkpoint Inhibitors [42,43] | Endothelial Dysfunction → Destabilization of atherosclerotic lesions → Plaque rupture → Cardiovascular events |
| Anti-microtubule | Paclitaxel [44] | Vasoconstriction, Endothelial injury → Cardiovascular events |
| Proteasome Inhibitor | Carfilzomib [45] Bortezomib [46] | Cardiac ubiquitin–proteasome dysfunction → Endothelial injury→ Cardiovascular events |
| Aromatase Inhibitors | Anastrozole [47] Letrozole [47] | Vasoconstriction, Endothelial injury → Cardiovascular events |
| Anti-metabolites | 5-fluorouracil (5-FU) [30] Capecitabine [48] Gemcitabine [49] Nilotinib [50] | Coronary Vasospasm Thrombus Formation Direct cardiomyocytes and endothelial cells damage → Increase of Von Willebrand Factor’s activity → Cardiovascular events |
| BRC-ABL tyrosine kinase inhibitors | Nilotinib [50] Ponatinib [51] | Coronary Atherosclerosis Endothelial Apoptosis → Increase of Factor VII Levels → Prothrombrotic state → Cardiovascular events (cardiac, cerebrovascular, and peripheral events) |
| Vascular Endothelial Growth Factor Inhibitors | Bevacizumab [52] Sorafenib [53] Sunitinib [54] Pazopanib [55] Regorafenib [56] Axitinib [57] Ramucirumab [58] Aflibercept [59] | Vasospasm Inflammation Platelet Activation → Cardiac ischemia and arterial thrombosis |
| Alkilating agents | Cyclophosphamide [60,61] | Endothelial dysfunction → platelet aggregation and activation → cardiovascular events |
| Vinca-alkaloids | Vincristine [62] | Thrombus Formation Endothelial Injury → Cardiovascular Events |
| Platinum | Cisplatin [63] | Endothelial dysfunction → Thromboxane Production → Thrombus Formation → Platelet aggregation and activation → Cardiovascular Events |
| Anti-tumor antibiotics | Bleomycin [64] | Endothelial dysfunction → Platelet aggregation and activation → Cardiovascular Events |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucà, F.; Parrini, I.; Abrignani, M.G.; Rao, C.M.; Piccioni, L.; Di Fusco, S.A.; Ceravolo, R.; Bisceglia, I.; Riccio, C.; Gelsomino, S.; et al. Management of Acute Coronary Syndrome in Cancer Patients: It’s High Time We Dealt with It. J. Clin. Med. 2022, 11, 1792. https://doi.org/10.3390/jcm11071792
Lucà F, Parrini I, Abrignani MG, Rao CM, Piccioni L, Di Fusco SA, Ceravolo R, Bisceglia I, Riccio C, Gelsomino S, et al. Management of Acute Coronary Syndrome in Cancer Patients: It’s High Time We Dealt with It. Journal of Clinical Medicine. 2022; 11(7):1792. https://doi.org/10.3390/jcm11071792
Chicago/Turabian StyleLucà, Fabiana, Iris Parrini, Maurizio Giuseppe Abrignani, Carmelo Massimiliano Rao, Laura Piccioni, Stefania Angela Di Fusco, Roberto Ceravolo, Irma Bisceglia, Carmine Riccio, Sandro Gelsomino, and et al. 2022. "Management of Acute Coronary Syndrome in Cancer Patients: It’s High Time We Dealt with It" Journal of Clinical Medicine 11, no. 7: 1792. https://doi.org/10.3390/jcm11071792
APA StyleLucà, F., Parrini, I., Abrignani, M. G., Rao, C. M., Piccioni, L., Di Fusco, S. A., Ceravolo, R., Bisceglia, I., Riccio, C., Gelsomino, S., Colivicchi, F., & Gulizia, M. M., on behalf of Management and Quality Working Group ANMCO. (2022). Management of Acute Coronary Syndrome in Cancer Patients: It’s High Time We Dealt with It. Journal of Clinical Medicine, 11(7), 1792. https://doi.org/10.3390/jcm11071792






