Unveiling the Pathogenesis of Adenomyosis through Animal Models
Abstract
1. Introduction
2. Methods
3. Results
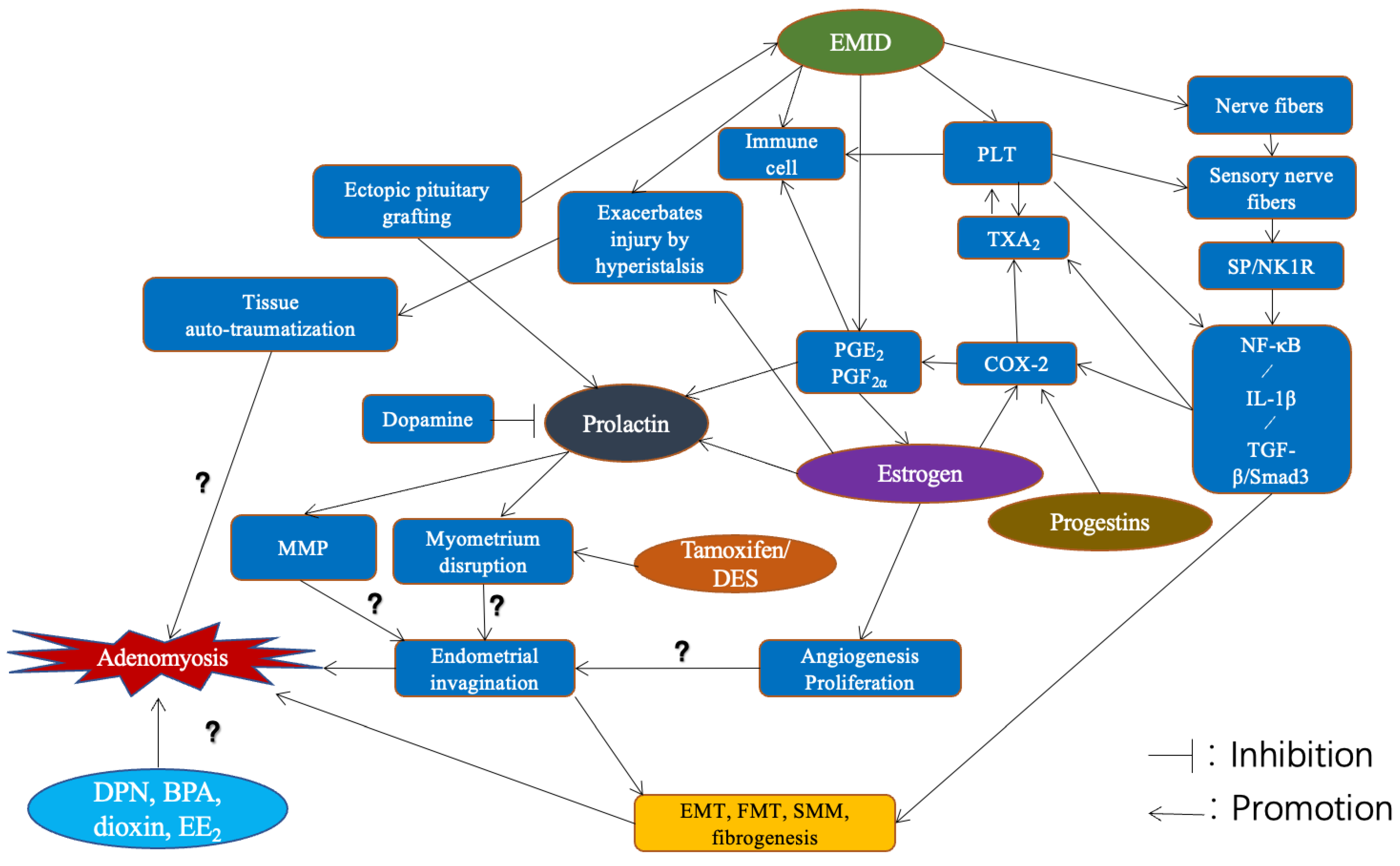
3.1. Pathogenetic Hypotheses and Theories
3.2. The Quest for the Primum Movens
3.3. Animal Models of Adenomyosis
3.3.1. Progestogens
3.3.2. Prolactin
3.4. Estrogens and Estrogenic Compounds
3.5. Evidence for More Than One Pathogenesis
3.6. In Utero Exposure to Exogenous Estrogens
3.7. Endometrial–Myometrial Interface Disruption
3.8. Other Models
3.9. The Root Causes for Pathogenesis
4. Discussion
4.1. Pathogenesis and Beyond
4.2. Knowledge Gaps
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGD | anogenital distance |
| DES | diethylstilbestrol |
| DPN | diarylpropionitrile |
| DRD2 | dopamine D2 receptor |
| D&C | dilatation and curettage |
| EMI | endometrial–myometrial interface |
| EMID | endometrial–myometrial interface disruption |
| EMT | epithelial–mesenchymal transition |
| FMT | fibroblast-to-myofibroblast transdifferentiation |
| FSH | follicular stimulating hormone |
| FSHR | follicular stimulating hormone receptor |
| HPA | hypothalamus–pituitary–adrenal |
| HSD17B1 | 17β-hydroxysteroid dehydrogenase type 1 |
| MMP | matrix metalloproteinase |
| NK1R | neurokinin receptor 1 |
| PGE2 | prostaglandin E2 |
| PGF2α | prostaglandin F2α |
| PPT | propylpyrazoletriol |
| PRL | prolactin |
| PRLR | prolactin receptor |
| ReTIAR | repeated tissue injury and repair |
| SERM | selective estrogen receptor modulator |
| SMM | smooth muscle metaplasia |
| SSRI | selective serotonin reuptake inhibitor |
| TIAR | tissue injury and repair |
References
- Bird, C.C.; McElin, T.W.; Manalo-Estrella, P. The elusive adenomyosis of the uterus—Revisited. Am. J. Obstet. Gynecol. 1972, 112, 583–593. [Google Scholar] [CrossRef]
- Emge, L.A. The elusive adenomyosis of the uterus. Its historical past and its present state of recognition. Am. J. Obstet. Gynecol. 1962, 83, 1541–1563. [Google Scholar] [CrossRef]
- Munro, M.G. Adenomyosis: A riddle, wrapped in mystery, inside an enigma. Fertil. Steril. 2021, 116, 89–90. [Google Scholar] [CrossRef]
- Seidman, J.D.; Kjerulff, K.H. Pathologic findings from the Maryland Women’s Health Study: Practice patterns in the diagnosis of adenomyosis. Int. J. Gynecol. Pathol. 1996, 15, 217–221. [Google Scholar] [CrossRef]
- Mark, A.S.; Hricak, H.; Heinrichs, L.W.; Hendrickson, M.R.; Winkler, M.L.; Bachica, J.A.; Stickler, J.E. Adenomyosis and leiomyoma: Differential diagnosis with MR imaging. Radiology 1987, 163, 527–529. [Google Scholar] [CrossRef]
- Fedele, L.; Bianchi, S.; Dorta, M.; Arcaini, L.; Zanotti, F.; Carinelli, S. Transvaginal ultrasonography in the diagnosis of diffuse adenomyosis. Fertil. Steril. 1992, 58, 94–97. [Google Scholar] [CrossRef]
- Peric, H.; Fraser, I.S. The symptomatology of adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, C.; Amant, F.; Ferenczy, A. Pathology and physiopathology of adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 511–521. [Google Scholar] [CrossRef]
- Alcalde, A.M.; Martinez-Zamora, M.A.; Gracia, M.; Ros, C.; Rius, M.; Carmona, F. Assessment of quality of sexual life in women with adenomyosis. Women Health 2021, 61, 520–526. [Google Scholar] [CrossRef]
- Harada, T.; Khine, Y.M.; Kaponis, A.; Nikellis, T.; Decavalas, G.; Taniguchi, F. The Impact of Adenomyosis on Women’s Fertility. Obstet. Gynecol. Surv. 2016, 71, 557–568. [Google Scholar] [CrossRef]
- Harada, T.; Taniguchi, F.; Amano, H.; Kurozawa, Y.; Ideno, Y.; Hayashi, K.; Harada, T.; Japan, E.; Children’s Study, G. Adverse obstetrical outcomes for women with endometriosis and adenomyosis: A large cohort of the Japan Environment and Children’s Study. PLoS ONE 2019, 14, e0220256. [Google Scholar] [CrossRef] [PubMed]
- Cope, A.G.; Ainsworth, A.J.; Stewart, E.A. Current and Future Medical Therapies for Adenomyosis. Semin. Reprod. Med. 2020, 38, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Vigano, P.; Somigliana, E.; Daguati, R.; Abbiati, A.; Fedele, L. Adenomyosis: Epidemiological factors. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 465–477. [Google Scholar] [CrossRef]
- Upson, K.; Missmer, S.A. Epidemiology of Adenomyosis. Semin. Reprod. Med. 2020, 38, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Yildiz, S.; Adli, M.; Wei, J.J. Adenomyosis pathogenesis: Insights from next-generation sequencing. Hum. Reprod. Update 2021, 27, 1086–1097. [Google Scholar] [CrossRef]
- Vannuccini, S.; Tosti, C.; Carmona, F.; Huang, S.J.; Chapron, C.; Guo, S.W.; Petraglia, F. Pathogenesis of adenomyosis: An update on molecular mechanisms. Reprod. Biomed. Online 2017, 35, 592–601. [Google Scholar] [CrossRef]
- Zhai, J.; Vannuccini, S.; Petraglia, F.; Giudice, L.C. Adenomyosis: Mechanisms and Pathogenesis. Semin. Reprod. Med. 2020, 38, 129–143. [Google Scholar] [CrossRef]
- Garcia-Solares, J.; Donnez, J.; Donnez, O.; Dolmans, M.M. Pathogenesis of uterine adenomyosis: Invagination or metaplasia? Fertil. Steril. 2018, 109, 371–379. [Google Scholar] [CrossRef]
- Stratopoulou, C.A.; Donnez, J.; Dolmans, M.M. Origin and Pathogenic Mechanisms of Uterine Adenomyosis: What Is Known So Far. Reprod. Sci. 2021, 28, 2087–2097. [Google Scholar] [CrossRef]
- Antero, M.F.; Ayhan, A.; Segars, J.; Shih, I.M. Pathology and Pathogenesis of Adenomyosis. Semin. Reprod. Med. 2020, 38, 108–118. [Google Scholar] [CrossRef]
- Vannuccini, S.; Petraglia, F. Recent advances in understanding and managing adenomyosis. F1000Res 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrovych, V.; Basta, P.; Gil, K. Current facts constituting an understanding of the nature of adenomyosis. Adv. Clin. Exp. Med. 2019, 28, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Greaves, P.; White, I.N. Experimental adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 503–510. [Google Scholar] [CrossRef]
- Nagasawa, H.; Kusakawa, S. Relationship between incidence and onset age of mammary tumours and uterine adenomyosis in four strains of mice: Comparison with the findings of 40 generations previously. In Vivo 2001, 15, 345–349. [Google Scholar] [PubMed]
- Kida, H. Histological analysis of spontaneous adenomyosis-like changes in recombinant inbred mouse uterus (SMXA mouse)—A novel animal model for adenomyosis. Nihon Sanka Fujinka Gakkai Zasshi 1994, 46, 323–330. [Google Scholar]
- DiGiacomo, R.F. Gynecologic pathology in the rhesus monkey (Macaca mulatta). II. Findings in laboratory and free-ranging monkeys. Vet. Pathol. 1977, 14, 539–546. [Google Scholar] [CrossRef]
- Gelberg, H.B.; McEntee, K. Pathology of the canine and feline uterine tube. Vet. Pathol. 1986, 23, 770–775. [Google Scholar] [CrossRef]
- Stocklin-Gautschi, N.M.; Guscetti, F.; Reichler, I.M.; Geissbuhler, U.; Braun, S.A.; Arnold, S. Identification of focal adenomyosis as a uterine lesion in two dogs. J. Small Anim. Pract. 2001, 42, 413–416. [Google Scholar] [CrossRef]
- Barrier, B.F.; Malinowski, M.J.; Dick, E.J., Jr.; Hubbard, G.B.; Bates, G.W. Adenomyosis in the baboon is associated with primary infertility. Fertil. Steril. 2004, 82 (Suppl. S3), 1091–1094. [Google Scholar] [CrossRef]
- Barrier, B.F.; Allison, J.; Hubbard, G.B.; Dick, E.J., Jr.; Brasky, K.M.; Schust, D.J. Spontaneous adenomyosis in the chimpanzee (Pan troglodytes): A first report and review of the primate literature: Case report. Hum. Reprod. 2007, 22, 1714–1717. [Google Scholar] [CrossRef][Green Version]
- Marquardt, R.M.; Jeong, J.W.; Fazleabas, A.T. Animal Models of Adenomyosis. Semin. Reprod. Med. 2020, 38, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Habiba, M. Animal models of adenomyosis. In Uterine Adenomyosis; Habiba, M., Benogiano, G., Eds.; Springer: New York, NY, USA, 2016; pp. 123–127. [Google Scholar]
- Hill, A.B. The Environment and Disease: Association or Causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E. Uterine stem cells: What is the evidence? Hum. Reprod. Update 2007, 13, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Ferenczy, A. Pathophysiology of adenomyosis. Hum. Reprod. Update 1998, 4, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Wildt, L.; Mall, G. The pathophysiology of endometriosis and adenomyosis: Tissue injury and repair. Arch. Gynecol. Obstet. 2009, 280, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Wildt, L. A new concept of endometriosis and adenomyosis: Tissue injury and repair (TIAR). Horm. Mol. Biol. Clin. Investig. 2011, 5, 125–142. [Google Scholar] [CrossRef]
- Leyendecker, G.; Bilgicyildirim, A.; Inacker, M.; Stalf, T.; Huppert, P.; Mall, G.; Bottcher, B.; Wildt, L. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch. Gynecol. Obstet. 2015, 291, 917–932. [Google Scholar] [CrossRef]
- Guo, S.W. The Pathogenesis of Adenomyosis vis-a-vis Endometriosis. J. Clin. Med. 2020, 9, 485. [Google Scholar] [CrossRef]
- de Miguel-Gomez, L.; Lopez-Martinez, S.; Frances-Herrero, E.; Rodriguez-Eguren, A.; Pellicer, A.; Cervello, I. Stem Cells and the Endometrium: From the Discovery of Adult Stem Cells to Pre-Clinical Models. Cells 2021, 10, 595. [Google Scholar] [CrossRef]
- Feng, W.; Madajka, M.; Kerr, B.A.; Mahabeleshwar, G.H.; Whiteheart, S.W.; Byzova, T.V. A novel role for platelet secretion in angiogenesis: Mediating bone marrow-derived cell mobilization and homing. Blood 2011, 117, 3893–3902. [Google Scholar] [CrossRef]
- Qi, Q.; Guo, S.-W.; Liu, X. Activated Platelets Induce Hypoxia-Inducible Factor-1α Expression Likely through Transforming Growth Factor-β1 in Human Endometrial Stromal Cells. Reprod. Dev. Med. 2019; in press. [Google Scholar]
- Qi, Q.; Liu, X.; Zhang, Q.; Guo, S.-W. Platelets induce increased estrogen production through NF-κB and TGF-β1 signaling pathways in endometriotic stromal cells. Sci. Rep. 2019; in press. [Google Scholar]
- Delaney, C.; Davizon-Castillo, P.; Allawzi, A.; Posey, J.; Gandjeva, A.; Neeves, K.; Tuder, R.M.; Di Paola, J.; Stenmark, K.R.; Nozik, E.S. Platelet activation contributes to hypoxia-induced inflammation. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2021, 320, L413–L421. [Google Scholar] [CrossRef] [PubMed]
- Chapron, C.; Tosti, C.; Marcellin, L.; Bourdon, M.; Lafay-Pillet, M.C.; Millischer, A.E.; Streuli, I.; Borghese, B.; Petraglia, F.; Santulli, P. Relationship between the magnetic resonance imaging appearance of adenomyosis and endometriosis phenotypes. Hum. Reprod. 2017, 32, 1393–1401. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, L.; Santulli, P.; Bortolato, S.; Morin, C.; Millischer, A.E.; Borghese, B.; Chapron, C. Anterior Focal Adenomyosis and Bladder Deep Infiltrating Endometriosis: Is There a Link? J. Minim. Invasive Gynecol. 2018, 25, 896–901. [Google Scholar] [CrossRef]
- Bourdon, M.; Oliveira, J.; Marcellin, L.; Santulli, P.; Bordonne, C.; Maitrot Mantelet, L.; Millischer, A.E.; Plu Bureau, G.; Chapron, C. Adenomyosis of the inner and outer myometrium are associated with different clinical profiles. Hum. Reprod. 2021, 36, 349–357. [Google Scholar] [CrossRef]
- Marcellin, L.; Santulli, P.; Bourdon, M.; Maignien, C.; Campin, L.; Lafay-Pillet, M.C.; Millischer, A.E.; Bordonne, C.; Borghese, B.; Dousset, B.; et al. Focal adenomyosis of the outer myometrium and deep infiltrating endometriosis severity. Fertil. Steril. 2020, 114, 818–827. [Google Scholar] [CrossRef]
- Khan, K.N.; Fujishita, A.; Koshiba, A.; Kuroboshi, H.; Mori, T.; Ogi, H.; Itoh, K.; Nakashima, M.; Kitawaki, J. Biological differences between intrinsic and extrinsic adenomyosis with coexisting deep infiltrating endometriosis. Reprod. Biomed. Online 2019, 39, 343–353. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M.; Fellah, L. What if deep endometriotic nodules and uterine adenomyosis were actually two forms of the same disease? Fertil. Steril. 2019, 111, 454–456. [Google Scholar] [CrossRef]
- Habiba, M.; Gordts, S.; Bazot, M.; Brosens, I.; Benagiano, G. Exploring the challenges for a new classification of adenomyosis. Reprod. Biomed. Online 2020, 40, 569–581. [Google Scholar] [CrossRef]
- Brosens, I.A. Endometriosis—A disease because it is characterized by bleeding. Am. J. Obstet. Gynecol. 1997, 176, 263–267. [Google Scholar] [CrossRef]
- Lacheta, J. Uterine adenomyosis: Pathogenesis, diagnostics, symptomatology and treatment. Ceská Gynekol. 2019, 84, 240–246. [Google Scholar]
- Guo, S.W. Fibrogenesis resulting from cyclic bleeding: The Holy Grail of the natural history of ectopic endometrium. Hum. Reprod. 2018, 33, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shen, M.; Qi, Q.; Zhang, H.; Guo, S.W. Corroborating evidence for platelet-induced epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis. Hum. Reprod. 2016, 31, 734–749. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Liu, X.; Zhang, H.; Guo, S.W. Transforming growth factor beta1 signaling coincides with epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis in mice. Hum. Reprod. 2016, 31, 355–369. [Google Scholar] [CrossRef]
- Yin, B.; Jiang, H.; Liu, X.; Guo, S.W. Enriched Environment Decelerates the Development of Endometriosis in Mouse. Reprod. Sci. 2020, 27, 1423–1435. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Liu, X.; Guo, S.W. Caloric Restriction Dramatically Stalls Lesion Growth in Mice With Induced Endometriosis. Reprod. Sci. 2018, 25, 1024–1036. [Google Scholar] [CrossRef]
- Guo, S.W.; Zhang, Q.; Liu, X. Social psychogenic stress promotes the development of endometriosis in mouse. Reprod. Biomed. Online 2017, 34, 225–239. [Google Scholar] [CrossRef]
- Long, Q.; Liu, X.; Qi, Q.; Guo, S.W. Chronic stress accelerates the development of endometriosis in mouse through adrenergic receptor beta2. Hum. Reprod. 2016, 31, 2506–2519. [Google Scholar] [CrossRef]
- Heard, M.E.; Melnyk, S.B.; Simmen, F.A.; Yang, Y.; Pabona, J.M.; Simmen, R.C. High-Fat Diet Promotion of Endometriosis in an Immunocompetent Mouse Model is Associated with Altered Peripheral and Ectopic Lesion Redox and Inflammatory Status. Endocrinology 2016, 157, 2870–2882. [Google Scholar] [CrossRef]
- Nodler, J.L.; Harris, H.R.; Chavarro, J.E.; Frazier, A.L.; Missmer, S.A. Dairy consumption during adolescence and endometriosis risk. Am. J. Obstet. Gynecol. 2020, 222, 257.e1–257.e16. [Google Scholar] [CrossRef]
- Liu, X.; Long, Q.; Guo, S.W. Surgical History and the Risk of Endometriosis: A Hospital-Based Case-Control Study. Reprod. Sci. 2016, 23, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Liu, X.; Guo, S.W. Surgery accelerates the development of endometriosis in mice. Am. J. Obstet. Gynecol. 2016, 215, 320.e1–320.e15. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Liu, X.; Guo, S.W. Early maternal separation accelerates the progression of endometriosis in adult mice. Reprod. Biol. Endocrinol. 2020, 18, 63. [Google Scholar] [CrossRef] [PubMed]
- Guttner, J. Adenomyosis in mice. Z Vers. 1980, 22, 249–251. [Google Scholar]
- Heinecke, H.; Klaus, S.; Guttner, J.; Oettel, M. References to the observation of the postnatal development of the F1-offspring from mice treated with oestrogen during pregnancy. Verh. Anat. Ges. 1977, 71, 655–658. [Google Scholar]
- Meissner, W.A.; Sommers, S.C.; Sherman, G. Endometrial hyperplasia, endometrial carcinoma, and endometriosis produced experimentally by estrogen. Cancer 1957, 10, 500–509. [Google Scholar] [CrossRef]
- Baskin, G.B.; Smith, S.M.; Marx, P.A. Endometrial hyperplasia, polyps, and adenomyosis associated with unopposed estrogen in rhesus monkeys (Macaca mulatta). Vet. Pathol. 2002, 39, 572–575. [Google Scholar] [CrossRef]
- Heinosalo, T. Characterization of the adenomyosis-like phenotype present in trangsgenic mice overexpressing human HSDB17. March 7, 2021.
- Hayashi, K.; Carpenter, K.D.; Spencer, T.E. Neonatal estrogen exposure disrupts uterine development in the postnatal sheep. Endocrinology 2004, 145, 3247–3257. [Google Scholar] [CrossRef]
- Parrott, E.; Butterworth, M.; Green, A.; White, I.N.; Greaves, P. Adenomyosis—A result of disordered stromal differentiation. Am. J. Pathol. 2001, 159, 623–630. [Google Scholar] [CrossRef]
- Green, A.R.; Styles, J.A.; Parrott, E.L.; Gray, D.; Edwards, R.E.; Smith, A.G.; Gant, T.W.; Greaves, P.; Al-Azzawi, F.; White, I.N. Neonatal tamoxifen treatment of mice leads to adenomyosis but not uterine cancer. Exp. Toxicol. Pathol. 2005, 56, 255–263. [Google Scholar] [CrossRef]
- Mehasseb, M.K.; Bell, S.C.; Habiba, M.A. Neonatal administration of tamoxifen causes disruption of myometrial development but not adenomyosis in the C57/BL6J mouse. Reproduction 2010, 139, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Huseby, R.A.; Thurlow, S. Effects of prenatal exposure of mice to “low-dose” diethylstilbestrol and the development of adenomyosis associated with evidence of hyperprolactinemia. Am. J. Obstet. Gynecol. 1982, 144, 939–949. [Google Scholar] [CrossRef]
- McLachlan, J.A.; Newbold, R.R.; Bullock, B.C. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 1980, 40, 3988–3999. [Google Scholar] [PubMed]
- Cao, Y.L.; Wang, X.; Liu, X.S.; Harada, T.; Guo, S.W. Neonatal feeding of an estrogen receptor β (ERβ) agonist induces external adenomyosis-like lesions in ICR mouse. Reprod. Dev. Med. 2022. [Google Scholar]
- Newbold, R.R.; Jefferson, W.N.; Padilla-Banks, E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod. Toxicol. 2007, 24, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Bruner-Tran, K.L.; Duleba, A.J.; Taylor, H.S.; Osteen, K.G. Developmental Toxicant Exposure Is Associated with Transgenerational Adenomyosis in a Murine Model. Biol. Reprod. 2016, 95, 73. [Google Scholar] [CrossRef]
- Koike, E.; Yasuda, Y.; Shiota, M.; Shimaoka, M.; Tsuritani, M.; Konishi, H.; Yamasaki, H.; Okumoto, K.; Hoshiai, H. Exposure to ethinyl estradiol prenatally and/or after sexual maturity induces endometriotic and precancerous lesions in uteri and ovaries of mice. Congenit. Anom. 2013, 53, 9–17. [Google Scholar] [CrossRef]
- Lipschutz, A.; Iglesias, R.; Panasevich, V.I.; Salinas, S. Pathological changes induced in the uterus of mice with the prolonged administration of progesterone and 19-nor-contraceptives. Br. J. Cancer 1967, 21, 160–165. [Google Scholar] [CrossRef]
- Mori, T.; Nagasawa, H. Mechanisms of development of prolactin-induced adenomyosis in mice. Acta Anat. 1983, 116, 46–54. [Google Scholar] [CrossRef]
- Mori, T.; Nagasawa, H.; Takahashi, S. The induction of adenomyosis in mice by intrauterine pituitary isografts. Life Sci. 1981, 29, 1277–1282. [Google Scholar] [CrossRef]
- Mori, T.; Kawashima, S.; Nagasawa, H. Induction of uterine adenomyosis by pituitary grafting and retardation of its development by bromocriptine-mesilate (CB-154) in BALB/c mice. In Vivo 1991, 5, 107–109. [Google Scholar] [PubMed]
- Mori, T.; Kyokuwa, M.; Nagasawa, H. Animal model of uterine adenomyosis: Induction of the lesion in rats by ectopic pituitary isografting. Lab. Anim. Sci. 1998, 48, 64–68. [Google Scholar]
- Huseby, R.A.; Soares, M.J.; Talamantes, F. Ectopic pituitary grafts in mice: Hormone levels, effects on fertility, and the development of adenomyosis uteri, prolactinomas, and mammary carcinomas. Endocrinology 1985, 116, 1440–1448. [Google Scholar] [CrossRef]
- Koujyo, T.; Hatakeyama, S.; Yamada, H.; Iwabuchi, K.; Kajino, K.; Ogasawara, K.; Onoe, K.; Fujimoto, S. Induction of endometriosis and adenomyosis by transvaginal pituitary transplantation in mice with and without natural killer cell activity. Am. J. Reprod. Immunol. 1998, 40, 441–446. [Google Scholar] [CrossRef]
- Singtripop, T.; Mori, T.; Park, M.K.; Sakamoto, S.; Kawashima, S. Development of uterine adenomyosis after treatment with dopamine antagonists in mice. Life Sci. 1991, 49, 201–206. [Google Scholar] [CrossRef]
- Ficicioglu, C.; Tekin, H.I.; Arioglu, P.F.; Okar, I. A murine model of adenomyosis: The effects of hyperprolactinemia induced by fluoxetine hydrochloride, a selective serotonin reuptake inhibitor, on adenomyosis induction in Wistar albino rats. Acta Eur. Fertil. 1995, 26, 75–79. [Google Scholar] [PubMed]
- Kelly, M.A.; Rubinstein, M.; Asa, S.L.; Zhang, G.; Saez, C.; Bunzow, J.R.; Allen, R.G.; Hnasko, R.; Ben-Jonathan, N.; Grandy, D.K.; et al. Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron 1997, 19, 103–113. [Google Scholar] [CrossRef]
- Hao, M.; Liu, X.; Guo, S.W. Adenomyosis in mice resulting from mechanically or thermally induced endometrial-myometrial interface disruption and its possible prevention. Reprod. Biomed. Online 2020, 41, 925–942. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, G.; Behringer, R.R. Dicer is required for female reproductive tract development and fertility in the mouse. Mol. Reprod. Dev. 2009, 76, 678–688. [Google Scholar] [CrossRef]
- Danilovich, N.; Roy, I.; Sairam, M.R. Emergence of uterine pathology during accelerated biological aging in FSH receptor-haploinsufficient mice. Endocrinology 2002, 143, 3618–3627. [Google Scholar] [CrossRef]
- Bellessort, B.; Bachelot, A.; Heude, E.; Alfama, G.; Fontaine, A.; Le Cardinal, M.; Treier, M.; Levi, G. Role of Foxl2 in uterine maturation and function. Hum. Mol. Genet. 2015, 24, 3092–3103. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, P.S.; Lee, H.J.; Zhang, L.; Zukerberg, L.R.; Taketo, M.M.; Rueda, B.R.; Teixeira, J.M. Constitutive activation of Beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol. Reprod. 2009, 81, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, J.; Luo, W.; Zhang, Y.; Li, J.; Li, H.; Chen, L.; Zhou, Y. Prostaglandin D2 is the major cyclooxygenase-1-derived product in prepartum mouse uteri where it mediates an enhanced in vitro myometrial contraction. Eur. J. Pharmacol. 2017, 813, 140–146. [Google Scholar] [CrossRef]
- Lee, N.C.; Dicker, R.C.; Rubin, G.L.; Ory, H.W. Confirmation of the preoperative diagnoses for hysterectomy. Am. J. Obstet. Gynecol. 1984, 150, 283–287. [Google Scholar] [CrossRef]
- Mathur, B.B.; Shah, B.S.; Bhende, Y.M. Adenomyosis uteri. A pathologic study of 290 cases. Am. J. Obstet. Gynecol. 1962, 84, 1820–1829. [Google Scholar] [CrossRef]
- Taran, F.A.; Wallwiener, M.; Kabashi, D.; Rothmund, R.; Rall, K.; Kraemer, B.; Brucker, S.Y. Clinical characteristics indicating adenomyosis at the time of hysterectomy: A retrospective study in 291 patients. Arch. Gynecol. Obstet. 2012, 285, 1571–1576. [Google Scholar] [CrossRef]
- Pinzauti, S.; Lazzeri, L.; Tosti, C.; Centini, G.; Orlandini, C.; Luisi, S.; Zupi, E.; Exacoustos, C.; Petraglia, F. Transvaginal sonographic features of diffuse adenomyosis in 18-30-year-old nulligravid women without endometriosis: Association with symptoms. Ultrasound Obstet. Gynecol. 2015, 46, 730–736. [Google Scholar] [CrossRef]
- Dietrich, J.E. An update on adenomyosis in the adolescent. Curr. Opin. Obstet. Gynecol. 2010, 22, 388–392. [Google Scholar] [CrossRef]
- Uduwela, A.S.; Perera, M.A.; Aiqing, L.; Fraser, I.S. Endometrial-myometrial interface: Relationship to adenomyosis and changes in pregnancy. Obstet. Gynecol. Surv. 2000, 55, 390–400. [Google Scholar] [CrossRef]
- Maia, H., Jr.; Casoy, J.; Pimentel, K.; Correia, T.; Athayde, C.; Cruz, T.; Coutinho, E.M. Effect of oral contraceptives on vascular endothelial growth factor, Cox-2 and aromatase expression in the endometrium of uteri affected by myomas and associated pathologies. Contraception 2008, 78, 479–485. [Google Scholar] [CrossRef]
- Maia, H., Jr.; Haddad, C.; Pinheiro, N.; Casoy, J. The effect of oral contraceptives on aromatase and Cox-2 expression in the endometrium of patients with idiopathic menorrhagia or adenomyosis. Int. J. Womens Health 2013, 5, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Monsivais, D.; Kakinuma, T.; Furukawa, Y.; Bernardi, L.; Pavone, M.E.; Dyson, M. Molecular biology of endometriosis: From aromatase to genomic abnormalities. Semin. Reprod. Med. 2015, 33, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Li, H.Y.; Huang, C.H.; Twu, N.F.; Yen, M.S.; Wang, P.H.; Chou, T.Y.; Liu, Y.N.; Chao, K.C.; Yang, M.H. Oestrogen-induced epithelial-mesenchymal transition of endometrial epithelial cells contributes to the development of adenomyosis. J. Pathol. 2010, 222, 261–270. [Google Scholar] [CrossRef]
- Hardy, D.B.; Janowski, B.A.; Corey, D.R.; Mendelson, C.R. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol. Endocrinol. 2006, 20, 2724–2733. [Google Scholar] [CrossRef]
- Esquifino, A.I.; Pazo, D.; Cutrera, R.A.; Cardinali, D.P. Seasonally dependent effect of ectopic pituitary grafts on 24-hour rhythms in serum prolactin and gonadotropins in rats. Chronobiol. Int. 1999, 16, 451–460. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, J.J.; Ubeda, E.; Cebeira, M.; Agrasal, C.; Tresguerres, J.A.; Ramos, J.A.; Esquifino, A.I. Modifications of plasma prolactin levels and catecholamine content in an ectopic anterior pituitary gland transplanted under the kidney capsule. Horm. Res. 1987, 25, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P.; Dinan, T.G. Prolactin and dopamine: What is the connection? A review article. J. Psychopharmacol. 2008, 22, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ruiz, J.J.; Cebeira, M.; Agrasal, C.; Tresguerres, J.A.; Bartke, A.; Esquifino, A.I.; Ramos, J.A. Possible role of dopamine and noradrenaline in the regulation of prolactin secretion from an ectopic anterior pituitary gland in female rats. J. Endocrinol. 1987, 113, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Singtripop, T.; Kawashima, S. Animal model of uterine adenomyosis: Is prolactin a potent inducer of adenomyosis in mice? Am. J. Obstet. Gynecol. 1991, 165, 232–234. [Google Scholar] [CrossRef]
- Sengupta, P.; Sharma, A.; Mazumdar, G.; Banerjee, I.; Tripathi, S.K.; Bagchi, C.; Das, N. The possible role of fluoxetine in adenomyosis: An animal experiment with clinical correlations. J. Clin. Diagn. Res. 2013, 7, 1530–1534. [Google Scholar] [CrossRef]
- Yamashita, M.; Matsuda, M.; Mori, T. Increased expression of prolactin receptor mRNA in adenomyotic uterus in mice. Life Sci. 1997, 60, 1437–1446. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, J.J.; Agrasal, C.; Cebeira, M.; Tresguerres, J.A.; Esquifino, A.I.; Ramos, J.A. Effects of estradiol on circulating levels of prolactin in female rats bearing ectopic pituitaries. Rev. Esp. Fisiol. 1988, 44, 205–210. [Google Scholar]
- Bernard, V.; Young, J.; Binart, N. Prolactin—A pleiotropic factor in health and disease. Nat. Rev. Endocrinol. 2019, 15, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Walters, C.A.; Daly, D.C.; Chapitis, J.; Kuslis, S.T.; Prior, J.C.; Kusmik, W.F.; Riddick, D.H. Human myometrium: A new potential source of prolactin. Am. J. Obstet. Gynecol. 1983, 147, 639–644. [Google Scholar] [CrossRef]
- Bonhoff, A.; Gellersen, B. Modulation of prolactin secretion in human myometrium by cytokines. Eur. J. Obstet. Gynecol. Reprod. Biol. 1994, 54, 55–62. [Google Scholar] [CrossRef]
- Gerlo, S.; Verdood, P.; Hooghe-Peters, E.L.; Kooijman, R. Modulation of prolactin expression in human T lymphocytes by cytokines. J. Neuroimmunol. 2005, 162, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Frasor, J.; Gaspar, C.A.; Donnelly, K.M.; Gibori, G.; Fazleabas, A.T. Expression of prolactin and its receptor in the baboon uterus during the menstrual cycle and pregnancy. J. Clin. Endocrinol. Metab. 1999, 84, 3344–3350. [Google Scholar] [CrossRef]
- Jikihara, H.; Kessler, C.A.; Cedars, M.I.; Brar, A.K. Up-regulation of the human prolactin receptor in the endometrium. Endocrine 1996, 5, 157–162. [Google Scholar] [CrossRef]
- Tseng, L.; Mazella, J. Prolactin and its receptor in human endometrium. Semin. Reprod. Endocrinol. 1999, 17, 23–27. [Google Scholar] [CrossRef]
- Trott, J.F.; Horigan, K.C.; Gloviczki, J.M.; Costa, K.M.; Freking, B.A.; Farmer, C.; Hayashi, K.; Spencer, T.; Morabito, J.E.; Hovey, R.C. Tissue-specific regulation of porcine prolactin receptor expression by estrogen, progesterone, and prolactin. J. Endocrinol. 2009, 202, 153–166. [Google Scholar] [CrossRef]
- Bozic, I.; Antal, T.; Ohtsuki, H.; Carter, H.; Kim, D.; Chen, S.; Karchin, R.; Kinzler, K.W.; Vogelstein, B.; Nowak, M.A. Accumulation of driver and passenger mutations during tumor progression. Proc. Natl. Acad. Sci. USA 2010, 107, 18545–18550. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.A.; Mora, S.; Diehl, T.; Rhoades, A.R.; Stewart, E.A. Prolactin is an autocrine or paracrine growth factor for human myometrial and leiomyoma cells. Gynecol. Obstet. Investig. 1999, 48, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.A.; Rein, M.S.; Heffner, L.J.; Friedman, A.J.; Tashjian, A.H., Jr. Production of prolactin by smooth muscle cells cultured from human uterine fibroid tumors. J. Clin. Endocrinol. Metab. 1993, 76, 1308–1313. [Google Scholar] [CrossRef]
- Matsuda, M.; Sasabe, H.; Adachi, Y.; Suzuki, T.; Mori, T. Increased invasion activity of endometrial stromal cells and elevated expression of matrix metalloproteinase messenger RNA in the uterine tissues of mice with experimentally induced adenomyosis. Am. J. Obstet. Gynecol. 2001, 185, 1374–1380. [Google Scholar] [CrossRef]
- O’Leary, K.A.; Rugowski, D.E.; Shea, M.P.; Sullivan, R.; Moser, A.R.; Schuler, L.A. Prolactin synergizes with canonical Wnt signals to drive development of ER+ mammary tumors via activation of the Notch pathway. Cancer Lett. 2021, 503, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Shin, J.H.; Kim, T.H.; Lee, H.S.; Yoo, J.Y.; Ahn, J.Y.; Broaddus, R.R.; Taketo, M.M.; Lydon, J.P.; Leach, R.E.; et al. beta-Catenin activation contributes to the pathogenesis of adenomyosis through epithelial-mesenchymal transition. J. Pathol. 2013, 231, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Zhao, X.; Li, M.; Zhang, X.; Lu, Z.; Yang, C.; Zhang, C.; Zhang, H.; Zhang, N. Aberrant expression of Notch1/numb/snail signaling, an epithelial mesenchymal transition related pathway, in adenomyosis. Reprod. Biol. Endocrinol. 2015, 13, 96. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Ku, B.J.; Kim, T.H.; Ahn, J., II; Ahn, J.Y.; Yang, W.S.; Lim, J.M.; Taketo, M.M.; Shin, J.H.; Jeong, J.W. Beta-catenin activates TGF-beta-induced epithelial-mesenchymal transition in adenomyosis. Exp. Mol. Med. 2020, 52, 1754–1765. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Duan, H.; Wang, S.; Quan, Y.J.; Huang, J.H.; Guo, Z.C. Talin1 Induces Epithelial-Mesenchymal Transition to Facilitate Endometrial Cell Migration and Invasion in Adenomyosis Under the Regulation of microRNA-145-5p. Reprod. Sci. 2021, 28, 1523–1539. [Google Scholar] [CrossRef]
- Taran, F.A.; Weaver, A.L.; Coddington, C.C.; Stewart, E.A. Understanding adenomyosis: A case control study. Fertil. Steril. 2010, 94, 1223–1228. [Google Scholar] [CrossRef]
- Patil, M.J.; Henry, M.A.; Akopian, A.N. Prolactin receptor in regulation of neuronal excitability and channels. Channels 2014, 8, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.J.; Green, D.P.; Henry, M.A.; Akopian, A.N. Sex-dependent roles of prolactin and prolactin receptor in postoperative pain and hyperalgesia in mice. Neuroscience 2013, 253, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.; Belugin, S.; Mecklenburg, J.; Wangzhou, A.; Paige, C.; Barba-Escobedo, P.A.; Boyd, J.T.; Goffin, V.; Grattan, D.; Boehm, U.; et al. Prolactin Regulates Pain Responses via a Female-Selective Nociceptor-Specific Mechanism. iScience 2019, 20, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Belugin, S.; Diogenes, A.R.; Patil, M.J.; Ginsburg, E.; Henry, M.A.; Akopian, A.N. Mechanisms of transient signaling via short and long prolactin receptor isoforms in female and male sensory neurons. J. Biol. Chem. 2013, 288, 34943–34955. [Google Scholar] [CrossRef]
- Liu, T.T.; Qu, Z.W.; Ren, C.; Gan, X.; Qiu, C.Y.; Hu, W.P. Prolactin potentiates the activity of acid-sensing ion channels in female rat primary sensory neurons. Neuropharmacology 2016, 103, 174–182. [Google Scholar] [CrossRef]
- Lupicka, M.; Socha, B.M.; Szczepanska, A.A.; Korzekwa, A.J. Prolactin role in the bovine uterus during adenomyosis. Domest. Anim. Endocrinol. 2017, 58, 1–13. [Google Scholar] [CrossRef]
- Chakroborty, D.; Goswami, S.; Basu, S.; Sarkar, C. Catecholamines in the regulation of angiogenesis in cutaneous wound healing. FASEB J. 2020, 34, 14093–14102. [Google Scholar] [CrossRef]
- Vaughn, A.R.; Davis, M.J.; Sivamani, R.K.; Isseroff, R.R. A Concise Review of the Conflicting Roles of Dopamine-1 versus Dopamine-2 Receptors in Wound Healing. Molecules 2017, 23, 50. [Google Scholar] [CrossRef]
- Pellicer, N.; Galliano, D.; Herraiz, S.; Bagger, Y.Z.; Arce, J.C.; Pellicer, A. Use of dopamine agonists to target angiogenesis in women with endometriosis. Hum. Reprod. 2021, 36, 850–858. [Google Scholar] [CrossRef]
- Chen, Y.; Moutal, A.; Navratilova, E.; Kopruszinski, C.; Yue, X.; Ikegami, M.; Chow, M.; Kanazawa, I.; Bellampalli, S.S.; Xie, J.; et al. The prolactin receptor long isoform regulates nociceptor sensitization and opioid-induced hyperalgesia selectively in females. Sci. Transl. Med. 2020, 12, eaay7550. [Google Scholar] [CrossRef]
- Andersson, J.K.; Khan, Z.; Weaver, A.L.; Vaughan, L.E.; Gemzell-Danielsson, K.; Stewart, E.A. Vaginal bromocriptine improves pain, menstrual bleeding and quality of life in women with adenomyosis: A pilot study. Acta Obstet. Gynecol. Scand. 2019, 98, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Zhang, X.; Luo, H.; Xu, L.; Lu, X.; Lu, J. Adrenaline promotes epithelial-to-mesenchymal transition via HuR-TGFbeta regulatory axis in pancreatic cancer cells and the implication in cancer prognosis. Biochem. Biophys. Res. Commun. 2017, 493, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Cui, X.; Li, W.; Lin, W.; Li, Y.; Chen, X.; Wu, T. Novel regulatory program for norepinephrine-induced epithelial-mesenchymal transition in gastric adenocarcinoma cell lines. Cancer Sci. 2014, 105, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zuo, S.; Hou, Y.; Shang, W.; Liu, N.; Yin, Z. Inhibition of alpha1-adrenoceptor reduces TGF-beta1-induced epithelial-to-mesenchymal transition and attenuates UUO-induced renal fibrosis in mice. FASEB J. 2020, 34, 14892–14904. [Google Scholar] [CrossRef]
- Archer, M.; Dogra, N.; Dovey, Z.; Ganta, T.; Jang, H.S.; Khusid, J.A.; Lantz, A.; Mihalopoulos, M.; Stockert, J.A.; Zahalka, A.; et al. Role of alpha- and beta-adrenergic signaling in phenotypic targeting: Significance in benign and malignant urologic disease. Cell Commun. Signal. 2021, 19, 78. [Google Scholar] [CrossRef]
- Vercellini, P.; Parazzini, F.; Oldani, S.; Panazza, S.; Bramante, T.; Crosignani, P.G. Adenomyosis at hysterectomy: A study on frequency distribution and patient characteristics. Hum. Reprod. 1995, 10, 1160–1162. [Google Scholar] [CrossRef]
- Jensen, E.V.; Jordan, V.C. The estrogen receptor: A model for molecular medicine. Clin. Cancer Res. 2003, 9, 1980–1989. [Google Scholar]
- Pinkerton, J.V.; Goldstein, S.R. Endometrial safety: A key hurdle for selective estrogen receptor modulators in development. Menopause 2010, 17, 642–653. [Google Scholar] [CrossRef]
- Jordan, V.C. Tamoxifen: A most unlikely pioneering medicine. Nat. Rev. Drug Discov. 2003, 2, 205–213. [Google Scholar] [CrossRef]
- Parandin, R.; Behnam-Rassouli, M.; Mahdavi-Shahri, N. Oestrogenic action of neonatal tamoxifen on the hypothalamus and reproductive system in female mice. Reprod. Fertil. Dev. 2016, 29, 1012–1020. [Google Scholar] [CrossRef]
- Mehasseb, M.K.; Bell, S.C.; Habiba, M.A. The effects of tamoxifen and estradiol on myometrial differentiation and organization during early uterine development in the CD1 mouse. Reproduction 2009, 138, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Beyth, Y.; Shapira, J.; Tepper, R.; Fishman, A.; Cordoba, M.; Bernheim, J.; Yigael, D.; Altaras, M.M. High frequency of adenomyosis in postmenopausal breast cancer patients treated with tamoxifen. Gynecol. Obstet. Investig. 1997, 44, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Street, M.E.; Angelini, S.; Bernasconi, S.; Burgio, E.; Cassio, A.; Catellani, C.; Cirillo, F.; Deodati, A.; Fabbrizi, E.; Fanos, V.; et al. Current Knowledge on Endocrine Disrupting Chemicals (EDCs) from Animal Biology to Humans, from Pregnancy to Adulthood: Highlights from a National Italian Meeting. Int. J. Mol. Sci. 2018, 19, 1647. [Google Scholar] [CrossRef] [PubMed]
- Carthew, P.; Rich, K.J.; Martin, E.A.; De Matteis, F.; Lim, C.K.; Manson, M.M.; Festing, M.F.; White, I.N.; Smith, L.L. DNA damage as assessed by 32P-postlabelling in three rat strains exposed to dietary tamoxifen: The relationship between cell proliferation and liver tumour formation. Carcinogenesis 1995, 16, 1299–1304. [Google Scholar] [CrossRef]
- Newbold, R.R.; Jefferson, W.N.; Padilla-Burgos, E.; Bullock, B.C. Uterine carcinoma in mice treated neonatally with tamoxifen. Carcinogenesis 1997, 18, 2293–2298. [Google Scholar] [CrossRef][Green Version]
- Herbst, A.L. Diethylstilbestrol and other sex hormones during pregnancy. Obstet. Gynecol. 1981, 58, 35S–40S. [Google Scholar]
- Matsubara, M.; Harigaya, T.; Nogami, H. Effects of diethylstilbestrol on the cytogenesis of prolactin cells in the pars distalis of the pituitary gland of the mouse. Cell Tissue Res. 2001, 306, 301–307. [Google Scholar] [CrossRef]
- Murphy, E. Estrogen signaling and cardiovascular disease. Circ. Res. 2011, 109, 687–696. [Google Scholar] [CrossRef]
- Burns, K.A.; Korach, K.S. Estrogen receptors and human disease: An update. Arch. Toxicol. 2012, 86, 1491–1504. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Enmark, E.; Pelto-Huikko, M.; Nilsson, S.; Gustafsson, J.A. Cloning of a novel receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA 1996, 93, 5925–5930. [Google Scholar] [CrossRef]
- Mosselman, S.; Polman, J.; Dijkema, R. ER beta: Identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996, 392, 49–53. [Google Scholar] [CrossRef]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J.A. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.; Carlsson, B.; Grandien, K.; Enmark, E.; Haggblad, J.; Nilsson, S.; Gustafsson, J.A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Makela, S.; Treuter, E.; Tujague, M.; Thomsen, J.; Andersson, G.; Enmark, E.; Pettersson, K.; Warner, M.; Gustafsson, J.A. Mechanisms of estrogen action. Physiol. Rev. 2001, 81, 1535–1565. [Google Scholar] [CrossRef]
- Pettersson, K.; Delaunay, F.; Gustafsson, J.A. Estrogen receptor beta acts as a dominant regulator of estrogen signaling. Oncogene 2000, 19, 4970–4978. [Google Scholar] [CrossRef]
- Makela, S.; Savolainen, H.; Aavik, E.; Myllarniemi, M.; Strauss, L.; Taskinen, E.; Gustafsson, J.A.; Hayry, P. Differentiation between vasculoprotective and uterotrophic effects of ligands with different binding affinities to estrogen receptors alpha and beta. Proc. Natl. Acad. Sci. USA 1999, 96, 7077–7082. [Google Scholar] [CrossRef]
- Liu, M.M.; Albanese, C.; Anderson, C.M.; Hilty, K.; Webb, P.; Uht, R.M.; Price, R.H., Jr.; Pestell, R.G.; Kushner, P.J. Opposing action of estrogen receptors alpha and beta on cyclin D1 gene expression. J. Biol. Chem. 2002, 277, 24353–24360. [Google Scholar] [CrossRef]
- Cheng, G.; Weihua, Z.; Warner, M.; Gustafsson, J.A. Estrogen receptors ER alpha and ER beta in proliferation in the rodent mammary gland. Proc. Natl. Acad. Sci. USA 2004, 101, 3739–3746. [Google Scholar] [CrossRef]
- Matthews, J.; Wihlen, B.; Tujague, M.; Wan, J.; Strom, A.; Gustafsson, J.A. Estrogen receptor (ER) beta modulates ERalpha-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters. Mol. Endocrinol. 2006, 20, 534–543. [Google Scholar] [CrossRef]
- Chang, E.C.; Frasor, J.; Komm, B.; Katzenellenbogen, B.S. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology 2006, 147, 4831–4842. [Google Scholar] [CrossRef]
- Jordan, V.C. Tamoxifen (ICI46,474) as a targeted therapy to treat and prevent breast cancer. Br. J. Pharmacol. 2006, 147 (Suppl. S1), S269–S276. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.K. Tamoxifen in the treatment of breast cancer. N. Engl. J. Med. 1998, 339, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Bentrem, D.J.; Craig Jordan, V. Tamoxifen, raloxifene and the prevention of breast cancer. Minerva Endocrinol. 2002, 27, 127–139. [Google Scholar] [PubMed]
- Mann, S.; Laucirica, R.; Carlson, N.; Younes, P.S.; Ali, N.; Younes, A.; Li, Y.; Younes, M. Estrogen receptor beta expression in invasive breast cancer. Hum. Pathol. 2001, 32, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Honma, N.; Horii, R.; Iwase, T.; Saji, S.; Younes, M.; Takubo, K.; Matsuura, M.; Ito, Y.; Akiyama, F.; Sakamoto, G. Clinical importance of estrogen receptor-beta evaluation in breast cancer patients treated with adjuvant tamoxifen therapy. J. Clin. Oncol. 2008, 26, 3727–3734. [Google Scholar] [CrossRef] [PubMed]
- Rouhimoghadam, M.; Safarian, S.; Carroll, J.S.; Sheibani, N.; Bidkhori, G. Tamoxifen-Induced Apoptosis of MCF-7 Cells via GPR30/PI3K/MAPKs Interactions: Verification by ODE Modeling and RNA Sequencing. Front. Physiol. 2018, 9, 907. [Google Scholar] [CrossRef] [PubMed]
- Ignatov, A.; Ignatov, T.; Roessner, A.; Costa, S.D.; Kalinski, T. Role of GPR30 in the mechanisms of tamoxifen resistance in breast cancer MCF-7 cells. Breast Cancer Res. Treat. 2010, 123, 87–96. [Google Scholar] [CrossRef]
- Mo, Z.; Liu, M.; Yang, F.; Luo, H.; Li, Z.; Tu, G.; Yang, G. GPR30 as an initiator of tamoxifen resistance in hormone-dependent breast cancer. Breast Cancer Res. 2013, 15, R114. [Google Scholar] [CrossRef]
- Liu, L.; Liu, S.; Luo, H.; Chen, C.; Zhang, X.; He, L.; Tu, G. GPR30-mediated HMGB1 upregulation in CAFs induces autophagy and tamoxifen resistance in ERalpha-positive breast cancer cells. Aging 2021, 13, 16178–16197. [Google Scholar] [CrossRef]
- Ignatov, T.; Eggemann, H.; Semczuk, A.; Smith, B.; Bischoff, J.; Roessner, A.; Costa, S.D.; Kalinski, T.; Ignatov, A. Role of GPR30 in endometrial pathology after tamoxifen for breast cancer. Am. J. Obstet. Gynecol. 2010, 203, 595.e9–595.e16. [Google Scholar] [CrossRef]
- Lin, B.C.; Suzawa, M.; Blind, R.D.; Tobias, S.C.; Bulun, S.E.; Scanlan, T.S.; Ingraham, H.A. Stimulating the GPR30 estrogen receptor with a novel tamoxifen analogue activates SF-1 and promotes endometrial cell proliferation. Cancer Res. 2009, 69, 5415–5423. [Google Scholar] [CrossRef]
- Tsai, C.L.; Wu, H.M.; Lin, C.Y.; Lin, Y.J.; Chao, A.; Wang, T.H.; Hsueh, S.; Lai, C.H.; Wang, H.S. Estradiol and tamoxifen induce cell migration through GPR30 and activation of focal adhesion kinase (FAK) in endometrial cancers with low or without nuclear estrogen receptor alpha (ERalpha). PLoS ONE 2013, 8, e72999. [Google Scholar] [CrossRef]
- Bazot, M.; Darai, E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil. Steril. 2018, 109, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Kishi, Y.; Suginami, H.; Kuramori, R.; Yabuta, M.; Suginami, R.; Taniguchi, F. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am. J. Obstet. Gynecol. 2012, 207, 114.e1–114.e7. [Google Scholar] [CrossRef]
- Patisaul, H.B.; Burke, K.T.; Hinkle, R.E.; Adewale, H.B.; Shea, D. Systemic administration of diarylpropionitrile (DPN) or phytoestrogens does not affect anxiety-related behaviors in gonadally intact male rats. Horm. Behav. 2009, 55, 319–328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mendiola, J.; Sanchez-Ferrer, M.L.; Jimenez-Velazquez, R.; Canovas-Lopez, L.; Hernandez-Penalver, A.I.; Corbalan-Biyang, S.; Carmona-Barnosi, A.; Prieto-Sanchez, M.T.; Nieto, A.; Torres-Cantero, A.M. Endometriomas and deep infiltrating endometriosis in adulthood are strongly associated with anogenital distance, a biomarker for prenatal hormonal environment. Hum. Reprod. 2016, 31, 2377–2383. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ferrer, M.L.; Mendiola, J.; Jimenez-Velazquez, R.; Canovas-Lopez, L.; Corbalan-Biyang, S.; Hernandez-Penalver, A.I.; Carmona-Barnosi, A.; Maldonado-Carceles, A.B.; Prieto-Sanchez, M.T.; Machado-Linde, F.; et al. Investigation of anogenital distance as a diagnostic tool in endometriosis. Reprod. Biomed. Online 2017, 34, 375–382. [Google Scholar] [CrossRef]
- Sanchez-Ferrer, M.L.; Jimenez-Velazquez, R.; Mendiola, J.; Prieto-Sanchez, M.T.; Canovas-Lopez, L.; Carmona-Barnosi, A.; Corbalan-Biyang, S.; Hernandez-Penalver, A.I.; Adoamnei, E.; Nieto, A.; et al. Accuracy of anogenital distance and anti-Mullerian hormone in the diagnosis of endometriosis without surgery. Int. J. Gynaecol. Obstet. 2019, 144, 90–96. [Google Scholar] [CrossRef]
- Crestani, A.; Arfi, A.; Ploteau, S.; Breban, M.; Boudy, A.S.; Bendifallah, S.; Ferrier, C.; Darai, E. Anogenital distance in adult women is a strong marker of endometriosis: Results of a prospective study with laparoscopic and histological findings. Hum. Reprod. Open 2020, 2020, hoaa023. [Google Scholar] [CrossRef]
- Crestani, A.; Abdel Wahab, C.; Arfi, A.; Ploteau, S.; Kolanska, K.; Breban, M.; Bendifallah, S.; Ferrier, C.; Darai, E. A short anogenital distance on MRI is a marker of endometriosis. Hum. Reprod. Open 2021, 2021, hoab003. [Google Scholar] [CrossRef]
- Brody, J.R.; Cunha, G.R. Histologic, morphometric, and immunocytochemical analysis of myometrial development in rats and mice: II. Effects of DES on development. Am. J. Anat. 1989, 186, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Moller, F.J.; Ledwig, C.; Zierau, O.; Hertrampf, T.; Degen, G.H.; Diel, P.; Vollmer, G. The rat prepubertal uterine myometrium and not the luminal epithelium is predominantly affected by a chronic dietary genistein exposure. Arch. Toxicol. 2012, 86, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, T.; Takasugi, N. Postnatal development of uterine abnormalities in mice exposed to DES in utero. Biol. Neonate 1987, 52, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.; Kitajima, M.; Hiraki, K.; Fujishita, A.; Nakashima, M.; Masuzaki, H. Involvement of hepatocyte growth factor-induced epithelial-mesenchymal transition in human adenomyosis. Biol. Reprod. 2015, 92, 35. [Google Scholar] [CrossRef]
- Curtis, K.M.; Hillis, S.D.; Marchbanks, P.A.; Peterson, H.B. Disruption of the endometrial-myometrial border during pregnancy as a risk factor for adenomyosis. Am. J. Obstet. Gynecol. 2002, 187, 543–544. [Google Scholar] [CrossRef]
- Levgur, M.; Abadi, M.A.; Tucker, A. Adenomyosis: Symptoms, histology, and pregnancy terminations. Obstet. Gynecol. 2000, 95, 688–691. [Google Scholar] [CrossRef]
- Panganamamula, U.R.; Harmanli, O.H.; Isik-Akbay, E.F.; Grotegut, C.A.; Dandolu, V.; Gaughan, J.P. Is prior uterine surgery a risk factor for adenomyosis? Obstet. Gynecol. 2004, 104, 1034–1038. [Google Scholar] [CrossRef]
- Parazzini, F.; Vercellini, P.; Panazza, S.; Chatenoud, L.; Oldani, S.; Crosignani, P.G. Risk factors for adenomyosis. Hum. Reprod. 1997, 12, 1275–1279. [Google Scholar] [CrossRef]
- Parazzini, F.; Mais, V.; Cipriani, S.; Busacca, M.; Venturini, P.; GISE. Determinants of adenomyosis in women who underwent hysterectomy for benign gynecological conditions: Results from a prospective multicentric study in Italy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 143, 103–106. [Google Scholar] [CrossRef]
- Bulun, S.E.; Imir, G.; Utsunomiya, H.; Thung, S.; Gurates, B.; Tamura, M.; Lin, Z. Aromatase in endometriosis and uterine leiomyomata. J. Steroid Biochem. Mol. Biol. 2005, 95, 57–62. [Google Scholar] [CrossRef]
- Philibert, P.; Dejardin, S.; Pirot, N.; Pruvost, A.; Nguyen, A.L.; Bernex, F.; Poulat, F.; Boizet-Bonhoure, B. In the mouse, prostaglandin D2 signalling protects the endometrium against adenomyosis. Mol. Hum. Reprod. 2021, 27, gaab029. [Google Scholar] [CrossRef]
- Liaqat, I.; Arifa, N.A.; Asif, S.; Lone, K.P. Association of FSHR gene polymorphisms with endometriosis in women visiting tertiary-care hospitals of Lahore, Pakistan. J. Pak. Med. Assoc. 2021, 71, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Andre, G.M.; Martins Trevisan, C.; Pedruzzi, I.N.; Fernandes, R.F.M.; Oliveira, R.; Christofolini, D.M.; Bianco, B.; Barbosa, C.P. The Impact of FSHR Gene Polymorphisms Ala307Thr and Asn680Ser in the Endometriosis Development. DNA Cell Biol. 2018, 37, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Vigano, P.; Somigliana, E.; Parazzini, F.; Vercellini, P. Bias versus causality: Interpreting recent evidence of association between endometriosis and ovarian cancer. Fertil. Steril. 2007, 88, 588–593. [Google Scholar] [CrossRef]
- Nisolle, M.; Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef]
- Tempest, N.; Jansen, M.; Baker, A.M.; Hill, C.J.; Hale, M.; Magee, D.; Treanor, D.; Wright, N.A.; Hapangama, D.K. Histological 3D reconstruction and in vivo lineage tracing of the human endometrium. J. Pathol. 2020, 251, 440–451. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yoshihara, K.; Suda, K.; Nakaoka, H.; Yachida, N.; Ueda, H.; Sugino, K.; Mori, Y.; Yamawaki, K.; Tamura, R.; et al. Three-dimensional understanding of the morphological complexity of the human uterine endometrium. iScience 2021, 24, 102258. [Google Scholar] [CrossRef]
- Mantyh, P.W. Substance P and the inflammatory and immune response. Ann. N. Y. Acad. Sci. 1991, 632, 263–271. [Google Scholar] [CrossRef]
- Kirchhoff, C.; Biberthaler, P.; Mutschler, W.E.; Faist, E.; Jochum, M.; Zedler, S. Early down-regulation of the pro-inflammatory potential of monocytes is correlated to organ dysfunction in patients after severe multiple injury: A cohort study. Crit. Care 2009, 13, R88. [Google Scholar] [CrossRef]
- Goldfarb, Y.; Sorski, L.; Benish, M.; Levi, B.; Melamed, R.; Ben-Eliyahu, S. Improving postoperative immune status and resistance to cancer metastasis: A combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann. Surg. 2011, 253, 798–810. [Google Scholar] [CrossRef]
- Alm, P.; Lundberg, L.M. Co-existence and origin of peptidergic and adrenergic nerves in the guinea pig uterus. Retrograde tracing and immunocytochemistry, effects of chemical sympathectomy, capsaicin treatment and pregnancy. Cell Tissue Res. 1988, 254, 517–530. [Google Scholar] [CrossRef]
- Bae, S.E.; Corcoran, B.M.; Watson, E.D. Immunohistochemical study of the distribution of adrenergic and peptidergic innervation in the equine uterus and the cervix. Reproduction 2001, 122, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Yoshihara, K.; Yachida, N.; Suda, K.; Tamura, R.; Ishiguro, T.; Enomoto, T. The New Era of Three-Dimensional Histoarchitecture of the Human Endometrium. J. Pers. Med. 2021, 11, 713. [Google Scholar] [CrossRef] [PubMed]
- Gaetje, R.; Kotzian, S.; Herrmann, G.; Baumann, R.; Starzinski-Powitz, A. Nonmalignant epithelial cells, potentially invasive in human endometriosis, lack the tumor suppressor molecule E-cadherin. Am. J. Pathol. 1997, 150, 461–467. [Google Scholar] [PubMed]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noe, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-Associated Mutations in Endometriosis without Cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef]
- Gabbutt, C.; Schenck, R.O.; Weisenberger, D.J.; Kimberley, C.; Berner, A.; Househam, J.; Lakatos, E.; Robertson-Tessi, M.; Martin, I.; Patel, R.; et al. Fluctuating methylation clocks for cell lineage tracing at high temporal resolution in human tissues. Nat. Biotechnol. 2022. [Google Scholar] [CrossRef] [PubMed]
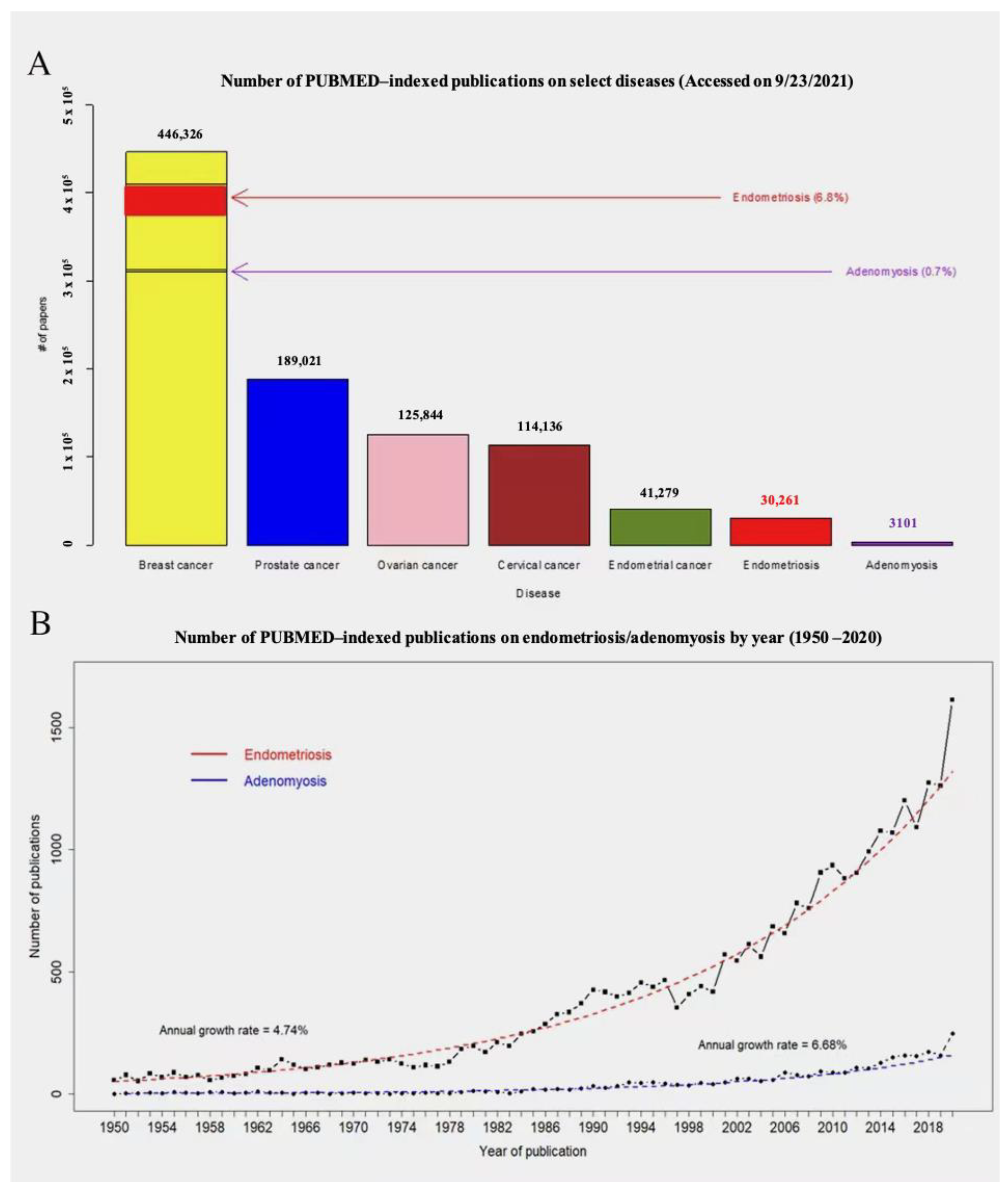
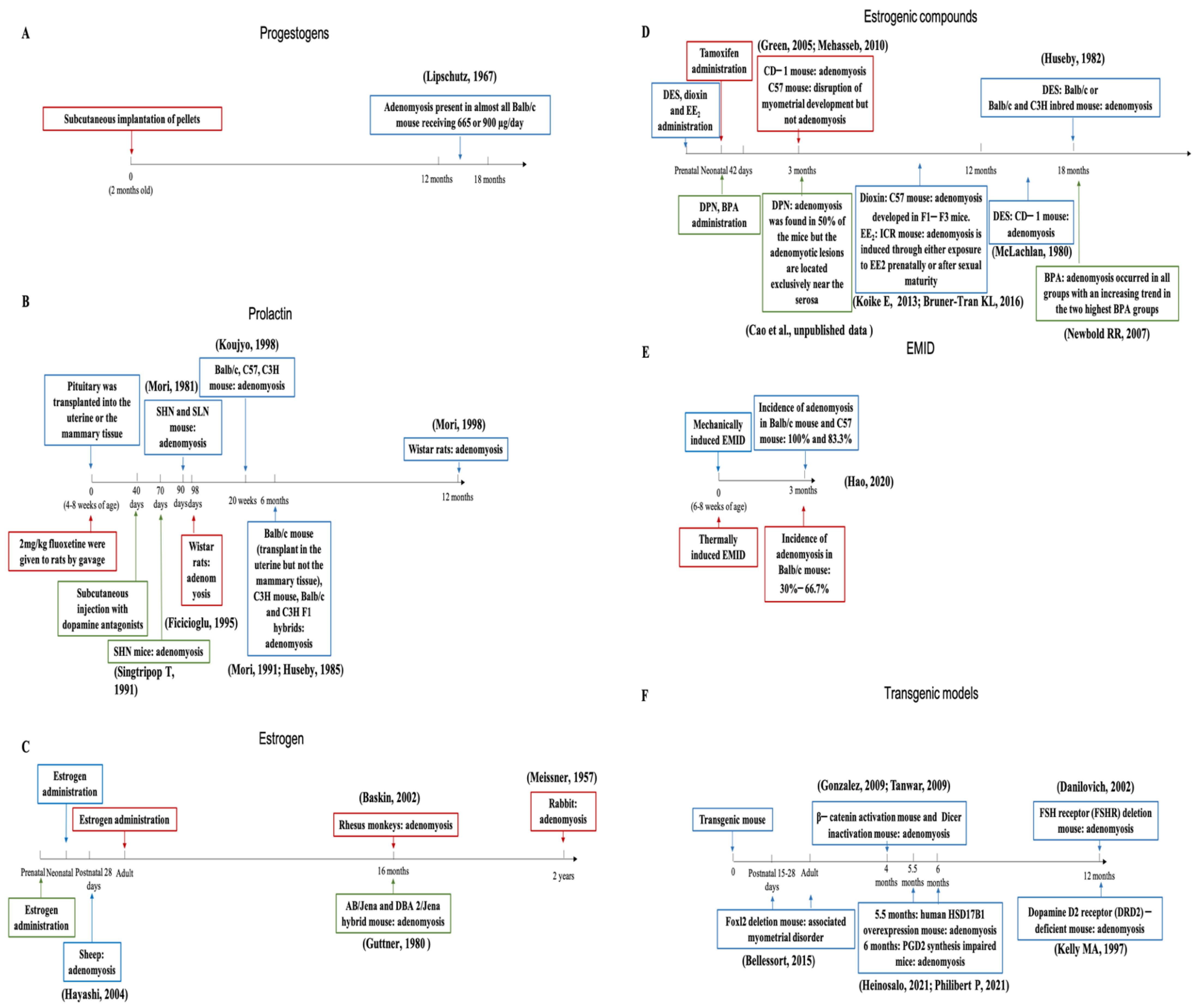
| Pathogenesis | Species/Strain | Induction Method | Duration of Induction | Outcome | References | |
|---|---|---|---|---|---|---|
| Estrogen or estrogenic compounds | Estrogen | AB/Jena and DBA 2/Jena hybrid mouse | Pregnant F1 animals were orally given 1 mg/kg of 17β-phenylaminocarbonyloxyestra-1,3,5(10)-triene-3-methyl ether daily on days 12 to 16 post coitus | ≥10 months | Adenomyosis was found in 10 out of 27 virgin female offspring of estrogen-treated dams from 16 to 33 months of age | [66,67] |
| Rabbit | Stilbestrol (5 mg/mL) was injected i.m. | 2 years | Adenomyosis | [68] | ||
| Rhesus monkeys (Macaca mulatta) | S.c. implants containing 200 mg estradiol | 16 months | Adenomyosis | [69] | ||
| Transgenic mouse | Overexpressing human HSD17B1 | 5–12 months | Adenomyosis appeared at the age of 5.5 months and became more severe at 12 months | [70] | ||
| Sheep | Postnatal daily i.m. injections of estradiol-17β benzoate at a dose of either 0, 0.01, 0.1, 1, or 10 μg/kg body weight from PND 14–27 (period one) or PND 42–55 (period two) | PND 28, PND 56, PND 112 | Immediate responses to EB treatment included dose- and age-dependent increases in uterine wet weight, thickness of the endometrium, myometrium, and LE, but decreases in endometrial glands on PND 28 and 56. Transient exposure to EB decreased gland number and thickness of the endometrium and LE on PND 112 | [71] | ||
| Tamoxifen | CD-1 mouse | Tamoxifen, toremifene, and raloxifene dosed orally 2–5 days after birth consecutively | 42–90 days | Uterine adenomyosis was found in all (14 out of 14) mice dosed with tamoxifen and most mice (12 out of 14) treated with toremifene, in only one animal treated with raloxifene | [72,73] | |
| C57 mouse | Female C57/BL6J pups (n = 20) were treated with oral tamoxifen (1 mg/kg) from age 1 to 5 days | 5, 10, 15, and 42 days of age | Causes disruption of myometrial development but not adenomyosis | [74] | ||
| Diethylstilbestrol | Balb/c or Balb/c and C3H inbred mouse | Pregnant mice were fed a diet containing 0.2 μg/g (of bodyweight) of DES continuously on the seventh day of pregnancy until the morning after delivery of the young | 18 months of age | Resembled adenomyosis occurred in Balb/c mice with the lesser frequency encountered in the hybrid strain | [75] | |
| CD-1 mouse | Pregnant outbred mice were treated s.c. with daily doses of DES ranging from 0.01 to 100 jug/kg on days 9 to 16 of gestation | 12 to 18 months of age | 1/22 adenomyosis in 5 ug/kg group | [76] | ||
| Diarylpropionitrile (DPN) | ICR mouse | Mice in DPN group were dosed orally with 5 mg/kg DPN from day 2 to day 5 after birth | 3 months | Neonatal feeding of DPN resulted in adenomyosis in 50% of the mice, but the adenomyotic lesions were located exclusively near the serosa | [77] | |
| Bisphenol A (BPA) | CD-1 mouse | Outbred female CD-1 mice were treated on days 1–5 with subcutaneous injections of BPA (10, 100, or 1000 μg/kg/day) dissolved in corn oil or corn oil alone (Control) | 18 months | Adenomyosis occurred in all groups with an increasing trend in the two highest BPA groups (6% (1/18) Controls, 9% (2/23) BPA-10, 20% (4/20) BPA-100, and 19% (3/16) BPA-1000) | [78] | |
| Dioxin | C57 mouse | Pregnant mice (F0) were exposed to dioxin (10 μg/kg) in corn oil or vehicle alone by gavage on E15.5 (when organogenesis is complete) | 10–12 weeks | Adenomyosis was identified in most animals with a history of direct (F1–F2) or indirect (F3) dioxin exposure. However, although 70% (n = 10) of F1 animals exhibited deep adenomyosis, the incidence of advanced disease was slightly lower in F2 mice (63%; n = 11) and F3 animals (56%; n = 9) | [79] | |
| Ethinyl estradiol (EE2) | ICR mouse | Pregnant mice were exposed to 0.01 mg ethinyl estradiol (EE2)/kg per day or vehicle (olive oil) through oral intubation from day 11 to 17 of gestation. They delivered their offspring and raised them. When the experimental female F1 mice were at 8 weeks of age, they were not exposed to EE2 or to the same dose of EE2 or to vehicle twice a week until 20 weeks of age | 28 weeks | These findings indicate that adenomyosis is induced through either exposure to EE2 prenatally or after sexual maturity, but the highest frequency is seen through the combined exposures | [80] | |
| Progesterone | Balb/c mouse | S.c. implantation of pellets | 12–18 months | Present in almost all animals receiving 665 or 900 µg/day | [80,81] | |
| Prolactin | Pituitary grafts | SHN and SLN mouse | Ectopic (intrauterine and under the renal capsule) pituitary transplantation | 90 days | Incidence: 100% | [82,83] |
| Balb/c mouse | Anterior pituitary (AP) isografting at 8 weeks of age | 36 weeks | Increased the incidence of adenomyosis in mice | [84] | ||
| Wistar rat | Transplantation of a single anterior pituitary gland into the uterine lumen | 12 months | Adenomyosis was induced in six out of eight Wistar rats | [85] | ||
| Balb/c mouse, C3H mouse, or Balb/c and C3H F1 hybrids | Transplantation of pituitary into the mammary tissue | 6 months | Lesions of adenomyosis were frequent in uteri of C3H and F1 hybrids but essentially absent from Balb/c animals | [86] | ||
| Balb/c, C57, C3H mouse | Pituitary was transplanted into the uterine cavity | 20 weeks | Adenomyosis had formed in the uteri of 22 (91.7%) mice out of 24 Balb/c mice after the transplantation of pituitary glands. Similar findings were obtained by experiments with C3H and C57 mice | [87] | ||
| Dopamine antagonists | SHN mouse | SHN female mice were subcutaneously injected with dopamine antagonists for 30 days or 50 days. | 70 or 90 days of age | The incidences of adenomyosis in the experimental groups of mice for 50 days rose up to over 70% | [88] | |
| Fluoxetine | Wistar rat | 2 mg/kg fluoxetine were given to rats by gavage | 98 days | Histological studies revealed 11 cases of adenomyosis in the noncastrated group receiving fluoxetine | [89] | |
| Transgenic mouse | Dopamine D2 receptor (DRD2)-deficient mouse | Mice that are deficient in functional D2 receptors were generated | One year old | A large proportion of the female DRD2 deficient mice developed uterine adenomyosis, most commonly in mice greater than one year of age | [90] | |
| Endometrial–myometrial interface disruption (EMID) | Balb/c and C57 mouse | Mechanically induced EMID or thermally induced EMID | 8–12 weeks | Adenomyosis developed in the majority of mice in the EMID groups (83.3% in C57BL/6 mice, 100% in Balb/c mice); adenomyosis was found in 66.7% of the EMID mice 10 weeks later | [91] | |
| Other transgenic models | Dicer | Dicer inactivated mutant mice | Dicer was inactivated in Müllerian duct mesenchyme-derived tissues of the reproductive tract of the mouse, using an Amhr2-Cre allele | >4 months of age | The glands were found within the myometrium. | [92] |
| FSHR | FSH receptor-haplo insufficient mice | The animals of the required genotype were produced by breeding 129T2/SV EmsJ Fshr−/− male and females of 3–5 months | 12 months of age | Some uteri showed endometrial glands deeply penetrating the myometrium | [93] | |
| Foxl2 | Foxl2 deleted mice | Conditional deletion of Foxl2 in the PN uterus using PR-Cre (Pgrcre/+) mice | PN15, PN25, adult | Myometrial disorder | [94] | |
| β-catenin | Conditionally stabilized β-catenin mouse | Mice that expressed a dominant stabilized β-catenin in the uterus were used by crossing PR-Cre mice with Ctnnb1f(ex3)/+ mice | 4 months of age | The incidence of 40% at 4 months of age and 80% at 6 months of age | [95] | |
| PGD2 | PGD2 synthesis impaired mice | PGD2 is not produced due to invalidation of both lipocalin hematopoietic type (L-PGDS and H-PGDS) genes | 6 months of age | HE staining showed the presence of focal adenomyosis in 35% (n = 9 from 28) of knockout mice | [96] | |
| Criterion | Comment |
|---|---|
| Strength of association (Sa) | If the relative risk is “strong”, there is less likelihood that there are other adequate explanations for the observed association. |
| Consistency (Cs) | Is the association consistent over the various studies? |
| Biological gradient (Bg) | Is there an exposure–response relationship exhibited over the range of studies? |
| Specificity (Sp) | Is the association limited to a particular outcome? |
| Temporality (Tm) | Does the exposure precede the outcome? |
| Biological plausibility (Bp) | Is the proposed association explained by a biologically plausible mechanism? |
| Experimental evidence (Ee) | Are there experimental studies that support the association? |
| Analogy (An) | Is the proposed causal relationship analogous to some other accepted cause and effect? |
| Coherence (Ch) | Does the proposed relationship seriously conflict with generally known facts about the natural history and biology of the disease? |
| Induction Agent | Evidence in Humans? | References | Which Hill’s Criterion or Criteria Are Satisfied |
|---|---|---|---|
| Estrogen | No direct support | [149,189,190,191,192,193] | Bg, Tm, Bp, Ee, An |
| Tamoxifen | No | No | As, Bg, Tm, Ee, An |
| Diethylstilbestrol (DES) | No | No | Tm, Ee, An |
| Diarylpropionitrile (DPN) Bisphenol A (BPA) Dioxin Ethinyl estradiol (EE2) | No | No | Tm Ee |
| Progestins | No direct support | [97] | Sa, Tm, Bp, Ee, An |
| Prolactin | No direct support | [133,144] | Sa, Cs, Tm, Bp, Ee, An |
| Fluoxetine | No | No | Sa, Tm, Ee, An |
| Endometrial–myometrial interface disruption (EMID) | Yes | [133,198,199,200,201,202] | Sa, Cs, Bg, Sp, Tm, Bp, Ee, An, Ch |
| Other models | No | No | Sa, Cs, Sp, Tm, Bp, Ee, An for the conditionally stabilized β-catenin mouse Tm, Ee for the others |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Benagiano, G.; Liu, X.; Guo, S.-W. Unveiling the Pathogenesis of Adenomyosis through Animal Models. J. Clin. Med. 2022, 11, 1744. https://doi.org/10.3390/jcm11061744
Wang X, Benagiano G, Liu X, Guo S-W. Unveiling the Pathogenesis of Adenomyosis through Animal Models. Journal of Clinical Medicine. 2022; 11(6):1744. https://doi.org/10.3390/jcm11061744
Chicago/Turabian StyleWang, Xi, Giuseppe Benagiano, Xishi Liu, and Sun-Wei Guo. 2022. "Unveiling the Pathogenesis of Adenomyosis through Animal Models" Journal of Clinical Medicine 11, no. 6: 1744. https://doi.org/10.3390/jcm11061744
APA StyleWang, X., Benagiano, G., Liu, X., & Guo, S.-W. (2022). Unveiling the Pathogenesis of Adenomyosis through Animal Models. Journal of Clinical Medicine, 11(6), 1744. https://doi.org/10.3390/jcm11061744








