Clinical Application of Factor VIII:C to VWF:Ag Ratio for the Screening of Haemophilia A Carriers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Coagulation Assay
2.3. Mutation Analysis
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Bleeding Diathesis
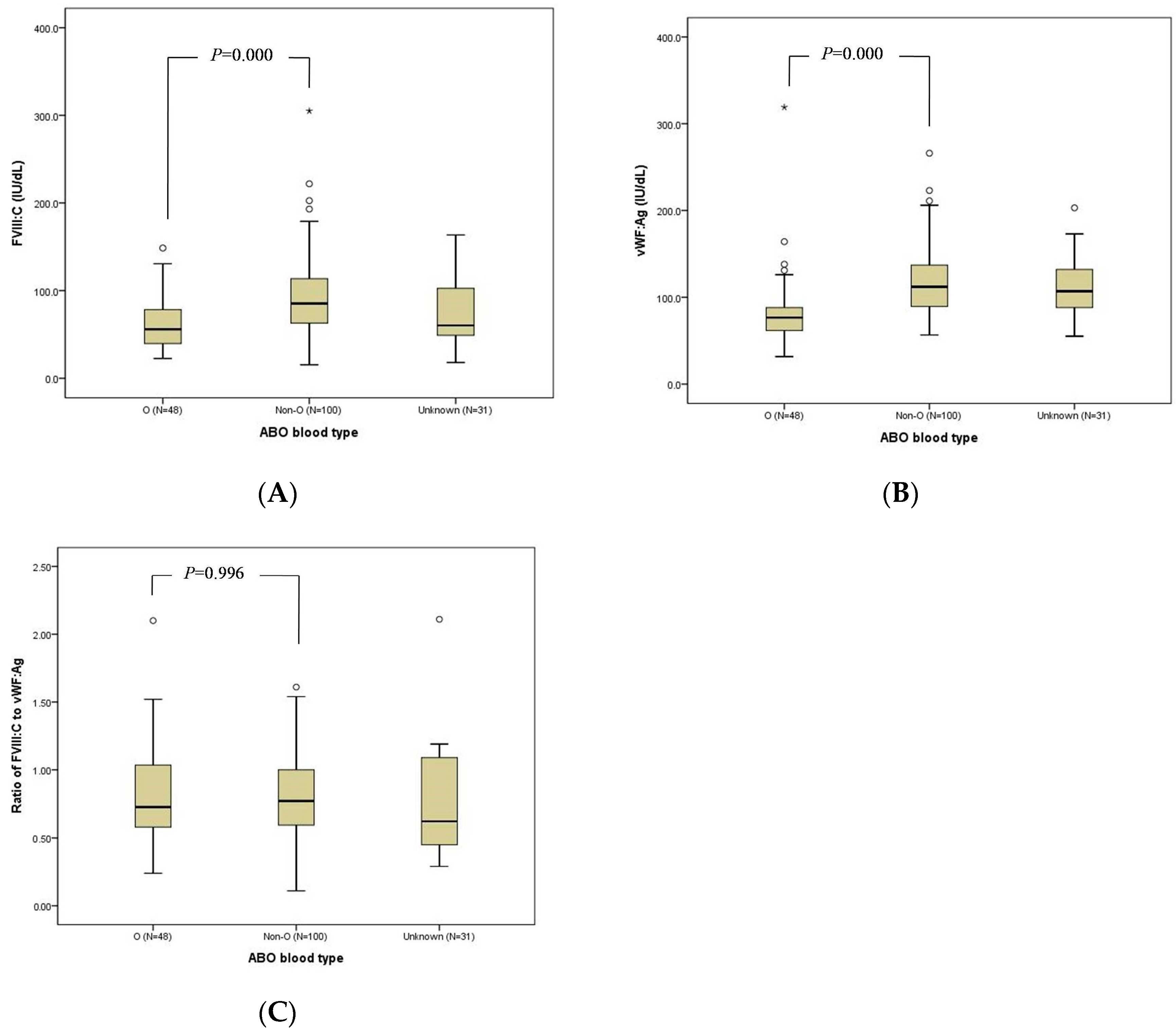
3.3. FVIII:C, VWF:Ag, and FVIII:C/VWF:Ag Ratio
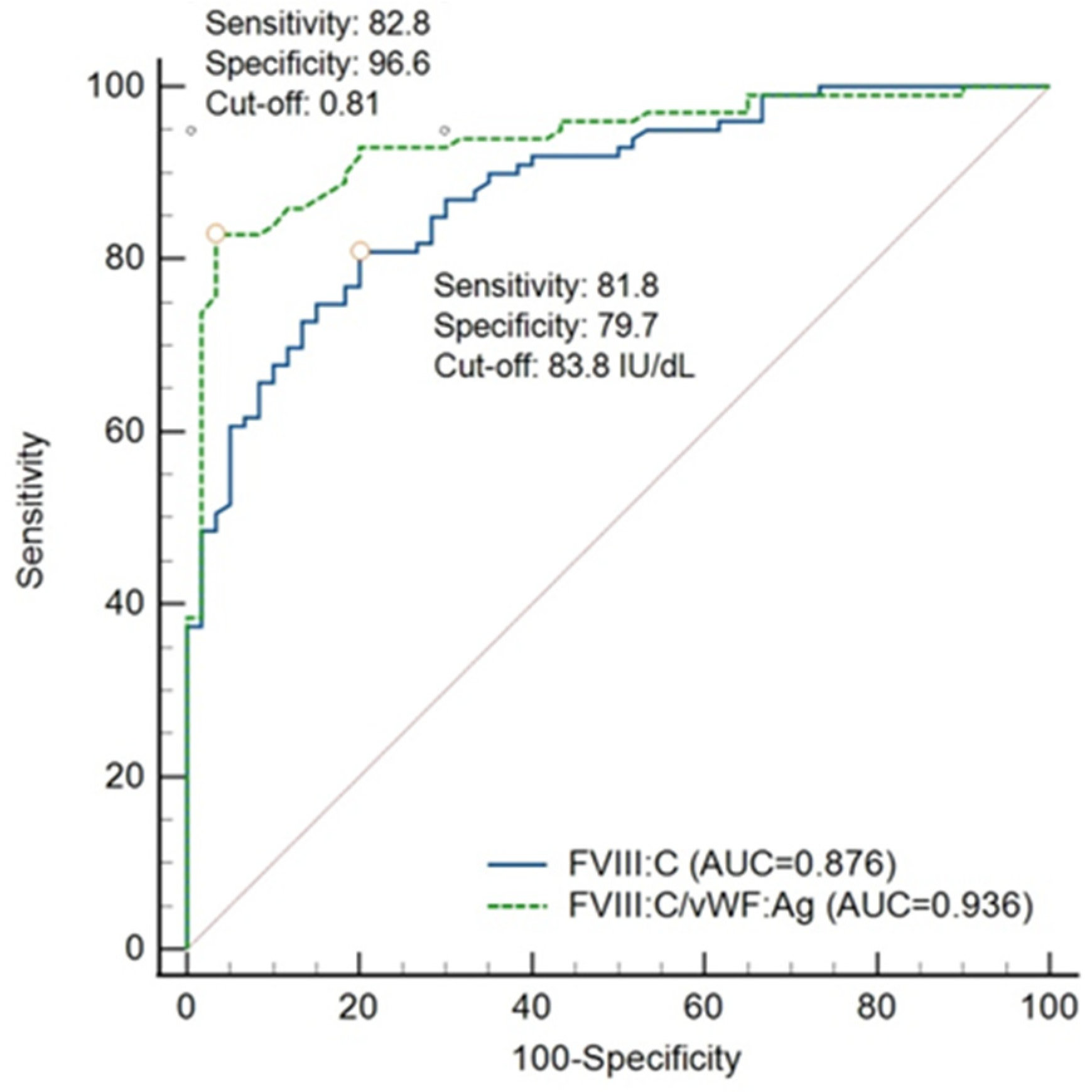
3.4. Validity of FVIII:C/VWF:Ag Ratio and FVIII:C
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kasper, C.K.; Lin, J.C. Prevalence of sporadic and familial haemophilia. Haemophilia 2007, 13, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Lee, H. Current status and comprehensive care of Korean hemophiliacs. Blood Res. 2000, 35, 222–232. [Google Scholar]
- World Federation of Hemophilia. Carriers and Women with Hemophilia; World Federation of Hemophilia: Montreal, QC, Canada, 2012; Available online: https://www1.wfh.org/publication/files/pdf-1471.pdf (accessed on 20 January 2021).
- Rizza, C.R.; Rhymes, I.L.; Austen, D.E.G.; Kernoff, P.B.A.; Aroni, S.A. Detection of carriers of haemophilia: A ‘blind’ study. Br. J. Haematol. 1975, 30, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Keeney, S.; Mitchell, M.; Goodeve, A. The molecular analysis of haemophilia A: A guideline from the UK haemophilia centre doctors’ organization haemophilia genetics laboratory network. Haemophilia 2005, 11, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kim, E.; Lee, M.; Son, Y.; Choe, J.; Hwang, D.; Jun, J.; Jee, B.; Ku, S.; Suh, C.; et al. Factor VIII gene mutations in Korean patients with hemophilia A. Korean J. Obstet. Gynecol. 2004, 47, 1975–1981. [Google Scholar]
- Kim, H.-J.; Chung, H.-S.; Kim, S.K.; Yoo, K.Y.; Jung, S.-Y.; Park, I.-A.; Lee, K.-O.; Kim, S.-H.; Kim, H.-J. Mutation spectrum and inhibitor risk in 100 korean patients with severe hemophilia A. Haemophilia 2012, 18, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, Z.; Rodgers, S.E.; Sheffield, L.J.; Lloyd, J.V. Detection of carriers of haemophilia A: Use of bioassays and restriction fragment length polymorphisms (RFLP). Aust. N. Z. J. Med. 1996, 26, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Ghosh, K.; Pathare, A.; Mohanty, D. Carrier detection in haemophilia A families: Comparison of conventional coagulation parameters with DNA polymorphism analysis-first report from India. Haemophilia 1999, 5, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Labarque, V.; Perinparajah, V.; Bouskill, V.; Stain, A.M.; Wakefield, C.; Manuel, C.; Blanchette, V.S.; James, P.D.; Lillicrap, D.; Carcao, M.D. Utility of factor VIII and factor VIII to von Willebrand factor ratio in identifying 277 unselected carriers of hemophilia A. Am. J. Hematol. 2017, 92, e94–e96. [Google Scholar] [CrossRef] [PubMed]
- Lillicrap, D.; Holden, J.J.A.; Giles, A.R.; White, B.N.; The Ontario Haemophilia Study Group. Carrier detection strategy in haemophilia A: The benefits of combined DNA marker analysis and coagulation testing in sporadic haemophilic familes. Br. J. Haematol. 1988, 70, 321–326. [Google Scholar] [CrossRef]
- Lee, G.; Lee, M.; Park, S.; Choi, Y.; Yoo, S.; Min, E.; Hwang, D.; Choe, J.; Jun, J.; Jee, B.; et al. Diagnosis of factor VIII gene inversion by PCR in Korean patients with hemophilia A and its application to carrier detection. Korean J. Obstet. Gynecol. 2007, 50, 976–981. [Google Scholar]
- Rossetti, L.C.; Radic, C.P.; Larripa, I.B.; De Brasi, C.D. Developing a new generation of tests for genotyping hemophilia-causative rearrangements involving int22h and int1h hot spots in the factor VIII gene. J. Thromb. Haemost. 2008, 6, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Method 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden index and its associated cutoff point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korea Hemophilia Foundation. 2019 Annual Report; Korea Hemophilia Foundation: Seoul, Korea, 2019; Available online: http://www.kohem.org/_data/board_list_file/8/2020/2006091214021.pdf (accessed on 15 November 2021).
- Paroskie, A.; Oso, O.; DeBun, M.R.; Sidonio, R.F. Both hemophilia health care providers and hemophilia A carriers report that carriers have excessive bleeding. J. Pediatr. Hematol. Oncol. 2014, 36, e224–e230. [Google Scholar] [CrossRef] [Green Version]
- Seck, M.; Faye, B.F.; Sall, A.; Sy, D.; Touré, S.A.; Dieng, N.; Guéye, Y.B.; Gadji, M.; Touré, A.O.; Costa, C.; et al. Bleeding risk assessment in hemophilia A carriers from Dakar, Senegal. Blood Coagul. Fibrinolysis 2017, 28, 642–645. [Google Scholar] [CrossRef]
- Olsson, A.; Hellgren, M.; Berntorp, E.; Ljung, R.; Baghaei, F. Clotting factor level is not a good predictor of bleeding in carriers of haemophilia A and B. Blood Coagul. Fibrinolysis 2014, 25, 471–475. [Google Scholar] [CrossRef]
- Simurda, T.; Dobrotova, M.; Skornova, I.; Sokol, J.; Kubisz, P.; Stasko, J. Successful Use of a Highly Purified Plasma von Willebrand Factor Concentrate Containing Little FVIII for the Long-Term Prophylaxis of Severe (Type 3) von Willebrand’s Disease. Semin. Thromb. Hemost. 2017, 43, 639–641. [Google Scholar]
- Favaloro, E.J. Towards personalised therapy for von Willebrand disease: A future role for recombinant products. Blood Transfus. 2016, 14, 262–276. [Google Scholar]
- Laffan, M.; Sathar, J.; Johnsen, J.M. Von Willebrand disease: Diagnosis and treatment, treatment of women, and genomic approach to diagnosis. Haemophilia 2021, 27, 66–74. [Google Scholar] [CrossRef]
- O’Donnell, J.; Laffan, M.A. The relationship between ABO histo-blood group, factor VIIII and von Willebrand factor. Transfus. Med. 2001, 11, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Seo, J.Y.; Bang, S.H.; Park, I.A.; Kim, H.J.; Kim, S.H. Establishment of reference intervals for won Willebrand factor antigen and eight coagulation factors in a Korean population following the clinical and laboratory standards institute guidelines. Blood Coagul. Fibrinolysis 2010, 21, 251–255. [Google Scholar] [CrossRef]
- Skornova, I.; Simurda, T.; Stasko, J.; Zolkova, J.; Sokol, J.; Holly, P.; Dobrotova, M.; Plamenova, I.; Hudecek, J.; Brunclikova, M.; et al. Multimer Analysis of Von Willebrand Factor in Von Willebrand Disease with a Hydrasys Semi-Automatic Analyzer—Single-Center Experience. Diagnostics 2021, 11, 2153. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, P.V.; O’Donnell, J.S. ABO blood group determines plasma von Willebrand factor levels: A biologic function after all? Transfusion 2006, 46, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M. Comparison of Methods for Finding the Optimal Cutoff Value in MRMC Data. Master’s Thesis, Yonsei University Graduate School, Seoul, Korea, 2016. Available online: https://ir.ymlib.yonsei.ac.kr/handle/22282913/154759 (accessed on 18 November 2021).
- Simundic, A.M. Diagnostic AccuracyVPart 1 Basic Concepts: Sensitivity and Specificity, ROC Analysis, STARD Statement. Point Care 2012, 11, 6–8. [Google Scholar] [CrossRef]
| Overall (n = 179) | Carrier (n = 110) | Non-Carrier (n = 69) | p | ||
|---|---|---|---|---|---|
| Median (range) age, years | 31 (3–68) | 32 (5–68) | 28 (3–67) | 0.003 | |
| Relationship with proband, n (%) | Mother | 55 | 51 (92.7) | 4 (7.3) | |
| Sibling | 78 | 35 (44.9) | 43 (55.1) | ||
| Ascendant or descendant | 46 | 24 (52.2) | 22 (47.8) | ||
| ABO Blood type, n (%) | O | 48 | 33 (68.8) | 15 (31.2) | 0.138 |
| Non-O | 100 | 56 (56.0) | 44 (44.0) | ||
| Unknown | 31 | 21 (67.7) | 10 (32.3) | ||
| F8 mutation types of proband, n (%) | Intron 22 inversion | 58 | 38 (65.5) | 20 (34.5) | 0.489 |
| Missense | 61 | 35 (57.4) | 26 (42.6) | ||
| Nonsense | 25 | 15 (60.0) | 10 (40.0) | ||
| Frameshift | 24 | 18 (75.0) | 6 (25.0) | ||
| Splicing | 8 | 2 (25.0) | 6 (75.0) | ||
| Intron 1 inversion | 3 | 2 (66.7) | 1 (33.3) | ||
| Bleeding diathesis, n (%) | Asymptomatic | 68 | 36 (52.9) | 32 (47.1) | 0.052 |
| Hypermenorrhoea | 35 | 22 (62.9) | 13 (37.1) | ||
| Subcutaneous Haematoma | 26 | 18 (69.2) | 8 (30.8) | ||
| Epistaxis | 12 | 11 (91.7) | 1 (8.3) | ||
| Traumatic bleeding | 7 | 7 (100.0) | 0 | ||
| Gum bleeding | 1 | 1 (100.0) | 0 | ||
| Multiple diathesis | 14 | 12 (85.7) | 2 (14.3) | ||
| Unknown | 45 | 29 (64.4) | 16 (33.6) | ||
| Variables | ABO Blood Type | Total | Carrier | Non-Carrier | p * |
|---|---|---|---|---|---|
| Median FVIII:C, IU/dL, (n, range) | Overall | 74.5 (179, 15.4–305.0) | 59.3 (110, 15.4–130.6) | 106.1 (69, 50.0–305.0) | 0.000 |
| O | 56.0 (48, 22.6–148.5) | 47.5 (33, 22.6–130.6) | 86.4 (15, 52.0–148.5) | 0.000 | |
| Non-O | 85.3 (100, 15.4–305.0) | 67.0 (56, 15.4–126.2) | 112.6 (44, 65.8–305.0) | 0.000 | |
| Unknown | 60.3 (31, 18.0–163.4) | 54.3 (21, 18.0–126.0) | 112.1 (10, 50–163.4) | NA | |
| Median VWF:Ag, IU/dL, (n, range) | Overall | 103.0 (160, 31.7–319.0) | 101.0 (99, 31.7–319.0) | 105.0 (61, 41.2–223.0) | 0.671 |
| O | 76.6 (48, 31.7–319.0) | 77.2 (33, 317–319.0) | 69.6 (15, 41.2–138.0) | 0.468 | |
| Non-O | 112.0 (95, 56.6–266.0) | 113.0 (54, 56.6–266.0) | 110.6 (41, 70.8–223.0) | 0.887 | |
| Unknown | 107.0 (17, 55.2–203.0) | 106.5 (12, 55.2–203.0) | 116.0 (5, 65.3–124.0) | NA | |
| Median FVIII:C/VWF:Ag, (n, range) | Overall | 0.77 (160, 0.11–2.11) | 0.62 (99, 0.11–1.43) | 1.08 (61, 0.57–2.11) | 0.000 |
| O | 0.73 (48, 0.24–2.10) | 0.62 (33, 0.24–1.19) | 1.09 (15, 0.87–2.10) | 0.000 | |
| Non-O | 0.77 (95, 0.11–1.61) | 0.63 (54, 0.11–1.43) | 1.06 (41, 0.57–1.61) | 0.000 | |
| Unknown | 0.62 (17, 0.29–2.11) | 0.48 (12, 0.2—1.19) | 1.12 (5, 1.02–2.11) | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, K.-Y.; Jung, S.-Y.; Choi, J.-Y.; Park, H.-R.; Park, Y.-S. Clinical Application of Factor VIII:C to VWF:Ag Ratio for the Screening of Haemophilia A Carriers. J. Clin. Med. 2022, 11, 1686. https://doi.org/10.3390/jcm11061686
Yoo K-Y, Jung S-Y, Choi J-Y, Park H-R, Park Y-S. Clinical Application of Factor VIII:C to VWF:Ag Ratio for the Screening of Haemophilia A Carriers. Journal of Clinical Medicine. 2022; 11(6):1686. https://doi.org/10.3390/jcm11061686
Chicago/Turabian StyleYoo, Ki-Young, Soo-Young Jung, Jin-Young Choi, Hye-Ryeon Park, and Young-Shil Park. 2022. "Clinical Application of Factor VIII:C to VWF:Ag Ratio for the Screening of Haemophilia A Carriers" Journal of Clinical Medicine 11, no. 6: 1686. https://doi.org/10.3390/jcm11061686
APA StyleYoo, K.-Y., Jung, S.-Y., Choi, J.-Y., Park, H.-R., & Park, Y.-S. (2022). Clinical Application of Factor VIII:C to VWF:Ag Ratio for the Screening of Haemophilia A Carriers. Journal of Clinical Medicine, 11(6), 1686. https://doi.org/10.3390/jcm11061686






