Additional Value of [18F]FDG PET/CT in Detection of Suspected Malignancy in Patients with Paraneoplastic Neurological Syndromes Having Negative Results of Conventional Radiological Imaging
Abstract
:1. Introduction
| Brain, Cranial Nerves and Retina | Spinal Cord | Peripheral Nerves or Muscle | Neuromuscular Junction | |
|---|---|---|---|---|
| Non-Classic PNS | Brainstem encephalitis | Stiff -person syndrome | Sensorimotor neuropathy | Myasthenia gravis |
| Optic neuritis | Myelitis | Neuropathy and paraproteinaemia | ||
| Cancer-associated retinopathy | Necrotising myelopathy | Neuropathy with vasculitis | ||
| Melanoma-associated retinopathy | Motor-neuron syndromes | Polymyositis | ||
| Acute necrotising myopathy | ||||
| Acquired neuromyotonia | ||||
| Autonomic neuropathies | ||||
| Classic PNS | Cerebellar degeneration | Sensory neuronopathy | Lambert-Eaton myasthenic syndrome | |
| Limbic encephalitis | Intestinal pseudo-obstruction | |||
| Encephalomyelitis | Dermatomyositis | |||
| Opsoclonus-myoclonus |
| Antibody | Common Neurological Phenotypes | Common Associated Malignancies |
|---|---|---|
| Anti-AMPAR | Limbic encephalitis | Breast Lung Thymus |
| Anti-LGI1/Anti- CASPR2 | Limbic encephalitis Faciobrachial dystonic seizures Morvan’s syndrome | Thymoma (especially in patients positive for both antibodies) Other neoplasms (rare) |
| Anti-GABAbR | Limbic encephalitis, status epilepticus | Small-cell lung cancer |
| Anti-mGluR1 | Cerebellar degeneration | Hodgkin’s disease |
| Anti-mGlur2 | Cerebellar degeneration | Small-cell cancer; alveolar rhabdomyosarcoma |
| Anti-mGluR5 | Limbic encephalitis | Hodgkin’s disease |
| Anti-VGKC | Cerebellar degeneration (Lambert–Eaton myasthenic syndrome) | Small-cell lung cancer |
| Antibody | Common Neurological Phenotypes | Common Associated Malignancies |
|---|---|---|
| Anti-CRMP5 | Optic neuritis Cerebellar degeneration Encephalomyelitis | Small-cell lung cancer Breast carcinoma |
| Anti-GAD65 | Stiff person syndrome Limbic encephalitis Cerebellar ataxia | Thymoma Renal cell carcinoma |
| Anti-Hu (ANNA-1) | Limbic encephalitis, encephalomyelitis, dorsal sensory neuropathy | Small-cell lung cancer Neuroendocrine tumors Retinoblastoma (infants) |
| Anti-Ma1 | Limbic or brain-stem encephalitis | Non-small-cell lung cancer; other |
| Anti-Ma2 | Limbic or brain-stem encephalitis | Testicular or other germ cell tumors Non-small-cell lung cancer |
| Anti-Ri (ANNA-2) | Cerebellar degeneration, opsoclonus myoclonus, brain-stem encephalitis | Breast Small-cell lung cancer |
| Anti-Tr | Cerebellar degeneration | Hodgkin’s disease |
| Anti-Yo (PCA-1) | Cerebellar degeneration | Ovary, uterus, adnexa Breast |
2. Methods
2.1. Image Acquisitions and Analysis
2.2. Statistical Analysis
3. Results
4. Discussion
5. Highlights
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devine, M.F.; Kothapalli, N.; Elkhooly, M.; Dubey, D. Paraneoplastic neurological syndromes: Clinical presentations and management. Ther. Adv. Neurol. Disord. 2021, 14, 1756286420985323. [Google Scholar] [CrossRef] [PubMed]
- Vogrig, A.; Gigli, G.L.; Segatti, S.; Corazza, E.; Marini, A.; Bernardini, A.; Valent, F.; Fabris, M.; Curcio, F.; Brigo, F.; et al. Epidemiology of paraneoplastic neurological syndromes: A population-based study. J. Neurol. 2020, 267, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Graus, F.; Delattre, J.Y.; Antoine, J.C.; Dalmau, J.; Giometto, B.; Grisold, W.; Honnorat, J.; Smitt, P.S.; Vedeler, C.; Verschuuren, J.J.; et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1135–1140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Zhang, J.; Ren, H.; Zhou, X.; Chen, J.; Cui, L.; Lang, J.; Guan, H.; Sun, D. Surgical outcomes in patients with anti-N-methyl D-aspartate receptor encephalitis with ovarian teratoma. Am. J. Obstet. Gynecol. 2019, 221, 485.e1–485.e10. [Google Scholar] [CrossRef] [PubMed]
- Baizabal-Carvallo, J.F.; Jankovic, J. Autoimmune and paraneoplastic movement disorders: An update. J. Neurol. Sci. 2018, 385, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, J.; Rosenfeld, M.R. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008, 7, 327–340. [Google Scholar] [CrossRef] [Green Version]
- Galli, J.; Greenlee, J. Paraneoplastic Diseases of the Central Nervous System. F1000Research 2020, 9, F1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Titulaer, M.J.; Soffietti, R.; Dalmau, J.; Gilhus, N.E.; Giometto, B.; Graus, F.; Grisold, W.; Honnorat, J.; Sillevis Smitt, P.A.; Tanasescu, R.; et al. Screening for tumours in paraneoplastic syndromes: Report of an EFNS task force. European Federation of Neurological Societies. Eur. J. Neurol. 2011, 18, 19-e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, D.; Pittock, S.J.; Kelly, C.R.; McKeon, A.; Lopez-Chiriboga, A.S.; Lennon, V.A.; Gadoth, A.; Smith, C.Y.; Bryant, S.C.; Klein, C.J.; et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann. Neurol. 2018, 83, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Sheikhbahaei, S.; Marcus, C.V.; Fragomeni, R.S.; Rowe, S.P.; Javadi, M.S.; Solnes, L.B. Whole-Body 18F-FDG PET and 18F-FDG PET/CT in Patients with Suspected Paraneoplastic Syndrome: A Systematic Review and Meta-Analysis of Diagnostic Accuracy. J. Nucl. Med. 2017, 58, 1031–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vedeler, C.A.; Antoine, J.C.; Giometto, B.; Graus, F.; Grisold, W.; Hart, I.K.; Honnorat, J.; Sillevis Smitt, P.A.; Verschuuren, J.J.; Voltz, R. Management of paraneoplastic neurological syndromes: Report of an EFNS Task Force. Paraneoplastic Neurological Syndrome Euronetwork. Eur. J. Neurol. 2006, 13, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Harlos, C.; Metser, U.; Poon, R.; MacCrostie, P.; Mason, W. 18 F-Fluorodeoxyglucose positron-emission tomography for the investigation of malignancy in patients with suspected paraneoplastic neurologic syndromes and negative or indeterminate conventional imaging: A retrospective analysis of the Ontario PET Access Program. with systematic review and meta-analysis. Curr. Oncol. 2019, 26, e458–e465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vatankulu, B.; Aksoy, S.Y.; Asa, S.; Sager, S.; Sayman, H.B.; Halac, M.; Sonmezoglu, K. Vccuracy of FDG-PET/CT and paraneoplastic antibodies in diagnosing cancer in paraneoplastic neurological syndromes. Rev. Esp. Med. Nucl. Imagen Mol. 2016, 35, 17–21. [Google Scholar] [CrossRef] [PubMed]
- García Vicente, A.M.; Delgado-Bolton, R.C.; Amo-Salas, M.; López-Fidalgo, J.; Caresia Aróztegui, A.P.; García Garzón, J.R.; Orcajo Rincón, J.; García Velloso, M.J.; de Arcocha Torres, M.; Alvárez Ruíz, S. 18F-fluorodeoxyglucose positron emission tomography in the diagnosis of malignancy in patients with paraneoplastic neurological syndrome: A systematic review and meta-analysis. Oncology Task Force of Spanish Society of Nuclear Medicine and Molecular Imaging. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1575–1587. [Google Scholar] [CrossRef] [PubMed]
| Classic PNS | No of Patients with PNS Included to Analysis | No of Patients with Positive [18F]F-FDG PET/CT Findings | Percentage of PET/CT Positive Examination |
|---|---|---|---|
| Cerebellar degeneration | 3 | 3 | 100 |
| Sensory polyneuropathy | 1 | 0 | 0 |
| Autoimmune encephalitis | 3 | 1 | 33 |
| Non-classic PNS | |||
| Myasthenia gravis | 1 | 1 | 100 |
| Myelitis | 1 | 1 | 100 |
| Sensorimotor polyneuropathy | 4 | 2 | 50 |
| Motor neuron disease | 1 | 0 | 0 |
| Others | |||
| Primary Angiitis of Central Nervous System (PACS) | 1 | 0 | 0 |
| Sum | 15 | 8 | 53 |
| Patient No | Sex, Age | Neuroimaging (CT, MR) | CSF Results | Antibodies | Metabolic Abnormalities of CNS | PET/CT Abnormalities |
|---|---|---|---|---|---|---|
| Cerebellar Degeneration | ||||||
| 1. | F. 88 | Brain MRI: nonspecific vascular demyelination; no clinical relevance | Cytosis 0.005 × 103/uL (0–0.005 × 103/uL) Protein 48.5 mg/dL (20.00–40.00) oligoclonal bands: positive | anti-Yo | none | metabolically active area in the pylorus and metabolically active lymph node next to the pylorus SUV max 7.8 |
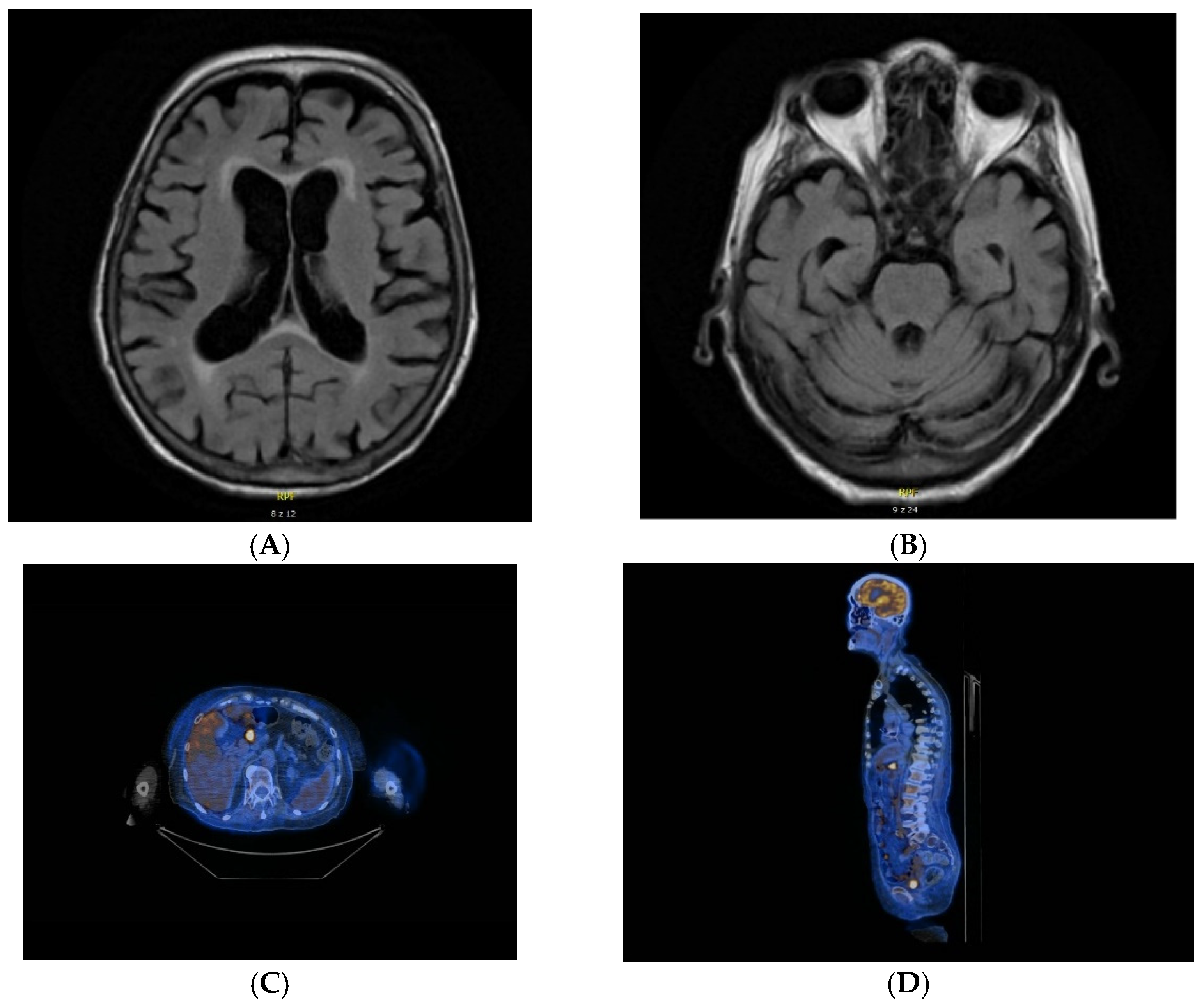
| 2. | F. 83 | Brain MRI: leukoaraiosis around lateral ventricles. nonspecific vascular demyelination. moderate cortical artrophy—mainly posterior. bilateral hyperintense changes in the thalami and caudate nuclei on diffusion weighted imaging (DWI). | Cytosis 0.004 × 103/uL (0–0.005 × 103/uL) Protein 34.8 mg/dL (20.00–40.00) oligoclonal bands: negative | anti-NMDA. anti-Yo | Generalized cortico-subcortical atrophy of the brain. | metabolically active lymph nodes in the mediastinum and chest—suspection of lymphoma SUV max 10.5 |
| 3. | M. 66 | Brain MRI: not performed (due to contraindications) Brain CT: normal | Cytosis 0.006 × 103/uL (0–0.005 × 103/uL) Protein 40.6 mg/dL (20.00–40.00) oligoclonal bands: positive | not detected | not detected | metabolically active tumor in the transverse colon SUV max 5.0 |
| Autoimmune Encephalitis | ||||||
| 4. | F. 32 | Brain MRI: normal | Cytosis 0.018 × 103/uL (0–0.005 × 103/uL) Protein 26.3 mg/dL (20.00–40.00) oligoclonal bands: negative | anti-NMDA | none | increased metabolism of FDG in the topography of numerous lesions in both lungs SUV max up to 7.5 |
| 5. | M. 30 | Brain MRI: demyelinating lesions located bilaterally in the white matter of the frontal and parietal lobe, in the periventricular and subcortical areas. | Cytosis 0.002 × 103/uL (0–0.005 × 103/uL) Protein 47.2 mg/dL (20.00–40.00) oligoclonal bands: negative | anti-NMDA | none | not significant |
| 6. | F. 74 | Brain MRI: epidermal cysts located anteriorly from the medulla oblongata, on the left side; numerous demyelinating lesions located in the white matter of the centrum semiovale, periventricular and paraventricular areas; numerous, small signalless zones on Susceptibility-Weighted Imaging (SWI) which correspond to the presence of haemosiderin deposits—after microchemorrhages | Cytosis 0.002 × 103/uL (0–0.005 × 103/uL) Protein 37.3 mg/dL (20.00–40.00) oligoclonal bands: positive | anti-NMDA | cortical-subcortical atrophy of the brain | not significant |
| Myasthenia Gravis | ||||||
| 7. | F. 79 | Brain MRI: nonspecific vascular demyelination; no clinical relevance | not performed | anti-AChR | areas of porencephaly with decreased FDG metabolism | not significant |
| Myelitis | ||||||
| 8. | F. 70 | Brain MRI: leukoaraiosis around lateral ventricles; few. small changes with increased signal in T2 in the radial corona radiata in the frontal lobes; moderate cortical atrophy of the brain and cerebellum. Cervical Spine MRI: The zone of increased signal in the T2 sequences dependent on the central part of the spinal cord. extending from the C2 / C3 to C6 / C7 level | Cytosis 0.008 × 103/uL (0–0.005 × 103/uL) Protein 74 mg/dL (20.00–40.00) oligoclonal bands: negative | anti-AQP4 | none | moderately metabolic active tumour in segment 4/5 of the right lung. SUV max 3.5 |
| Polyneuropathy | ||||||
| 9. | M. 59 | Brain MRI: not performed. Brain CT: normal | not performed | not detected | none | metabolically active tumor in the apex of the left lung with the involvement of homonymous mediastinal lymph nodes—SUV max 9.4 |
| 10. | F. 56 | MRI: not performed. Brain CT: normal | not performed | not detected | none | not significant |
| 11. | F. 73 | Brain MRI: not performed. Brain CT: normal | Cytosis 0.003 × 103/uL (0–0.005 × 103/uL) Protein 144.1 mg/dL (20.00–40.00) oligoclonal bands: negative | anti-PNMA2 (Ma2/Ta) anti-CV2.1 | None | metabolically active cervical lymph node and a metabolically active soft tissue mass in the lower part of the neck and in the upper mediastinum SUV max 5.7 |
| 12. | M. 69 | Brain MRI: nonspecific vascular demyelination; no clinical relevance. | not performed | anti-PNMA2 (Ma2/Ta) | none | not significant |
| 13. | M. 65 | Brain MRI: nonspecific vascular demyelination; no clinical relevance. | Cytosis 0.002 × 103/uL (0–0.005 × 103/uL) Protein 187 mg/dL (20.00–40.00) oligoclonal bands: negative | not detected | none | lesion in the 1 + 2 segment of left lung SUV max 2.2 |
| Primary Angiitis of Central Nervous System | ||||||
| 14. | M. 38 | Brain MRI: disseminated demyelinating lesions located in the cortico-subcortical area of the right insula. bilaterally in the corona radiata. and single irregular lesions with cortico-subcortical distribution. | Cytosis 0.004 × 103/uL (0–0.005 × 103/uL) Protein 38.2 mg/dL (20.00–40.00) oligoclonal bands: negative | anti-Yo | none | nonspecific segmental metabolic stimulation in the loops of the small intestine SUV max 7.7 |
| Motor Neuron Disease | ||||||
| 15. | F. 67 | Cervival Spine MRI: multi-level discopathy without pressure on the surrounding nerve roots. | Cytosis 0.001 × 103/uL (0–0.005 × 103/uL) Protein 67.96 mg/dL (20.00–40.00) oligoclonal bands: negative | anti-Yo | none | not significant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opalińska, M.; Sowa-Staszczak, A.; Wężyk, K.; Jagiełła, J.; Słowik, A.; Hubalewska-Dydejczyk, A. Additional Value of [18F]FDG PET/CT in Detection of Suspected Malignancy in Patients with Paraneoplastic Neurological Syndromes Having Negative Results of Conventional Radiological Imaging. J. Clin. Med. 2022, 11, 1537. https://doi.org/10.3390/jcm11061537
Opalińska M, Sowa-Staszczak A, Wężyk K, Jagiełła J, Słowik A, Hubalewska-Dydejczyk A. Additional Value of [18F]FDG PET/CT in Detection of Suspected Malignancy in Patients with Paraneoplastic Neurological Syndromes Having Negative Results of Conventional Radiological Imaging. Journal of Clinical Medicine. 2022; 11(6):1537. https://doi.org/10.3390/jcm11061537
Chicago/Turabian StyleOpalińska, Marta, Anna Sowa-Staszczak, Kamil Wężyk, Jeremiasz Jagiełła, Agnieszka Słowik, and Alicja Hubalewska-Dydejczyk. 2022. "Additional Value of [18F]FDG PET/CT in Detection of Suspected Malignancy in Patients with Paraneoplastic Neurological Syndromes Having Negative Results of Conventional Radiological Imaging" Journal of Clinical Medicine 11, no. 6: 1537. https://doi.org/10.3390/jcm11061537
APA StyleOpalińska, M., Sowa-Staszczak, A., Wężyk, K., Jagiełła, J., Słowik, A., & Hubalewska-Dydejczyk, A. (2022). Additional Value of [18F]FDG PET/CT in Detection of Suspected Malignancy in Patients with Paraneoplastic Neurological Syndromes Having Negative Results of Conventional Radiological Imaging. Journal of Clinical Medicine, 11(6), 1537. https://doi.org/10.3390/jcm11061537







