A Multicenter, Randomized, Double-Blinded, Parallel-Group, Placebo-Controlled Phase I/IIa Study to Evaluate the Efficacy and Safety of a Single Intra-Articular Injection of YYD302 in Patients with Knee Osteoarthritis
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject Population
2.2. Study Design
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
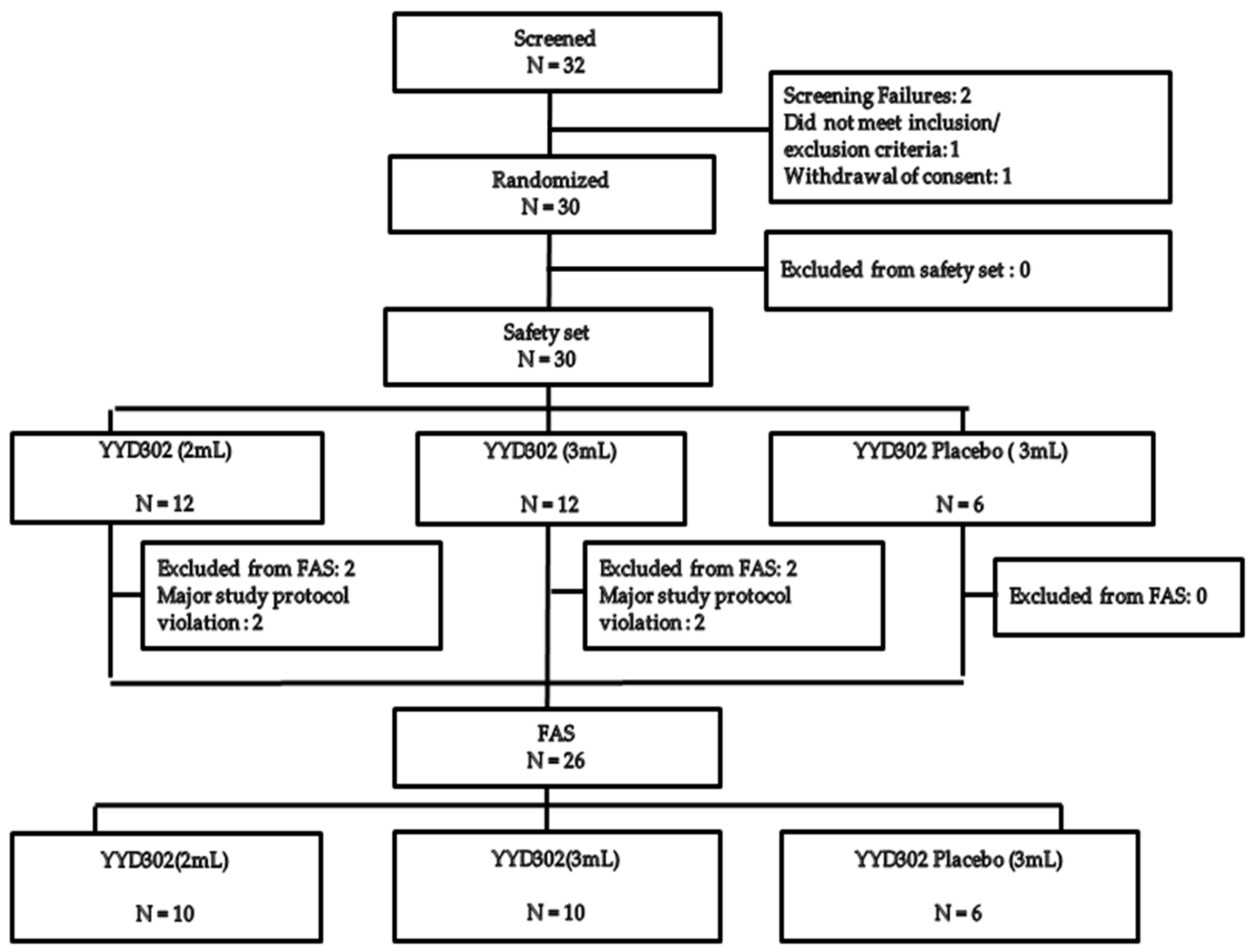
3.1. Subjects Distribution and Baseline Demographics
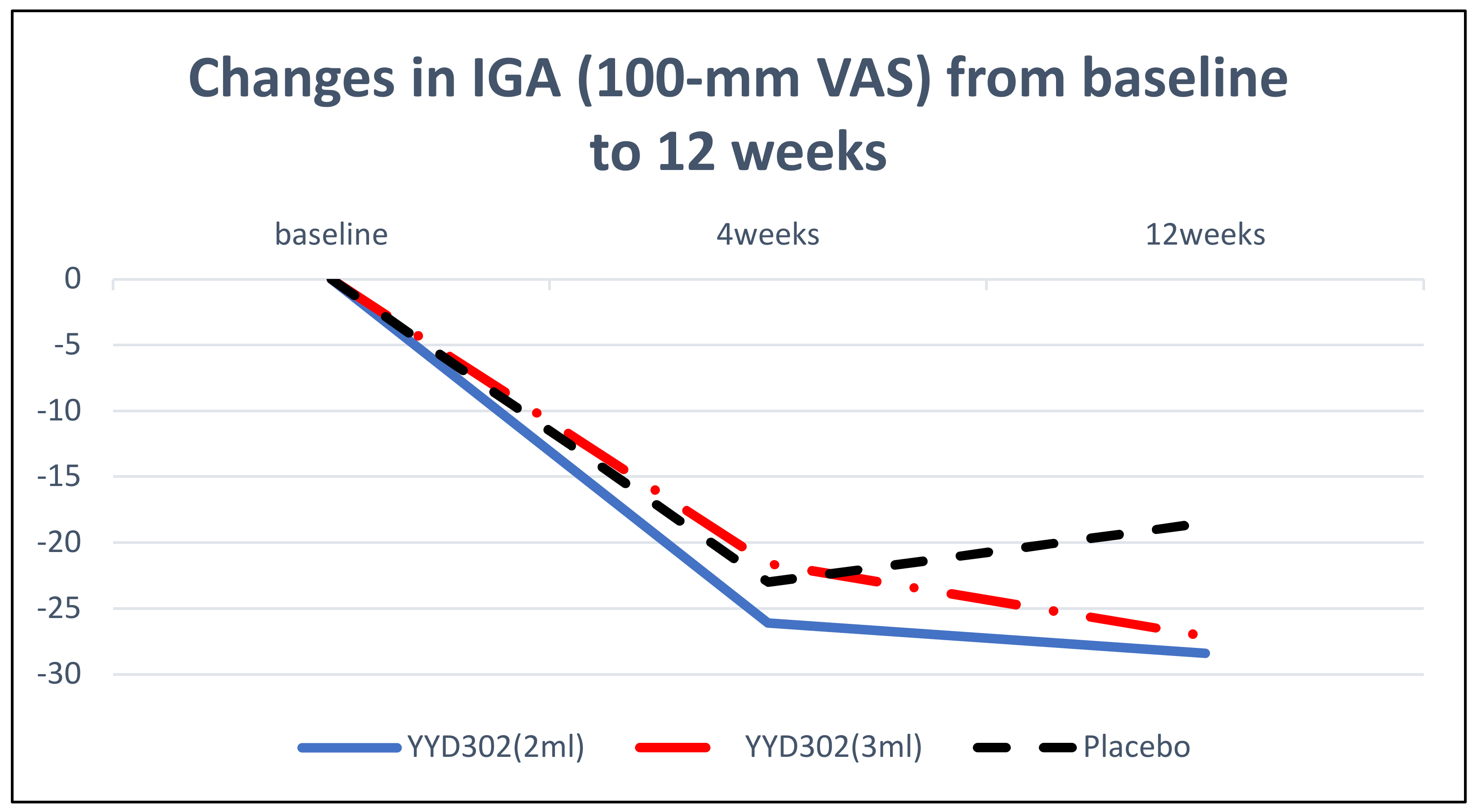
3.2. Efficacy Results
3.3. Safety Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Charlesworth, J.; Fitzpatrick, J.; Perera, N.K.P.; Orchard, J. Osteoarthritis—A systematic review of long-term safety implications for osteoarthritis of the knee. BMC Musculoskelet. Disord. 2019, 20, 151. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.M.; Maxwell, J.S.; Weng, L.; Angelos, M.G.; Golzarian, J. Intra-articular treatment of knee osteoarthritis: From anti-inflammatories to products of regenerative medicine. Physician Sportsmed. 2016, 44, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and treatment of hip and knee osteoarthritis: A review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Billesberger, L.M.; Fisher, K.M.; Qadri, Y.J.; Boortz-Marx, R.L. Procedural treatments for knee osteoarthritis: A review of current injectable therapies. Pain Res. Manag. 2020, 3873098. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Li, J.; Du, G.; Chen, J. Microbial production of hyaluronic acid: Current state, challenges, and perspectives. Microb. Cell Factories 2011, 10, 99. [Google Scholar] [CrossRef]
- Forster, M.; Straw, R. A prospective randomised trial comparing intra-articular Hyalgan injection and arthroscopic washout for knee osteoarthritis. Knee 2003, 10, 291–293. [Google Scholar] [CrossRef]
- Ha, C.-W.; Park, Y.-B.; Choi, C.-H.; Kyung, H.-S.; Lee, J.-H.; Yoo, J.D.; Yoo, J.-H.; Choi, C.-H.; Kim, C.-W.; Kim, H.-C. Efficacy and safety of single injection of cross-linked sodium hyaluronate vs. three injections of high molecular weight sodium hyaluronate for osteoarthritis of the knee: A double-blind, randomized, multi-center, non-inferiority study. BMC Musculoskelet. Disord. 2017, 18, 223. [Google Scholar] [CrossRef]
- Benke, M.; Shaffer, B. Viscosupplementation treatment of arthritis pain. Curr. Pain Headache Rep. 2009, 13, 440. [Google Scholar] [CrossRef]
- Bowman, S.; Awad, M.E.; Hamrick, M.W.; Hunter, M.; Fulzele, S. Recent advances in hyaluronic acid based therapy for osteoarthritis. Clin. Transl. Med. 2018, 7, 6. [Google Scholar] [CrossRef]
- Park, Y.-G.; Ha, C.-W.; Yoo, J.-H.; Lee, W.-S.; Lee, H.-J.; In, Y.; Bae, K.-C.; Shon, O.-J.; Kim, Y.-M.; Seon, J.-K. Intra-Articular Injection of a Novel DVS Cross-Linked Hyaluronic Acid Manufactured by Biological Fermentation (YYD302) in Patients With Knee Osteoarthritis: A Double-Blind, Randomized, Multicenter, Noninferiority Study. Clin. Ther. 2021, 43, 11. [Google Scholar] [CrossRef]
- Petrella, R.J.; Emans, P.J.; Alleyne, J.; Dellaert, F.; Gill, D.P.; Maroney, M. Safety and performance of Hydros and Hydros-TA for knee osteoarthritis: A prospective, multicenter, randomized, double-blind feasibility trial. BMC Musculoskelet. Disord. 2015, 16, 57. [Google Scholar] [CrossRef]
- Ohtsuki, T.; Asano, K.; Inagaki, J.; Shinaoka, A.; Kumagishi-Shinaoka, K.; Cilek, M.Z.; Hatipoglu, O.F.; Oohashi, T.; Nishida, K.; Komatsubara, I. High molecular weight hyaluronan protects cartilage from degradation by inhibiting aggrecanase expression. J. Orthop. Res. 2018, 36, 3247–3255. [Google Scholar] [CrossRef]
- Lázaro, B.; Alonso, P.; Rodriguez, A.; La Nuez, M.; Marzo, F.; Prieto, J.G. Characterization of the visco-elastic properties of hyaluronic acid. Biorheology 2018, 55, 41–50. [Google Scholar] [CrossRef]
- Moreland, L.W. Intra-articular hyaluronan (hyaluronic acid) and hylans for the treatment of osteoarthritis: Mechanisms of action. Arthritis Res. Ther. 2003, 5, 54. [Google Scholar] [CrossRef]
- Bannuru, R.; Natov, N.; Dasi, U.; Schmid, C.; McAlindon, T. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis—Meta-analysis. Osteoarthr. Cartil. 2011, 19, 611–619. [Google Scholar] [CrossRef]
- Chevalier, X.; Jerosch, J.; Goupille, P.; van Dijk, N.; Luyten, F.P.; Scott, D.L.; Bailleul, F.; Pavelka, K. Single, intra-articular treatment with 6 mL hylan GF 20 in patients with symptomatic primary osteoarthritis of the knee: A randomised, multicentre, double-blind, placebo controlled trial. Ann. Rheum. Dis. 2010, 69, 113–119. [Google Scholar] [CrossRef]
- Sun, S.-F.; Hsu, C.-W.; Lin, H.-S.; Liou, I.-H.; Chen, Y.-H.; Hung, C.-L. Comparison of single intra-articular injection of novel hyaluronan (HYA-JOINT Plus) with synvisc-one for knee osteoarthritis: A Randomized, Controlled, Double-Blind Trial of Efficacy and Safety. JBJS 2017, 99, 462–471. [Google Scholar] [CrossRef]
- Peat, G.; Thomas, E.; Duncan, R.; Wood, L.; Hay, E.; Croft, P. Clinical classification criteria for knee osteoarthritis: Performance in the general population and primary care. Ann. Rheum. Dis. 2006, 65, 1363–1367. [Google Scholar] [CrossRef]
- Kellgren, J.; Lawrence, J. Atlas of Standard Radiographs of Arthritis. The Epidemiology of Chronic Rheumatism; Blackwell Scientific Publications: Oxford, UK, 1963. [Google Scholar]
- Pavelka, K.; Uebelhart, D. Efficacy evaluation of highly purified intra-articular hyaluronic acid (Sinovial®) vs. hylan G-F20 (Synvisc®) in the treatment of symptomatic knee osteoarthritis. A double-blind, controlled, randomized, parallel-group non-inferiority study. Osteoarthr. Cartil. 2011, 19, 1294–1300. [Google Scholar] [CrossRef][Green Version]
- Roos, E.M.; Roos, H.P.; Lohmander, L.S.; Ekdahl, C.; Beynnon, B.D. Knee Injury and Osteoarthritis Outcome Score (KOOS)—Development of a self-administered outcome measure. J. Orthop. Sports Phys. Ther. 1998, 28, 88–96. [Google Scholar] [CrossRef]
- Pham, T.; van der Heijde, D.; Altman, R.; Anderson, J.; Bellamy, N.; Hochberg, M.; Simon, L.; Strand, V.; Woodworth, T.; Dougados, M. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthr. Cartil. 2004, 12, 389–399. [Google Scholar] [CrossRef]
- Hangody, L.; Szody, R.; Lukasik, P.; Zgadzaj, W.; Lénárt, E.; Dokoupilova, E.; Bichovsk, D.; Berta, A.; Vasarhelyi, G.; Ficzere, A. Intraarticular injection of a cross-linked sodium hyaluronate combined with triamcinolone hexacetonide (Cingal) to provide symptomatic relief of osteoarthritis of the knee: A randomized, double-blind, placebo-controlled multicenter clinical trial. Cartilage 2018, 9, 276–283. [Google Scholar] [CrossRef]
- Hermans, J.; Bierma-Zeinstra, S.M.; Bos, P.K.; Niesten, D.D.; Verhaar, J.A.; Reijman, M. The effectiveness of high molecular weight hyaluronic acid for knee osteoarthritis in patients in the working age: A randomised controlled trial. BMC Musculoskelet. Disord. 2019, 20, 196. [Google Scholar] [CrossRef]
- Vaquerizo, V.; Plasencia, M.Á.; Arribas, I.; Seijas, R.; Padilla, S.; Orive, G.; Anitua, E. Comparison of intra-articular injections of plasma rich in growth factors (PRGF-Endoret) versus Durolane hyaluronic acid in the treatment of patients with symptomatic osteoarthritis: A randomized controlled trial. Arthrosc. Arthrosc.—J. Arthrosc. Relat. Surg. 2013, 29, 1635–1643. [Google Scholar] [CrossRef]
- Leighton, R.; Fitzpatrick, J.; Smith, H.; Crandall, D.; Flannery, C.R.; Conrozier, T. Systematic clinical evidence review of NASHA (Durolane hyaluronic acid) for the treatment of knee osteoarthritis. Rheumatol. Res. Rev. 2018, 10, 43–54. [Google Scholar] [CrossRef]
- Danoff, J.R.; Goel, R.; Sutton, R.; Maltenfort, M.G.; Austin, M.S. How much pain is significant? Defining the minimal clinically important difference for the visual analog scale for pain after total joint arthroplasty. J. Arthroplast. 2018, 33, S71–S75. [Google Scholar] [CrossRef]
- Laigaard, J.; Pedersen, C.; Ronsbo, T.N.; Mathiesen, O.; Karlsen, A.P.H. Minimal clinically important differences in randomised clinical trials on pain management after total hip and knee arthroplasty: A systematic review. Br. J. Anaesth. 2021, 126, 1029–1037. [Google Scholar] [CrossRef]
- Balazs, E.A.; Denlinger, J.L. Viscosupplementation: A new concept in the treatment of osteoarthritis. J. Rheumatol. Suppl. 1993, 39, 3–9. [Google Scholar]
- Geborek, P.; Saxne, T.; Heinegård, D. Measurement of Synovial Fluid Volume Using Albumin Dilution upon lntraarticular Saline Injection. J. Rheumatol. 1988, 15, 91–94. [Google Scholar]
| Characteristics | YYD302 (2 mL) N = 12 | YYD302 (3 mL) N = 12 | YYD302 Placebo (3 mL) N = 6 | Total N = 30 | |
|---|---|---|---|---|---|
| Gender | N | 12 | 12 | 6 | 30 |
| Male, n (%) | 1 (8.3) | 4 (33.3) | 1 (16.7) | 6 (20.0) | |
| Female, n (%) | 11 (91.7) | 8 (66.7) | 5 (83.3) | 24 (80.0) | |
| p-value | 1.0000 *,2 | 0.6148 †,2 | 0.3816 ‡,2 | ||
| Age (year) | N | 12 | 12 | 6 | 30 |
| Mean ± SD | 60.7± 6.9 | 65.0 ± 8.3 | 61.7 ± 12.5 | 62.6 ± 8.7 | |
| Median | 60.5 | 63.5 | 59.0 | 61.5 | |
| Min, Max | 44.0, 71.0 | 52.0, 80.0 | 44.0, 78.0 | 44.0, 80.0 | |
| p-value | 0.8277 *,1 | 0.5064 †,1 | 0.4693 ‡,3 | ||
| OA site | One-side, n (%) | 12 | 12 | 6 | 30 |
| Left, n (%) | 2 (16.7) | 1 (8.3) | 1 (16.7) | 4 (13.3) | |
| Right, n (%) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 1 (3.3) | |
| Both, n (%) | 10 (83.3) | 10 (83.3) | 5 (83.3) | 25 (83.3) | |
| p-value | 1.0000 *,2 | 1.0000 †,2 | 1.0000 ‡,2 | ||
| History of previous administration of nonsteroidal anti-inflammatory agents, OA nutritional supplements, physical therapy, etc.; n (%) | N | 12 | 12 | 6 | 30 |
| Yes, n (%) | 0 (0.0) | 1 (8.3) | 0 (0.0) | 1 (3.3) | |
| No, n (%) | 12 (100.0) | 11 (91.7) | 6 (100.0) | 29 (96.7) | |
| p-value | - | 1.0000 †,2 | 1.0000 ‡,2 | ||
| Age at first diagnosis of OA (years) | N | 12 | 12 | 6 | 30 |
| Mean ± SD | 58.0 ± 8.5 | 59.5 ± 5.6 | 59.7 ± 11.5 | 58.9 ± 7.9 | |
| Median | 57.0 | 59.0 | 56.5 | 58.0 | |
| Min, Max | 43.0, 72.0 | 52.0, 71.0 | 45.0, 77.0 | 43.0, 77.0 | |
| p-value | 0.7306 *,1 | 0.9742 †,1 | 0.8765 ‡,3 | ||
| Disease duration of OA (years) | N | 12 | 12 | 6 | 30 |
| Mean ± SD | 3.3 ± 4.2 | 6.3 ± 5.7 | 2.5 ± 2.8 | 4.3 ± 4.8 | |
| Median | 1.5 | 4.0 | 1.5 | 2.0 | |
| Min, Max | 0.0, 13.0 | 0.0, 19.0 | 0.00, 6.00 | 0.00, 19.00 | |
| p-value | 0.7011 *,1 | 0.1501 †,1 | 0.1859 ‡,3 | ||
| Kellgren and Lawrence I–IV X-ray grading § | N | 12 | 12 | 6 | 30 |
| Stage I, n (%) | 2 (16.7) | 0 (0.0) | 0 (0.0) | 2 (6.7) | |
| Stage II, n (%) | 6 (50.0) | 6 (50.0) | 1 (16.7) | 13 (43.3) | |
| Stage III, n (%) | 4 (33.3) | 6 (50.0) | 5 (83.3) | 15 (50.0) | |
| Stage IV, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| p-value | 0.2104 *,2 | 0.3156 †,2 | 0.2193 ‡,2 | ||
| YYD302 (2 mL) N = 10 | YYD302 (3 mL) N = 10 | YYD302 Placebo (3 mL) N = 6 | ||
|---|---|---|---|---|
| Baseline | Mean ± SD | 59.8 ± 10.1 | 56.1 ± 11.1 | 64.0 ± 12.4 |
| Median | 58.0 | 56.5 | 65.0 | |
| Min, Max | 44.0, 72.0 | 40.0, 70.0 | 46.0, 77.0 | |
| W4 | Mean ± SD | 28.4 ± 18.4 | 35.6 ± 20.6 | 29.3 ± 26.1 |
| Median | 28.5 | 33.0 | 23.5 | |
| Min, Max | 4.0, 60.0 | 8.0, 77.0 | 4.0, 70.0 | |
| W12 | Mean ± SD | 25.6 ± 20.8 | 33.0 ± 21.5 | 35.0 ± 24.6 |
| Median | 20.5 | 28.0 | 31.0 | |
| Min, Max | 6.0, 69.0 | 8.0, 80.0 | 10.0, 70.0 | |
| Change (W4-Baseline) | Mean ± SD | −31.4 ± 22.3 | −20.5 ± 22.6 | −34.7 ± 27.9 |
| Median | −35.0 | −17.0 | −38.0 | |
| Min, Max | −68.0, −3.0 | −60.0, 18.0 | −72.0, 10.0 | |
| p-value (paired t-test) | 0.0016 | 0.0183 | 0.0287 | |
| p-value (t-test) * | 0.7997 | 0.2834 | ||
| Change (W12-Baseline) | Mean ± SD | −34.2 ± 24.1 | −23.1 ± 21.8 | −29.0 ± 33.2 |
| Median | −44.5 | −22.0 | −31.5 | |
| Min, Max | −61.0, 5.0 | −62.0, 16.0 | −67.0, 10.0 | |
| p-value (paired t-test) | 0.0015 | 0.0085 | 0.0850 | |
| p-value (t-test) * | 0.7214 | 0.6719 |
| YYD302 (2 mL) N = 10 | YYD302 (3 mL) N = 10 | YYD302 Placebo (3 mL) N = 6 | |||
|---|---|---|---|---|---|
| Total | Change (W4-Baseline) | Mean ± SD | 18.3 ± 13.2 | 9.6 ± 13.1 | 13.0 ± 18.8 |
| p-value (paired t-test) | 0.0018 | 0.0467 | 0.1528 | ||
| p-value (t-test) * | 0.5149 | 0.6756 | |||
| Change (W12-Baseline) | Mean ± SD | 19.8 ± 13.5 | 11.6 ± 15.2 | 8.4 ± 22.4 | |
| p-value (paired t-test) | 0.0012 | 0.0396 | 0.4014 | ||
| p-value (t-test) * | 0.2185 | 0.7386 | |||
| Symptoms | Change (W4-Baseline) | Mean ± SD | 21.0 ± 15.5 | 12.5 ± 14.2 | 17.3 ± 23.1 |
| p-value (paired t-test) | 0.0020 | 0.0214 | 0.1267 | ||
| p-value (t-test) * | 0.6998 | 0.6143 | |||
| Change (W12-Baseline) | Mean ± SD | 25.3 ± 11.7 | 17.1 ± 17.4 | 13.1 ± 26.2 | |
| p-value (paired t-test) | <0.0001 | 0.0124 | 0.2753 | ||
| p-value (t-test) * | 0.3201 | 0.7148 | |||
| Pain | Change (W4-Baseline) | Mean ± SD | 22.8 ± 14.8 | 13.9 ± 13.8 | 15.8 ± 20.1 |
| p-value (paired t-test) | 0.0009 | 0.0111 | 0.1126 | ||
| p-value (t-test) * | 0.4338 | 0.8285 | |||
| Change (W12-Baseline) | Mean ± SD | 22.0 ± 19.5 | 9.7 ± 18.1 | 12.07 ± 27.5 | |
| p-value (paired t-test) | 0.0061 | 0.1228 | 0.3320 | ||
| p-value (t-test) * | 0.4135 | 0.8387 | |||
| ADL | Change (W4-Baseline) | Mean ± SD | 21.3 ± 17.9 | 14.0 ± 15.04 | 7.1 ± 25.5 |
| p-value (paired t-test) | 0.0044 | 0.0164 | 0.5252 | ||
| p-value (t-test) * | 0.2103 | 0.5049 | |||
| Change (W12-Baseline) | Mean ± SD | 22.9 ± 16.6 | 13.6 ± 18.2 | 6.2 ± 28.4 | |
| p-value (paired t-test) | 0.0018 | 0.0426 | 0.6190 | ||
| p-value (t-test) * | 0.1542 | 0.5322 | |||
| Sports and Recreation function | Change (W4-Baseline) | Mean ± SD | 20.0 ± 17.6 | 10.5 ± 22.7 | 14.2 ± 21.5 |
| p-value (paired t-test) | 0.0059 | 0.1769 | 0.1682 | ||
| p-value (t-test) * | 0.5642 | 0.7546 | |||
| Change (W12-Baseline) | Mean ± SD | 19.0 ± 20.9 | 12.5 ± 26.5 | 7.5 ± 28.2 | |
| p-value (paired t-test) | 0.0184 | 0.1698 | 0.5441 | ||
| p-value (t-test) * | 0.3652 | 0.7264 | |||
| Quality of life | Change (W4-Baseline) | Mean ± SD | 6.2 ± 18.2 | −3.1 ± 15.7 | 10.4 ± 11.7 |
| p-value (paired t-test) | 0.3053 | 0.5428 | 0.0797 | ||
| p-value (t-test) * | 0.6226 | 0.0885 | |||
| Change (W12-Baseline) | Mean ± SD | 10.0 ± 14.2 | 5.0 ± 11.7 | 3.2 ± 12.4 | |
| p-value (paired t-test) | 0.0529 | 0.2130 | 0.5614 | ||
| p-value (t-test) * | 0.3439 | 0.7704 | |||
| YYD302 (2 mL) N = 10 | YYD302 (3 mL) N = 10 | YYD302 Placebo (3 mL) N = 6 | ||
|---|---|---|---|---|
| OMERACT-OARSI Response rate | n (%) | 7 (70.0) | 4 (40.0) | 3 (50.0) |
| p-value * | 0.6066 | 1.0000 |
| YYD302 (2 mL) N = 12 | YYD302 (3 mL) N = 12 | YYD302 Placebo (3 mL) N = 6 | ||||||
|---|---|---|---|---|---|---|---|---|
| System Organ Class * | Preferred Term * | Severity | No. of Patients (%) | Events | No. of Patients (%) | Events | No. of Patients (%) | Events |
| Overall local AEs | Overall | 10 (83.3) | 21 | 9 (75.0) | 19 | 3 (50.0) | 9 | |
| Mild | 4 (33.3) | 5 | 7 (58.3) | 10 | 2 (33.3) | 4 | ||
| Moderate | 8 (66.7) | 13 | 5 (41.7) | 7 | 3 (50.0) | 3 | ||
| Severe | 3 (25.0) | 3 | 2 (16.7) | 2 | 2 (33.3) | 2 | ||
| Overall local AEsat injection sites | Overall | 9 (75.0) | 19 | 9 (75.0) | 19 | 3 (50.0) | 8 | |
| Mild | 3 (25.0) | 4 | 7 (58.33 | 10 | 2 (33.3) | 3 | ||
| Moderate | 8 (66.7) | 12 | 5 (41.7) | 7 | 3 (50.0) | 3 | ||
| Severe | 3 (25.0) | 3 | 2 (16.7) | 2 | 2 (33.3) | 2 | ||
| Pain | Overall | 8 (66.7) | 8 | 9 (75.0) | 9 | 3 (50.0) | 3 | |
| Mild | 0 (0.0) | 0 | 2 (16.7) | 2 | 0 (0.0) | 0 | ||
| Moderate | 5 (41.7) | 5 | 5 (41.7) | 5 | 2 (33.3) | 2 | ||
| Severe | 3 (25.0) | 3 | 2 (16.7) | 2 | 1 (16.7) | 1 | ||
| Warmth | Overall | 4 (33.3) | 4 | 7 (58.3) | 7 | 2 (33.3) | 2 | |
| Mild | 1 (8.3) | 1 | 6 (50.0) | 6 | 1 (16.7) | 1 | ||
| Moderate | 3 (25.0) | 3 | 1 (8.3) | 1 | 1 (16.7) | 1 | ||
| Severe | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | ||
| Erythema | Overall | 2 (16.7) | 2 | 1 (8.3) | 1 | 1 (16.7) | 1 | |
| Mild | 1 (8.3) | 1 | 1 (8.3) | 1 | 1 (16.7) | 1 | ||
| Moderate | 1 (8.3) | 1 | 0 (0.0) | 0 | 0 (0.0) | 0 | ||
| Severe | 0 (0.0) | 0 | 0 (0.0) | 0 | 0 (0.0) | 0 | ||
| Swelling | Overall | 5 (41.7) | 5 | 2 (16.7) | 2 | 2 (33.3) | 2 | |
| Mild | 2 (16.7) | 2 | 1 (8.3) | 1 | 1 (16.7) | 1 | ||
| Moderate | 3 (25.0) | 3 | 1 (8.3) | 1 | 0 (0.0) | 0 | ||
| Severe | 0 (0.0) | 0 | 0 (0.0) | 0 | 1 (16.7) | 1 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
In, Y.; Ha, C.-W. A Multicenter, Randomized, Double-Blinded, Parallel-Group, Placebo-Controlled Phase I/IIa Study to Evaluate the Efficacy and Safety of a Single Intra-Articular Injection of YYD302 in Patients with Knee Osteoarthritis. J. Clin. Med. 2022, 11, 1482. https://doi.org/10.3390/jcm11061482
In Y, Ha C-W. A Multicenter, Randomized, Double-Blinded, Parallel-Group, Placebo-Controlled Phase I/IIa Study to Evaluate the Efficacy and Safety of a Single Intra-Articular Injection of YYD302 in Patients with Knee Osteoarthritis. Journal of Clinical Medicine. 2022; 11(6):1482. https://doi.org/10.3390/jcm11061482
Chicago/Turabian StyleIn, Yong, and Chul-Won Ha. 2022. "A Multicenter, Randomized, Double-Blinded, Parallel-Group, Placebo-Controlled Phase I/IIa Study to Evaluate the Efficacy and Safety of a Single Intra-Articular Injection of YYD302 in Patients with Knee Osteoarthritis" Journal of Clinical Medicine 11, no. 6: 1482. https://doi.org/10.3390/jcm11061482
APA StyleIn, Y., & Ha, C.-W. (2022). A Multicenter, Randomized, Double-Blinded, Parallel-Group, Placebo-Controlled Phase I/IIa Study to Evaluate the Efficacy and Safety of a Single Intra-Articular Injection of YYD302 in Patients with Knee Osteoarthritis. Journal of Clinical Medicine, 11(6), 1482. https://doi.org/10.3390/jcm11061482







