HIPEC in Peritoneal Metastasis of Gastric Origin: A Systematic Review of Regimens and Techniques
Abstract
:1. Introduction
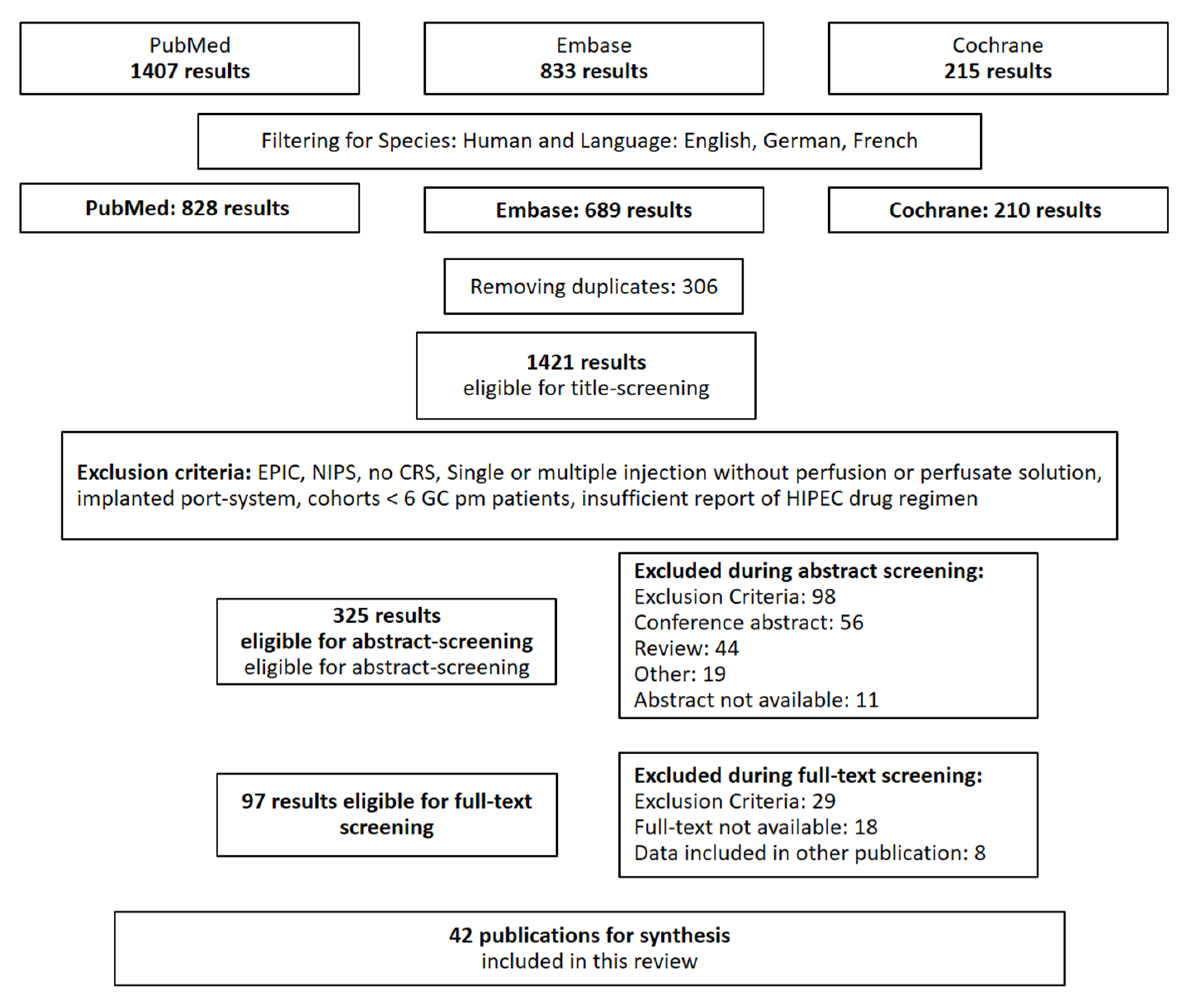
2. Materials and Methods
2.1. Literature Search and Selection of Records
- intraperitoneal: “intraperitonal” [All Fields] OR “intraperitonally” [All Fields] OR “intraperitoneal” [All Fields] OR “intraperitoneally” [All Fields]
- chemotherapy: “chemotherapy’s” [All Fields] OR “drug therapy” [MeSH Terms] OR (“drug” [All Fields] AND “therapy” [All Fields]) OR “drug therapy” [All Fields] OR “chemotherapies” [All Fields] OR “drug therapy” [Subheading] OR “chemotherapy” [All Fields]
- gastric cancer: “stomach neoplasms” [MeSH Terms] OR (“stomach” [All Fields] AND “neoplasms” [All Fields]) OR “stomach neoplasms” [All Fields] OR (“gastric” [All Fields] AND “cancer” [All Fields]) OR “gastric cancer” [All Fields]
2.2. Quality Assessment
2.3. Data Extraction
2.4. Data Synthesis
3. Results
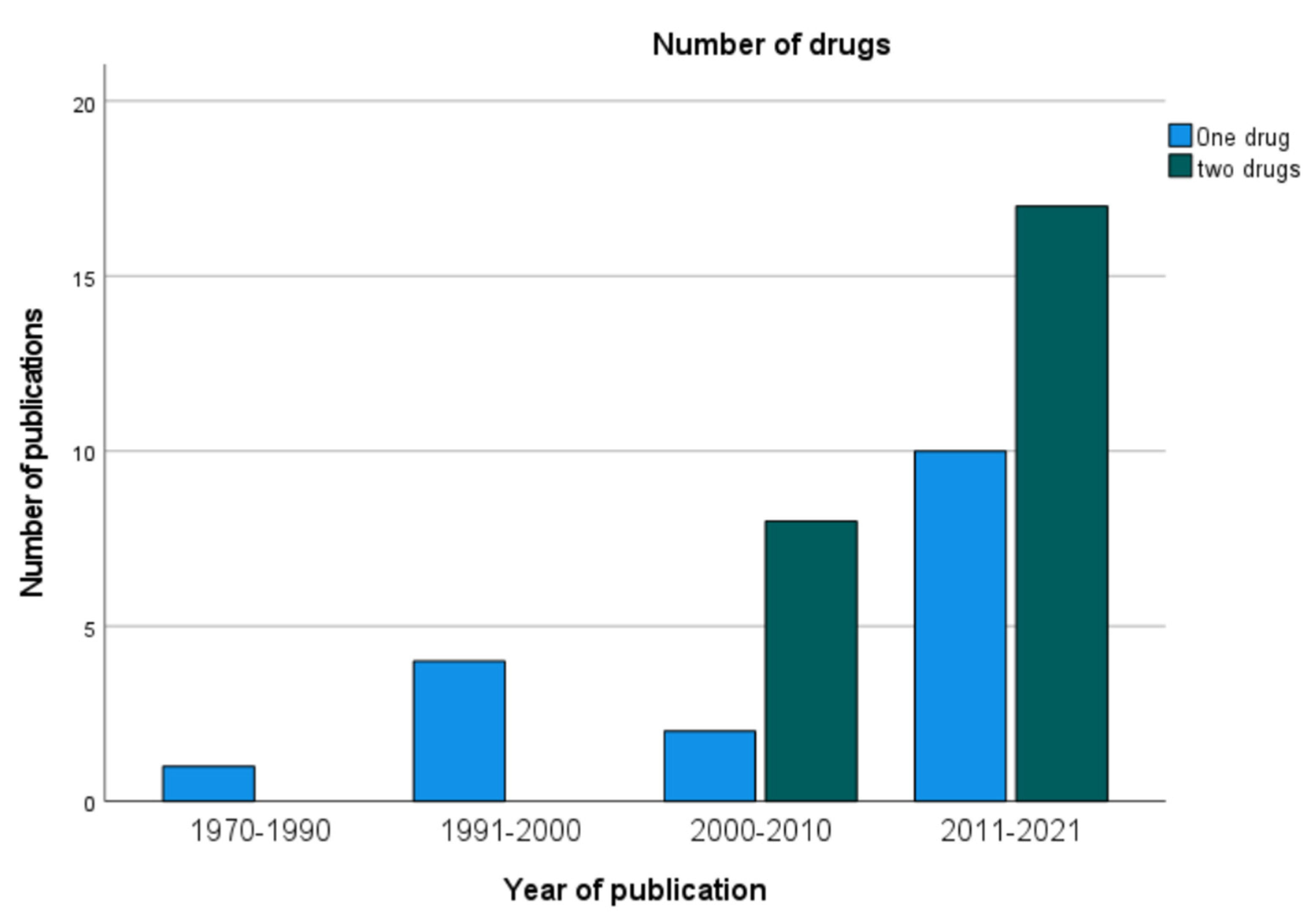
3.1. Publications on CRS and HIPEC for pmGC
3.2. Study Protocols
3.3. Open or Closed HIPEC before or after Anastomosis
3.4. Duration of HIPEC, Temperature, and Choice of Chemotherapeutic Regimen
3.5. Number of Tubes, Perfusate, and Flow Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, I.; van Gestel, Y.R.; van Ramshorst, B.; Luyer, M.D.; Bosscha, K.; Nienhuijs, S.W.; Lemmens, V.E.; de Hingh, I.H. Peritoneal carcinomatosis of gastric origin: A population-based study on incidence, survival and risk factors. Int. J. Cancer 2014, 134, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.H.; Noh, S.H.; Shin, D.W.; Choi, S.H.; Min, J.S. Recurrence following curative resection for gastric carcinoma. Br. J. Surg. 2000, 87, 236–242. [Google Scholar] [CrossRef]
- Rau, B.; Brandl, A.; Thuss-Patience, P.; Bergner, F.; Raue, W.; Arnold, A.; Horst, D.; Pratschke, J.; Biebl, M. The efficacy of treatment options for patients with gastric cancer and peritoneal metastasis. Gastric Cancer 2019, 22, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Solon, J.G.; O’Neill, M.; Chang, K.H.; Deady, S.; Cahill, R.; Moran, B.; Shields, C.; Mulsow, J. An 18 year population-based study on site of origin and outcome of patients with peritoneal malignancy in Ireland. Eur. J. Surg. Oncol. (EJSO) 2017, 43, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Mohamed, F.; Gilly, F.N. Peritoneal carcinomatosis from digestive tract cancer: New management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004, 5, 219–228. [Google Scholar] [CrossRef]
- Kok, H.P.; Beck, M.; Löke, D.R.; Helderman, R.; van Tienhoven, G.; Ghadjar, P.; Wust, P.; Crezee, H. Locoregional peritoneal hyperthermia to enhance the effectiveness of chemotherapy in patients with peritoneal carcinomatosis: A simulation study comparing different locoregional heating systems. Int. J. Hyperth. 2020, 37, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Helderman, R.F.C.P.A.; Löke, D.R.; Verhoeff, J.; Rodermond, H.M.; van Bochove, G.G.W.; Boon, M.; van Kesteren, S.; Garcia Vallejo, J.J.; Kok, H.P.; Tanis, P.J.; et al. The Temperature-Dependent Effectiveness of Platinum-Based Drugs Mitomycin-C and 5-FU during Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Colorectal Cancer Cell Lines. Cells 2020, 9, 1775. [Google Scholar] [CrossRef] [PubMed]
- Löke, D.R.; Helderman, R.F.C.P.A.; Franken, N.A.P.; Oei, A.L.; Tanis, P.J.; Crezee, J.; Kok, H.P. Simulating drug penetration during hyperthermic intraperitoneal chemotherapy. Drug Deliv. 2021, 28, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Brandl, A.; Yonemura, Y.; Glehen, O.; Sugarbaker, P.; Rau, B. Long term survival in patients with peritoneal metastasised gastric cancer treated with cytoreductive surgery and HIPEC: A multi-institutional cohort from PSOGI. Eur. J. Surg. Oncol. 2021, 47, 172–180. [Google Scholar] [CrossRef]
- Chia, C.S.; You, B.; Decullier, E.; Vaudoyer, D.; Lorimier, G.; Abboud, K.; Bereder, J.M.; Arvieux, C.; Boschetti, G.; Glehen, O. Patients with Peritoneal Carcinomatosis from Gastric Cancer Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Is Cure a Possibility? Ann. Surg. Oncol. 2016, 23, 1971–1979. [Google Scholar] [CrossRef]
- Kusamura, S.; Kepenekian, V.; Villeneuve, L.; Lurvink, R.J.; Govaerts, K.; De Hingh, I.; Moran, B.J.; Van der Speeten, K.; Deraco, M.; Glehen, O. Peritoneal mesothelioma: PSOGI/EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Eur. J. Surg. Oncol. 2021, 47, 36–59. [Google Scholar] [CrossRef] [PubMed]
- Govaerts, K.; Lurvink, R.J.; De Hingh, I.; Van der Speeten, K.; Villeneuve, L.; Kusamura, S.; Kepenekian, V.; Deraco, M.; Glehen, O.; Moran, B.J. Appendiceal tumours and pseudomyxoma peritonei: Literature review with PSOGI/EURACAN clinical practice guidelines for diagnosis and treatment. Eur. J. Surg. Oncol. 2021, 47, 11–35. [Google Scholar] [CrossRef] [PubMed]
- van Driel, W.J.; Koole, S.N.; Sikorska, K.; Schagen van Leeuwen, J.H.; Schreuder, H.W.R.; Hermans, R.H.M.; de Hingh, I.H.J.T.; van der Velden, J.; Arts, H.J.; Massuger, L.F.A.G.; et al. Hyperthermic Intraperitoneal Chemotherapy in Ovarian Cancer. N. Engl. J. Med. 2018, 378, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Hultman, B.; Lundkvist, J.; Glimelius, B.; Nygren, P.; Mahteme, H. Costs and clinical outcome of neoadjuvant systemic chemotherapy followed by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from gastric cancer. Acta Oncol. 2012, 51, 112–121. [Google Scholar] [CrossRef] [Green Version]
- Coccolini, F.; Gheza, F.; Lotti, M.; Virzi, S.; Iusco, D.; Ghermandi, C.; Melotti, R.; Baiocchi, G.; Giulini, S.M.; Ansaloni, L.; et al. Peritoneal carcinomatosis. World J. Gastroenterol. 2013, 19, 6979–6994. [Google Scholar] [CrossRef] [PubMed]
- Bonnot, P.E.; Piessen, G.; Kepenekian, V.; Decullier, E.; Pocard, M.; Meunier, B.; Bereder, J.M.; Abboud, K.; Marchal, F.; Quenet, F.; et al. Cytoreductive Surgery with or without Hyperthermic Intraperitoneal Chemotherapy for Gastric Cancer with Peritoneal Metastases (CYTO-CHIP study): A Propensity Score Analysis. J. Clin. Oncol. 2019, 37, 2028–2040. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; de Hingh, I.; Van Der Speeten, K.; Hubner, M.; Deraco, M.; Bakrin, N.; Villeneuve, L.; Kusamura, S.; Glehen, O. HIPEC Methodology and Regimens: The Need for an Expert Consensus. Ann. Surg. Oncol. 2021, 28, 9098–9113. [Google Scholar] [CrossRef] [PubMed]
- Kusamura, S.; Dominique, E.; Baratti, D.; Younan, R.; Deraco, M. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J. Surg. Oncol. 2008, 98, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Abdel Mageed, H.; Van Der Speeten, K.; Sugarbaker, P. The many faces of intraperitoneal chemotherapy. Surg. Oncol. 2021, 40, 101676. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.H.; Peng, K.W.; Yu, Y.; Li, X.B.; Yonemura, Y.; Liu, Y.; Sugarbaker, P.H.; Li, Y. Current status and future prospects of clinical trials on CRS + HIPEC for gastric cancer peritoneal metastases. Int. J. Hyperth. 2017, 33, 562–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemoine, L.; Sugarbaker, P.; Van der Speeten, K. Drugs, doses, and durations of intraperitoneal chemotherapy: Standardising HIPEC and EPIC for colorectal, appendiceal, gastric, ovarian peritoneal surface malignancies and peritoneal mesothelioma. Int. J. Hyperth. 2017, 33, 582–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- González-Moreno, S. Peritoneal Surface Oncology: A progress report. Eur. J. Surg. Oncol. 2006, 32, 593–596. [Google Scholar] [CrossRef]
- Brandl, A.; Prabhu, A. Intraperitoneal chemotherapy in the treatment of gastric cancer peritoneal metastases: An overview of common therapeutic regimens. J. Gastrointest. Oncol. 2021, 12, S32–S44. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Shrestha, R.D.; Kokubun, M.; Ohta, M.; Takahashi, M.; Kobayashi, K.; Kiuchi, S.; Okui, K.; Miyoshi, T.; Arimizu, N.; et al. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding. Ann. Surg. 1988, 208, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, S.; Takahashi, M.; Mutou, T.; Kobayashi, K.; Toyosawa, T.; Isawa, E.; Sumida, M.; Ohkubo, H. Improved mortality rate of gastric carcinoma patients with peritoneal carcinomatosis treated with intraperitoneal hyperthermic chemoperfusion combined with surgery. Cancer 1997, 79, 884–891. [Google Scholar] [CrossRef]
- Chen, M.Y.; Chiles, C.; Loggie, B.W.; Choplin, R.H.; Perini, M.A.; Fleming, R.A. Thoracic complications in patients undergoing intraperitoneal heated chemotherapy with mitomycin following cytoreductive surgery. J. Surg. Oncol. 1997, 66, 19–23. [Google Scholar] [CrossRef]
- Sayag-Beaujard, A.C.; Francois, Y.; Glehen, O.; Sadeghi-Looyeh, B.; Bienvenu, J.; Panteix, G.; Garbit, F.; Grandclément, E.; Vignal, J.; Gilly, F.N. Intraperitoneal chemo-hyperthermia with mitomycin C for gastric cancer patients with peritoneal carcinomatosis. Anticancer Res. 1999, 19, 1375–1382. [Google Scholar]
- Loggie, B.W.; Fleming, R.A.; McQuellon, R.P.; Russell, G.B.; Geisinger, K.R. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for disseminated peritoneal cancer of gastrointestinal origin. Am. Surg. 2000, 66, 561–568. [Google Scholar]
- Glehen, O.; Schreiber, V.; Cotte, E.; Sayag-Beaujard, A.C.; Osinsky, D.; Freyer, G.; Francois, Y.; Vignal, J.; Gilly, F.N. Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch. Surg. 2004, 139, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, Y.; Kawamura, T.; Bandou, E.; Takahashi, S.; Sawa, T.; Matsuki, N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br. J. Surg. 2005, 92, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Kusamura, S.; Younan, R.; Baratti, D.; Costanzo, P.; Favaro, M.; Gavazzi, C.; Deraco, M. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion: Analysis of morbidity and mortality in 209 peritoneal surface malignancies treated with closed abdomen technique. Cancer 2006, 106, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Roviello, F.; Marrelli, D.; Neri, A.; Cerretani, D.; de Manzoni, G.; Pedrazzani, C.; Cioppa, T.; Nastri, G.; Giorgi, G.; Pinto, E. Treatment of peritoneal carcinomatosis by cytoreductive surgery and intraperitoneal hyperthermic chemoperfusion (IHCP): Postoperative outcome and risk factors for morbidity. World J. Surg. 2006, 30, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Scaringi, S.; Kianmanesh, R.; Sabate, J.M.; Facchiano, E.; Jouet, P.; Coffin, B.; Parmentier, G.; Hay, J.M.; Flamant, Y.; Msika, S. Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: A single western center experience. Eur. J. Surg. Oncol. 2008, 34, 1246–1252. [Google Scholar] [CrossRef]
- Yang, X.J.; Li, Y.; al-shammaa Hassan, A.H.; Yang, G.L.; Liu, S.Y.; Lu, Y.L.; Zhang, J.W.; Yonemura, Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival in selected patients with peritoneal carcinomatosis from abdominal and pelvic malignancies: Results of 21 cases. Ann. Surg. Oncol. 2009, 16, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Piso, P.; Slowik, P.; Popp, F.; Dahlke, M.H.; Glockzin, G.; Schlitt, H.J. Safety of gastric resections during cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Ann. Surg. Oncol. 2009, 16, 2188–2194. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Li, Y.; Yonemura, Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy to treat gastric cancer with ascites and/or peritoneal carcinomatosis: Results from a Chinese center. J. Surg. Oncol. 2010, 101, 457–464. [Google Scholar] [CrossRef]
- Li, C.; Yan, M.; Chen, J.; Xiang, M.; Zhu, Z.G.; Yin, H.R.; Lin, Y.Z. Surgical resection with hyperthermic intraperitoneal chemotherapy for gastric cancer patients with peritoneal dissemination. J. Surg. Oncol. 2010, 102, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Gilly, F.N.; Arvieux, C.; Cotte, E.; Boutitie, F.; Mansvelt, B.; Bereder, J.M.; Lorimier, G.; Quenet, F.; Elias, D.; et al. Peritoneal carcinomatosis from gastric cancer: A multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann. Surg. Oncol. 2010, 17, 2370–2377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.J.; Huang, C.Q.; Suo, T.; Mei, L.J.; Yang, G.L.; Cheng, F.L.; Zhou, Y.F.; Xiong, B.; Yonemura, Y.; Li, Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: Final results of a phase III randomized clinical trial. Ann. Surg. Oncol. 2011, 18, 1575–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotte, E.; Passot, G.; Tod, M.; Bakrin, N.; Gilly, F.N.; Steghens, A.; Mohamed, F.; Glehen, O. Closed abdomen hyperthermic intraperitoneal chemotherapy with irinotecan and mitomycin C: A phase I study. Ann. Surg. Oncol. 2011, 18, 2599–2603. [Google Scholar] [CrossRef]
- Mizumoto, A.; Canbay, E.; Hirano, M.; Takao, N.; Matsuda, T.; Ichinose, M.; Yonemura, Y. Morbidity and mortality outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy at a single institution in Japan. Gastroenterol. Res. Pract. 2012, 2012, 836425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.J.; Yuan, P.; Li, Z.Y.; Bu, Z.D.; Zhang, L.H.; Wu, A.W.; Zong, X.L.; Li, S.X.; Shan, F.; Ji, X.; et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves the survival of gastric cancer patients with ovarian metastasis and peritoneal dissemination. Tumour Biol. 2013, 34, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Yarema, R.R.; Ohorchak, M.A.; Zubarev, G.P.; Mylyan, Y.P.; Oliynyk, Y.Y.; Zubarev, M.G.; Gyrya, P.I.; Kovalchuk, Y.J.; Safiyan, V.I.; Fetsych, T.G. Hyperthermic intraperitoneal chemoperfusion in combined treatment of locally advanced and disseminated gastric cancer: Results of a single-centre retrospective study. Int. J. Hyperth. 2014, 30, 159–165. [Google Scholar] [CrossRef]
- Tabrizian, P.; Shrager, B.; Jibara, G.; Yang, M.J.; Romanoff, A.; Hiotis, S.; Sarpel, U.; Labow, D.M. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis: Outcomes from a single tertiary institution. J. Gastrointest. Surg. 2014, 18, 1024–1031. [Google Scholar] [CrossRef]
- Rudloff, U.; Langan, R.C.; Mullinax, J.E.; Beane, J.D.; Steinberg, S.M.; Beresnev, T.; Webb, C.C.; Walker, M.; Toomey, M.A.; Schrump, D.; et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: Results of the GYMSSA trial. J. Surg. Oncol. 2014, 110, 275–284. [Google Scholar] [CrossRef]
- Magge, D.; Zenati, M.; Mavanur, A.; Winer, J.; Ramalingam, L.; Jones, H.; Zureikat, A.; Holtzman, M.; Lee, K.; Ahrendt, S.; et al. Aggressive locoregional surgical therapy for gastric peritoneal carcinomatosis. Ann. Surg. Oncol. 2014, 21, 1448–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, E.A.; Stewart, J.H.; Shen, P.; Russell, G.B.; Loggie, B.L.; Votanopoulos, K.I. Intraperitoneal chemotherapy for peritoneal surface malignancy: Experience with 1000 patients. J. Am. Coll. Surg. 2014, 218, 573–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.W.; Chow, O.; Parikh, K.; Blank, S.; Jibara, G.; Kadri, H.; Labow, D.M.; Hiotis, S.P. Peritoneal carcinomatosis in patients with gastric cancer, and the role for surgical resection, cytoreductive surgery, and hyperthermic intraperitoneal chemotherapy. Am. J. Surg. 2014, 207, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Königsrainer, I.; Horvath, P.; Struller, F.; Königsrainer, A.; Beckert, S. Initial clinical experience with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in signet-ring cell gastric cancer with peritoneal metastases. J. Gastric Cancer 2014, 14, 117–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graziosi, L.; Mingrone, E.; Marino, E.; Cavazzoni, E.; Donini, A. Analysis of operative morbidity in a single center initial experience with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Tumori 2014, 100, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Polanco, P.M.; Ding, Y.; Knox, J.M.; Ramalingam, L.; Jones, H.; Hogg, M.E.; Zureikat, A.H.; Holtzman, M.P.; Pingpank, J.; Ahrendt, S.; et al. Institutional learning curve of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for peritoneal malignancies. Ann. Surg. Oncol. 2015, 22, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Desantis, M.; Bernard, J.L.; Casanova, V.; Cegarra-Escolano, M.; Benizri, E.; Rahili, A.M.; Benchimol, D.; Bereder, J.M. Morbidity, mortality, and oncological outcomes of 401 consecutive cytoreductive procedures with hyperthermic intraperitoneal chemotherapy (HIPEC). Langenbecks Arch. Surg. 2015, 400, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.T.; Peng, K.W.; Ji, Z.H.; Sun, J.H.; Zhang, Q.; Yang, X.J.; Huang, C.Q.; Li, Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with lobaplatin and docetaxel to treat synchronous peritoneal carcinomatosis from gastric cancer: Results from a Chinese center. Eur. J. Surg. Oncol. 2016, 42, 1024–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopanakis, N.; Efstathiou, E.; Sarris, D.; Spiliotis, J. Does upfront therapy with cytoreductive surgery and HIPEC confer a survival benefit in patients with synchronous gastric peritoneal carcinomatosis when compared with patients with metachronous gastric peritoneal carcinomatosis? J. Buon 2017, 22, 1144–1147. [Google Scholar] [PubMed]
- Rihuete Caro, C.; Manzanedo, I.; Pereira, F.; Carrion-Alvarez, L.; Serrano, Á.; Pérez-Viejo, E. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with gastric cancer and peritoneal carcinomatosis. Eur. J. Surg. Oncol. 2018, 44, 1805–1810. [Google Scholar] [CrossRef]
- Montori, G.; Coccolini, F.; Fugazzola, P.; Ceresoli, M.; Tomasoni, M.; Rubicondo, C.; Raimondo, S.; Pinelli, D.; Colledan, M.; Frigerio, L.; et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in ovarian and gastrointestinal peritoneal carcinomatosis: Results from a 7-year experience. J. Gastrointest. Oncol. 2018, 9, 241–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarema, R.; Mielko, J.; Fetsych, T.; Ohorchak, M.; Skorzewska, M.; Rawicz-Pruszyński, K.; Mashukov, A.; Maksimovsky, V.; Jastrzębski, T.; Polkowski, W.; et al. Hyperthermic intraperitoneal chemotherapy (HIPEC) in combined treatment of locally advanced and intraperitonealy disseminated gastric cancer: A retrospective cooperative Central-Eastern European study. Cancer Med. 2019, 8, 2877–2885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, D.; DeNicola, N.; Feingold, D.; Liu, P.H.; Aycart, S.; Golas, B.J.; Sarpel, U.; Labow, D.M.; Magge, D.R. Signet ring cell features with peritoneal carcinomatosis in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy are associated with poor overall survival. J. Surg. Oncol. 2019, 119, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Manzanedo, I.; Pereira, F.; Rihuete Caro, C.; Perez-Viejo, E.; Serrano, A.; Gutierrez Calvo, A.; Regueira, F.M.; Casado-Adam, A.; Cascales-Campos, P.A.; Arteaga, X.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Gastric Cancer with Peritoneal Carcinomatosis: Multicenter Study of Spanish Group of Peritoneal Oncologic Surgery (GECOP). Ann. Surg. Oncol. 2019, 26, 2615–2621. [Google Scholar] [CrossRef]
- Kimbrough, C.W.; Beal, E.; Abdel-Misih, S.; Pawlik, T.M.; Cloyd, J.M. Survival Outcomes Among Patients with Gastric Adenocarcinoma Who Received Hyperthermic Intraperitoneal Chemotherapy with Cytoreductive Surgery. JAMA Surg. 2019, 154, 780–782. [Google Scholar] [CrossRef]
- Hotopp, T. HIPEC and CRS in peritoneal metastatic gastric cancer—Who really benefits? Surg. Oncol. 2019, 28, 159–166. [Google Scholar] [CrossRef]
- Braeuer, F.; Fischer, I.; Brammen, L.; Pressl, G.; Fuegger, R.; Rohregger, K.; Wundsam, H. Outcome in Patients Treated with Cytoreductive Surgery and HIPEC for Gastric Cancer with Peritoneal Carcinomatosis. Anticancer Res. 2020, 40, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Koemans, W.J.; van der Kaaij, R.T.; Wassenaar, E.C.E.; Boerma, D.; Boot, H.; Sikorska, K.; Los, M.; Grootscholten, C.; Hartemink, K.J.; Veenhof, A.; et al. Tumor characteristics and clinical outcome of peritoneal metastasis of gastric origin treated with a hyperthermic intraperitoneal chemotherapy procedure in the PERISCOPE I trial. J. Surg. Oncol. 2021, 123, 904–910. [Google Scholar] [CrossRef]
- Bouquet, W.; Ceelen, W.; Adriaens, E.; Almeida, A.; Quinten, T.; De Vos, F.; Pattyn, P.; Peeters, M.; Remon, J.P.; Vervaet, C. In vivo toxicity and bioavailability of Taxol and a paclitaxel/beta-cyclodextrin formulation in a rat model during HIPEC. Ann. Surg. Oncol. 2010, 17, 2510–2517. [Google Scholar] [CrossRef]
- Sugarbaker, P.H.; Van der Speeten, K. Surgical technology and pharmacology of hyperthermic perioperative chemotherapy. J. Gastrointest. Oncol. 2016, 7, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.R.; Mocellin, S.; Pilati, P.; Foletto, M.; Quintieri, L.; Palatini, P.; Lise, M. Pharmacokinetics of intraperitoneal cisplatin and doxorubicin. Surg. Oncol. Clin. N. Am. 2003, 12, 781–794. [Google Scholar] [CrossRef]
- Löke, D.R.; Helderman, R.; Sijbrands, J.; Rodermond, H.M.; Tanis, P.J.; Franken, N.A.P.; Oei, A.L.; Kok, H.P.; Crezee, J. A Four-Inflow Construction to Ensure Thermal Stability and Uniformity during Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in Rats. Cancers 2020, 12, 3516. [Google Scholar] [CrossRef] [PubMed]
- Leiting, J.L.; Cloyd, J.M.; Ahmed, A.; Fournier, K.; Lee, A.J.; Dessureault, S.; Felder, S.; Veerapong, J.; Baumgartner, J.M.; Clarke, C.; et al. Comparison of open and closed hyperthermic intraperitoneal chemotherapy: Results from the United States hyperthermic intraperitoneal chemotherapy collaborative. World J. Gastrointest. Oncol. 2020, 12, 756–767. [Google Scholar] [CrossRef]
- Dupont, S.; Schiffer, E.R.C.; White, M.J.; Diaper, J.R.A.; Licker, M.J.; Masouyé, P.C. Changes in Hepatic Blood Flow and Liver Function during Closed Abdominal Hyperthermic Intraperitoneal Chemotherapy following Cytoreduction Surgery. Gastroenterol. Res. Pract. 2018, 2018, 8063097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somashekhar, S.P.; Rohit, K.C.; Ramya, Y.; Zaveri, S.S.; Ahuja, V.; Namachivayam, A.K.; Ashwin, K.R. Bowel Anastomosis After or Before HIPEC: A Comparative Study in Patients Undergoing CRS+HIPEC for Peritoneal Surface Malignancy. Ann. Surg. Oncol. 2022, 29, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Woeste, M.R.; Philips, P.; Egger, M.E.; Scoggins, C.R.; McMasters, K.M.; Martin, R.C.G. Optimal perfusion chemotherapy: A prospective comparison of mitomycin C and oxaliplatin for hyperthermic intraperitoneal chemotherapy in metastatic colon cancer. J. Surg. Oncol. 2020, 121, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Montagnani, F.; Turrisi, G.; Marinozzi, C.; Aliberti, C.; Fiorentini, G. Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: A systematic review and meta-analysis. Gastric Cancer 2011, 14, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Koemans, W.J.; van der Kaaij, R.T.; Wassenaar, E.C.E.; Grootscholten, C.; Boot, H.; Boerma, D.; Los, M.; Imhof, O.; Schellens, J.H.M.; Rosing, H.; et al. Systemic exposure of oxaliplatin and docetaxel in gastric cancer patients with peritonitis carcinomatosis treated with intraperitoneal hyperthermic chemotherapy. Eur. J. Surg. Oncol. 2021, 47, 486–489. [Google Scholar] [CrossRef]
- Kuzuya, T.; Yamauchi, M.; Ito, A.; Hasegawa, M.; Hasegawa, T.; Nabeshima, T. Pharmacokinetic characteristics of 5-fluorouracil and mitomycin C in intraperitoneal chemotherapy. J. Pharm. Pharmacol. 1994, 46, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Nagourney, R.A.; Evans, S.; Tran, P.H.; Nagourney, A.J.; Sugarbaker, P.H. Colorectal cancer cells from patients treated with FOLFOX or CAPOX are resistant to oxaliplatin. Eur. J. Surg. Oncol. 2021, 47, 738–742. [Google Scholar] [CrossRef]
- Charrier, T.; Passot, G.; Peron, J.; Maurice, C.; Gocevska, S.; Quénet, F.; Eveno, C.; Pocard, M.; Goere, D.; Elias, D.; et al. Cytoreductive Surgery Combined with Hyperthermic Intraperitoneal Chemotherapy with Oxaliplatin Increases the Risk of Postoperative Hemorrhagic Complications: Analysis of Predictive Factors. Ann. Surg. Oncol. 2016, 23, 2315–2322. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Ren, Y.; Wei, Z.; Peng, J.; Chen, C.; Song, W.; Tan, M.; He, Y.; Yuan, Y. Nephrotoxicity and long-term survival investigations for patients with peritoneal carcinomatosis using hyperthermic intraperitoneal chemotherapy with cisplatin: A retrospective cohort study. Surg. Oncol. 2018, 27, 456–461. [Google Scholar] [CrossRef]
- Kurreck, A.; Gronau, F.; Alberto Vilchez, M.E.; Abels, W.; Enghard, P.; Brandl, A.; Francis, R.; Föhre, B.; Lojewski, C.; Pratschke, J.; et al. Sodium Thiosulfate Reduces Acute Kidney Injury in Patients Undergoing Cytoreductive Surgery Plus Hyperthermic Intraperitoneal Chemotherapy with Cisplatin: A Single-Center Observational Study. Ann. Surg. Oncol. 2021, 29, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.M.; Van den Hoven, J.M.; Rosing, H.; Hillebrand, M.J.; Nuijen, B.; Huitema, A.D.; Beijnen, J.H.; Verwaal, V.J. Stability of oxaliplatin in chloride-containing carrier solutions used in hyperthermic intraperitoneal chemotherapy. Int. J. Pharm. 2015, 479, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.S.; Al-Adra, D.P.; Nagendran, J.; Campbell, S.; Shi, X.; Haase, E.; Schiller, D. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: A systematic review of survival, mortality, and morbidity. J. Surg. Oncol. 2011, 104, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Eveno, C.; Pocard, M. Randomized controlled trials evaluating cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in prevention and therapy of peritoneal metastasis: A systematic review. Pleura Peritoneum 2016, 1, 169–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yap, D.R.Y.; Wong, J.S.M.; Tan, Q.X.; Tan, J.W.-S.; Chia, C.S.; Ong, C.-A.J. Effect of HIPEC on Peritoneal Recurrence in Peritoneal Metastasis Treated with Cytoreductive Surgery: A Systematic Review. Front. Oncol. 2021, 11, 795390. [Google Scholar] [CrossRef]
- Granieri, S.; Bonomi, A.; Frassini, S.; Chierici, A.P.; Bruno, F.; Paleino, S.; Kusamura, S.; Germini, A.; Facciorusso, A.; Deraco, M.; et al. Prognostic impact of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in gastric cancer patients: A meta-analysis of randomized controlled trials. Eur. J. Surg. Oncol. 2021, 47, 2757–2767. [Google Scholar] [CrossRef]
- Chia, C.S.; Seshadri, R.A.; Kepenekian, V.; Vaudoyer, D.; Passot, G.; Glehen, O. Survival outcomes after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis from gastric cancer: A systematic review. Pleura Peritoneum 2016, 1, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Dominic, J.L.; Kannan, A.; Tara, A.; Hakim Mohammed, A.R.; Win, M.; Khorochkov, A.; Sultan, W.; Ahmed, A.; Kantamaneni, K.; Syzmanski, M.W.; et al. Prophylactic hyperthermic intraperitoneal chemotherapy (HIPEC) for the prevention and control of peritoneal metastasis in patients with gastrointestinal malignancies: A systematic review of randomized controlled trials. EXCLI J. 2021, 20, 1328–1345. [Google Scholar] [CrossRef] [PubMed]
- Auer, R.C.; Sivajohanathan, D.; Biagi, J.; Conner, J.; Kennedy, E.; May, T. Indications for hyperthermic intraperitoneal chemotherapy with cytoreductive surgery: A systematic review. Eur. J. Cancer 2020, 127, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Kitai, T. The role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the treatment of peritoneal carcinomatosis: A systematic review including evidence from Japan. Surg. Today 2020, 51, 1085–1098. [Google Scholar] [CrossRef] [PubMed]
- Yurttas, C.; Hoffmann, G.; Tolios, A.; Haen, S.P.; Schwab, M.; Königsrainer, I.; Königsrainer, A.; Beckert, S.; Löffler, M.W. Systematic Review of Variations in Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Peritoneal Metastasis from Colorectal Cancer. J. Clin. Med. 2018, 7, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braam, H.J.; Schellens, J.H.; Boot, H.; van Sandick, J.W.; Knibbe, C.A.; Boerma, D.; van Ramshorst, B. Selection of chemotherapy for hyperthermic intraperitoneal use in gastric cancer. Crit. Rev. Oncol. Hematol. 2015, 95, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Helderman, R.F.C.P.A.; Löke, D.R.; Kok, H.P.; Oei, A.L.; Tanis, P.J.; Franken, N.A.P.K.; Crezee, J. Variation in Clinical Application of Hyperthermic Intraperitoneal Chemotherapy: A Review. Cancers 2019, 11, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Reference | Year | Single- or Multi-Institutional | Type of Study | Number of Patients Included | Number of Patients with pmGC and CRS + HIPEC |
|---|---|---|---|---|---|
| Fujimoto [29] | 1988 | single | PCS | 15 | 9 |
| Fujimoto [30] | 1997 | single | RCS | 48 | 30 |
| Chen [31] | 1997 | single | RCS | 42 | 6 |
| Sayag-Beaujard [32] | 1999 | single | pCTII | 83 | 18 |
| Loggie [33] | 2000 | single | pCTII | 84 | 22 |
| Glehen [34] | 2004 | single | RCS | 49 | 21 |
| Yonemura [35] | 2005 | single | RCS | 107 | 42 |
| Kusamura [36] | 2006 | single | RCS | 209 | 12 |
| Roviello [37] | 2006 | single | RCS | 59 | 6 |
| Scaringi [38] | 2008 | single | RCS | 37 | 26 |
| Yang [39] | 2009 | single | RCS | 21 | 12 |
| Piso [40] | 2009 | single | RCS | 37 | 11 |
| Yang [41] | 2010 | single | pCTII | 28 | 28 |
| Li [42] | 2010 | single | RCS | 128 | 10 |
| Glehen [43] | 2010 | multiple | RCS | 159 | 147 |
| Yang [44] | 2011 | single | RCT | 68 | 34 |
| Cotte [45] | 2011 | single | pCTI | 12 | 12 |
| Mizumoto [46] | 2012 | single | RCS | 250 | 16 |
| Wu [47] | 2013 | single | RCS | 62 | 32 |
| Yarema [48] | 2014 | single | RCS | 98 | 20 |
| Tabrizian [49] | 2014 | single | RCS | 170 | 12 |
| Rudloff [50] | 2014 | single | RCT | 17 | 9 |
| Magge [51] | 2014 | single | RCS | 23 | 23 |
| Levine [52] | 2014 | single | RCS | 1000 | 46 |
| Kim [53] | 2014 | single | RCS | 112 | 9 |
| Königsrainer [54] | 2014 | single | RCS | 18 | 13 |
| Graziosi [55] | 2014 | single | RCS | 36 | 15 |
| Polanco [56] | 2015 | single | PCS | 370 | 24 |
| Desantis [57] | 2015 | single | RCS | 356 | 14 |
| Wu [58] | 2016 | single | RCS | 50 | 50 |
| Kopanakis [59] | 2017 | single | RCS | 14 | 14 |
| Rihuete Caro [60] | 2018 | single | RCS | 35 | 32 |
| Montori [61] | 2018 | single | RCS | 150 | 26 |
| Yarema [62] | 2019 | multiple | RCS | 117 | 70 |
| Solomon [63] | 2019 | single | RCS | 268 | 18 |
| Rau [4] | 2019 | single | RCS | 88 | 58 |
| Manzanedo [64] | 2019 | multiple | RCS | 88 | 84 |
| Kimbrough [65] | 2019 | multiple | RCS | 28 | 28 |
| Hotopp [66] | 2019 | single | RCS | 26 | 26 |
| Bonnot [17] | 2019 | multiple | RCS-PSm | 275 | 180 |
| Braeuer [67] | 2020 | single | RCS | 109 | 37 |
| Koemans [68] | 2021 | single | pCT I–II | 25 | 23 |
| NCT Number | Patients | End Until | Location | Arm Intervention | Arm Control | Open/Closed | HIPEC Drug | HIPEC Solution/Duration | Temperature | Primary Outcome Measures | Secondary Outcome Measures |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NCT03023436 | 220 | 22 June | China | CRS + HIPEC + sCTx | single arm | closed | DTX 120 mg | 5 L saline; 70 min | 43 ± 0.5 °C | MS 2-year (24 months) | 1. 2-year OS; 2. 2-year PFS; 3. M&M (30 d; 24 months) |
| NCT02158988 | 105 | 21 September | Germany | CRS + HIPEC + sCTx | CRS + sCTx | open/closed | MMC 15 mg/m2 CDDP 75 mg/m2 | 5 L saline; 60 min | 41–42 °C | OS (2.5 years) | 1. PFS; 2. M&M (30 d; 24 months) 3. MFS; 4. QoL (every 6 months) |
| NCT03348150 | 182 | 22 October | The Netherlands | CRS + HIPEC + sCTx | palliative sCTx | open | OX 460 mg/m2 DTX 50 mg/m2 | ns; 30 + 90 min | 41–42 °C + 37 °C | OS (5 years) | 1. PFS 2. toxicity 3.cost and health benefits |
| Reference | Before/After Anastomosis | Duration (min) | Open/Closed | Max. Heat (°C) | Drug First i.p. (mg/m2) | Drug Second i.p. (mg/m2) | Perfusate | Flow Rate | Number of Tubes | Bidirectional Drugs i.v. |
|---|---|---|---|---|---|---|---|---|---|---|
| Fujimoto et al. [29] | ns | 120 | ns | 44.7–48.7 | MMC 10 µg/mL; 30 mg td | 3–5 L | ns | ns | ||
| Fujimoto et al. [30] | ns | 120 | closed | 43–45 | MMC 10 µg/mL; 100 mg/L | 3–4 L MWS | ns | 2 | ||
| Chen et al. [31] | after | 120 | closed | 40.5 | MMC 30–40 m td | 2–3 L RL | ns | 2|2 | ||
| Sayag-Beaujard et al. [32] | ns | 90 | ns | 46–49 | MMC 10 mg/L | 4–6 L | 400–500 mL/min | 2 | ||
| Loggie et al. [33] | ns | 120 | ns | 40.5 | MMC | ns | ns | ns | ||
| Glehen et al. [34] | after | 90 | closed | 46–48 | MMC 10 mg/mL | 4–6 L | 500 mL/min | 2|1 | ||
| Yonemura et al. [35] | after | 60 | open | 42–43 | MMC 30 mg td | td: CDDP 300 mg, Etoposid 150 mg | 8 L saline | 10 L/min | ns | |
| Kusamura et al. [36] | after | 60–90 | closed | 42–43 | CDDP 25 mg/m2/L | MMC 3.3 mg/m2/L | ns | ns | 4 | |
| Roviello et al. [37] | before | 60 | closed | 41–43 | MMC 25 | CDDP 100 | ns | 700–800 | 5 | |
| Scaringi et al. [38] | since 1998 before | 90–120 | o/c | 41–43 | MMC 120 | CDDP 200 | 12 L saline | ns | 2 | |
| Yang et al. [39] | after | 60–90 | open | 43 ± 0.5 | HCPT 20 mg td | MMC 30 mg td | 12 L saline | 200 mL/min | 1|1 | |
| Piso et al. [40] | after | 60 | closed | 42.5–43 | CDDP 75 | Doxorubicin 15 | ns | ns | ns | |
| Yang et al. [41] | after | 90–120 | open | 43 ± 0.5 | td: HCPT 20 mg CDDP 120 mg | MMC 30 mg td | 12 L saline | 200 mL/min | 1|1 | |
| Li et al. [42] | after | 60 | closed | 43 ± 1 | CDDP 50 µg/mL | MMC 5 µg/mL | 5–6 L | ns | 2|1 | |
| Glehen et al. [43] | ns | (1) 60–120 (2) 30 mean: 80.1 | o/c | 40–43; mean: 42.6 | (1) MMC 30–50 (2) OX 360–460 | (1) ±CDDP 100–200 (2) ±IRI 50–100 | ns | ns | ns | 5-FU + FA |
| Yang et al. [44] | after | 60–90 | open | 43 ± 0.5 | CDDP 120 mg td | MMC 30 mg td | 6 L saline | 500 mL/min | 1|1 | ns |
| Cotte et al. [45] | after | 90 | closed | 44–46 | MMC 0.7 mg/kg | IRI 100 | 3–4 L GLC | 500 mL/min | 2|1 | |
| Mizumoto et al. [46] | after | 60 | ns | 41–42 | MMC 20 mg td | CDDP 100 mg td | saline | ns | 2|1 | |
| Wu et al. [47] | ns | 60 | ns | 43 ±0.5 | OX 460 | 3–4 L GLC | 500–800 mL/min | 3 | ||
| Yarema et al. [48] | after | 90 | open | 43 ± 1.3 | MMC 12.5 | CDDP 75 | ns | ns | ns | 5-FU |
| Tabrizian et al. [49] | ns | 60 + 30 | closed | 41–43 | MMC † | ns | ns | ns | ||
| Rudloff et al. [50] | before | 30 | closed | 41 | OX 460 | 3–4 L GLC | 2 l/min | ns | ||
| Magge et al. [51] | before | 100 | closed | 42 | MMC 30–40 mg td | 3 L saline | 800 mL/min | 2|1 | ||
| Levine et al. [52] | after | 60 + 60 | closed | 43 | MMC † | 3 L RL | 1 L /min | 2|2 | ||
| Kim et al. [53] | ns | 60 + 30 | ns | 41 | MMC † | ns | ns | ns | ||
| Königsrainer I. et al. [54] | after | 90 | open | 42 | CDDP 50 | ns | ns | ns | ||
| Graziosi et al. [55] | after | 60 | closed | 42 | CDDP 25 mg/L/m2 | MMC 3.3 mg/L/m2 | ns | ns | ns | |
| Polanco et al. [56] | ns | ns | closed | 42 | MMC 40 CDDP 50 | ns | ns | ns | ||
| Desantis, M. [57] | before | 60 | open | 43 | CDDP 50 | ns | 800 mL/min | 5 | ||
| Wu, H. T. [58] | before | 60 | open | 43 ± 0.5 | Lobaplatin 50 | DTX 60 | 6 L saline | 400 mL/min | ns | |
| Kopanakis [59] | ns | 90 | ns | ns | CDDP 50 | Doxorubicin 50 | ns | ns | ns | |
| Rihuete Caro [60] | after | 90 | open | 42–43 | CDDP 100 | Doxorubicin 15 | ns | ns | ns | |
| Montori et al. [61] | before | 90 | open | 42–43 | CDDP 100 | Paclitaxel 175 | ns | ns | 1|4 | |
| Yarema, R. [62] | ns | 30–90 | closed | 42.7 ± 0.78 | (1) MMC 10–15 (2) OX 460 (3) CDDP 75 | (1) CDDP 75 (2) Doxorubicin 15 | ns | ns | ns | 5-FU |
| Solomon, D. [63] | before | 90 | closed | 41–43 | MMC 40 mg td | ns | ns | ns | ||
| Rau [4] | after | 60 | o/c | 41 | MMC 15 | CDDP 75 | ns | ns | ns | |
| Manzanedo [64] | ns | ns | open | CDDP (50%) | Doxorubicin (50%) | |||||
| Kimbrough, C. W. [65] | ns | ns | o/c | ns | MMC | ns | ns | ns | ||
| Hotopp [66] | ns | ns | open | OX 200 | DTX 80 | ns | 1500 mL/min | ns | ||
| Bonnot, P. E. [17] | ns | 30–120 | o/c | 41–43 | (1) MMC 30–50 (2) CDDP: 50–100 (3) OX: 300–460 | (1) or (3) ±IRI 200 (1) +CDDP 100 (2) ±doxorubicin 15 | ns | 500 mL/min | ns | 5-FU + FA |
| Braeuer, F. [67] | ns | 45–60 | closed | 42 | OX 400 | ns | ns | ns | ||
| Koemans, Willem J. [68] | before | 30 + 90 | open | 41–42 (OX), 37 (DTX) | OX 460 | DTX 0; 50, 75 | ns | ns |
| Reference | Year | Number of Patients with pmGC and CRS + HIPEC | Median PCI | Median OS | Clavien–Dindo ≥III° | Anastomotic Leakage Rate |
|---|---|---|---|---|---|---|
| Fujimoto et al. [29] | 1988 | 9 | ns | ns | ns | ns |
| Fujimoto et al. [30] | 1997 | 30 | ns | ns | ns | ns |
| Chen et al. [31] | 1997 | 6 | ns | ns | ns | ns |
| Sayag-Beaujard et al. [32] | 1999 | 18 | ns | ns | ns | ns |
| Loggie et al. [33] | 2000 | 22 | ns | ns | ns | ns |
| Glehen et al. [34] | 2004 | 21 | ns | 10.3 months 1-year: 48.1% 2-year: 19.0% 5-year: 16.0% | 13 (27%) | 0/49 (0%) |
| Yonemura et al. [35] | 2005 | 42 | ns | 11.5 months 5-year: 6.7% | ns | 7/107 (6.5%) |
| Kusamura et al. [36] | 2006 | 12 | ns | ns | ns | ns |
| Roviello et al. [37] | 2006 | 6 | ns | ns | ns | ns |
| Scaringi et al. [38] | 2008 | 26 | ns | 15 months | ns | ns |
| Yang et al. [39] | 2009 | 12 | ns | ns | ns | ns |
| Piso et al. [40] | 2009 | 11 | ns | ns | ns | 0/15 (0%) |
| Yang et al. [41] | 2010 | 28 | 12 | 1-year: 50.0% 2-year: 42.8% | ns | ns |
| Li et al. [42] | 2010 | 10 | ns | 11.8 months 1-year: 52.5% 3-year: 13.2% 5-year: 5.5% | ns | 0/10 (0%) |
| Glehen et al. [43] | 2010 | 147 | 9.4 (±7.7) | 9.2 months 1-year: 43% 3-year: 18% 5-year: 13% | 34.30% | ns |
| Yang et al. [44] | 2011 | 34 | 15 | 11 months 1-year: 41.2% 2-year: 14.7% 3-year: 5.9% | ns | 0/35 (0%) |
| Cotte et al. [45] | 2011 | 12 | ns | ns | ns | 0/12 (0%) |
| Mizumoto et al. [46] | 2012 | 16 | 10 (±10) * | ns | 38% | ns |
| Wu et al. [47] | 2013 | 32 | ns | 15.5 months | ns | ns |
| Yarema et al. [48] | 2014 | 20 | 3.40 | 12 ± 1.6 months | ns | 1 (2%) |
| Tabrizian et al. [49] | 2014 | 12 | ns | 3-year: 16.6% | ns | ns |
| Rudloff et al. [50] | 2014 | 9 | ns | 11.3 months | 8 (89%) | 2 (22%) |
| Magge et al. [51] | 2014 | 23 | 10.5 | 9.5 months 1-year: 49.6% 3-year: 17.9% | 52% | 3 (13%) |
| Levine et al. [52] | 2014 | 46 | ns | 6.1 months | see Ref [53] | ns |
| Kim et al. [53] | 2014 | 9 | ns | 16 months | ns | ns |
| Königsrainer et al. [54] | 2014 | 13 | ns | 8.9 months | 11 (Grade 1–5) | 0 (0%) |
| Graziosi et al. [55] | 2014 | 15 | ns | ns | 4 (11.1%) | 0 (0%) |
| Polanco et al. [56] | 2015 | 24 | 13 | ns | see Ref [57] | ns |
| Desantis [57] | 2015 | 14 | ns | ns | ns | ns |
| Wu [58] | 2016 | 50 | 15 | 24.8 months | 12 (23.1%) | 1 (1%) |
| Kopanakis [59] | 2017 | 14 | 15 | ns | ns | ns |
| Rihuete Caro [60] | 2018 | 32 | ns | ns | 38% | ns |
| Montori et al. [61] | 2018 | 26 | 8 | 16 months 1-year: 70.8% 3-year: 21.3% | 9 (25.7%) | ns |
| Yarema [62] | 2019 | 70 | ns | PCI 0–6: 15 months PCI > 6: 8.2 months | ns | 7 (6.5%) |
| Solomon [63] | 2019 | 18 | ns | 12 months | see Ref [63] | ns |
| Rau [4] | 2019 | 58 | 8.3 (±5.7) * | 9.8 months | 14 (62%) | ns |
| Manzanedo [64] | 2019 | 84 | 6 | 21.2 months 1-year: 79.9% 3-year: 30.9% | 30 (34.4%) | ns |
| Kimbrough [65] | 2019 | 28 | 12 | 10 months | 5 (18%) | 2 (7%) |
| Hotopp [66] | 2019 | 26 | 10 | 17 months | ns | 2 (7.7%) |
| Bonnot [17] | 2019 | 180 | 6 | 18.6 months | 53.70% | ns |
| Braeuer [67] | 2020 | 37 | 3.75 ±1.9 * | 33.8 months | 3 (37.5%) | ns |
| Koemans [68] | 2021 | 23 | 2 | 15 months | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gronau, F.; Feldbruegge, L.; Oberwittler, F.; Gonzalez-Moreno, S.; Villeneuve, L.; Eveno, C.; Glehen, O.; Kusamura, S.; Rau, B. HIPEC in Peritoneal Metastasis of Gastric Origin: A Systematic Review of Regimens and Techniques. J. Clin. Med. 2022, 11, 1456. https://doi.org/10.3390/jcm11051456
Gronau F, Feldbruegge L, Oberwittler F, Gonzalez-Moreno S, Villeneuve L, Eveno C, Glehen O, Kusamura S, Rau B. HIPEC in Peritoneal Metastasis of Gastric Origin: A Systematic Review of Regimens and Techniques. Journal of Clinical Medicine. 2022; 11(5):1456. https://doi.org/10.3390/jcm11051456
Chicago/Turabian StyleGronau, Felix, Linda Feldbruegge, Frauke Oberwittler, Santiago Gonzalez-Moreno, Laurent Villeneuve, Clarisse Eveno, Olivier Glehen, Shigeki Kusamura, and Beate Rau. 2022. "HIPEC in Peritoneal Metastasis of Gastric Origin: A Systematic Review of Regimens and Techniques" Journal of Clinical Medicine 11, no. 5: 1456. https://doi.org/10.3390/jcm11051456
APA StyleGronau, F., Feldbruegge, L., Oberwittler, F., Gonzalez-Moreno, S., Villeneuve, L., Eveno, C., Glehen, O., Kusamura, S., & Rau, B. (2022). HIPEC in Peritoneal Metastasis of Gastric Origin: A Systematic Review of Regimens and Techniques. Journal of Clinical Medicine, 11(5), 1456. https://doi.org/10.3390/jcm11051456








