OCT Angiography Fractal Analysis of Choroidal Neovessels Secondary to Central Serous Chorioretinopathy, in a Caucasian Cohort
Abstract
:1. Introduction
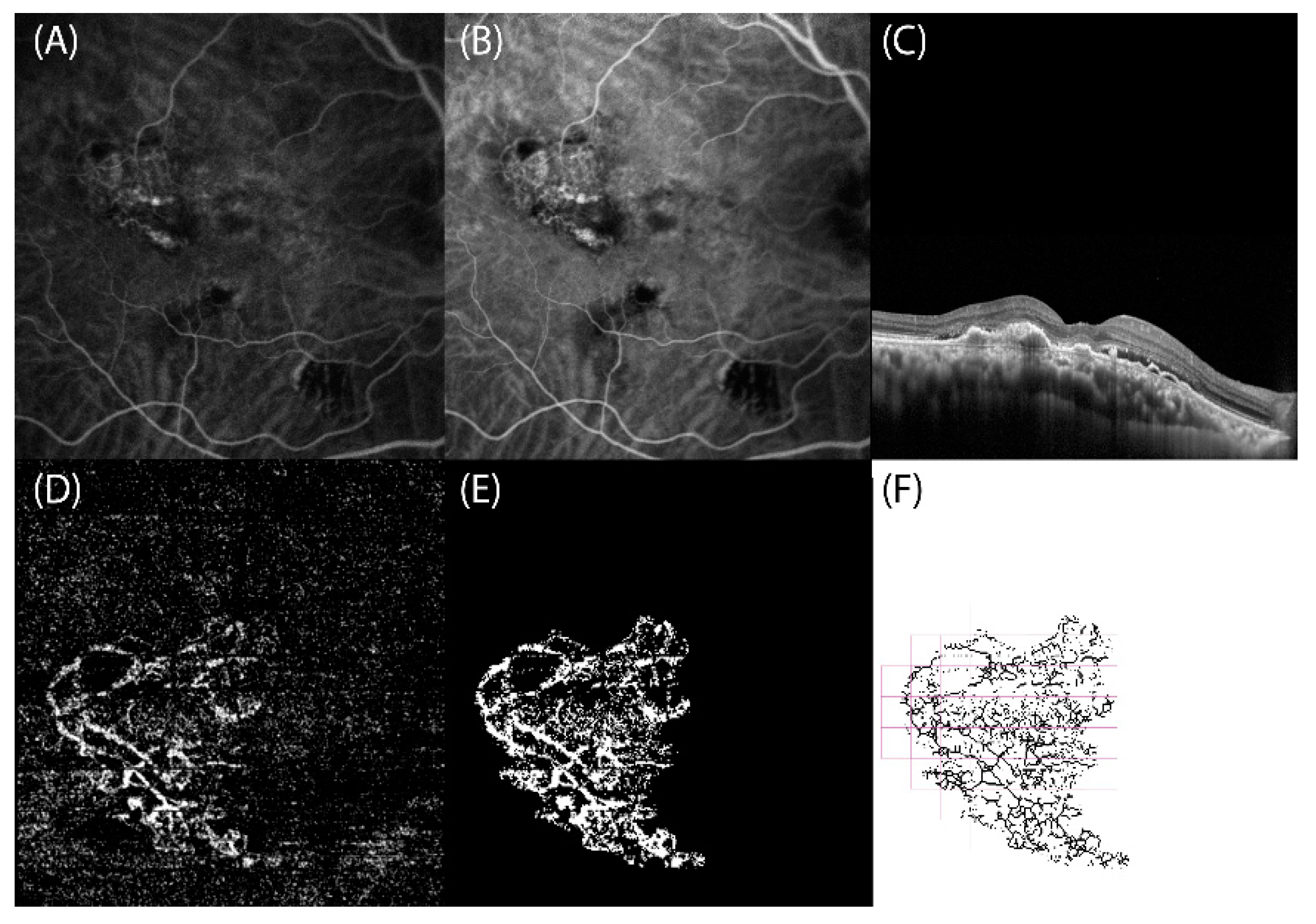
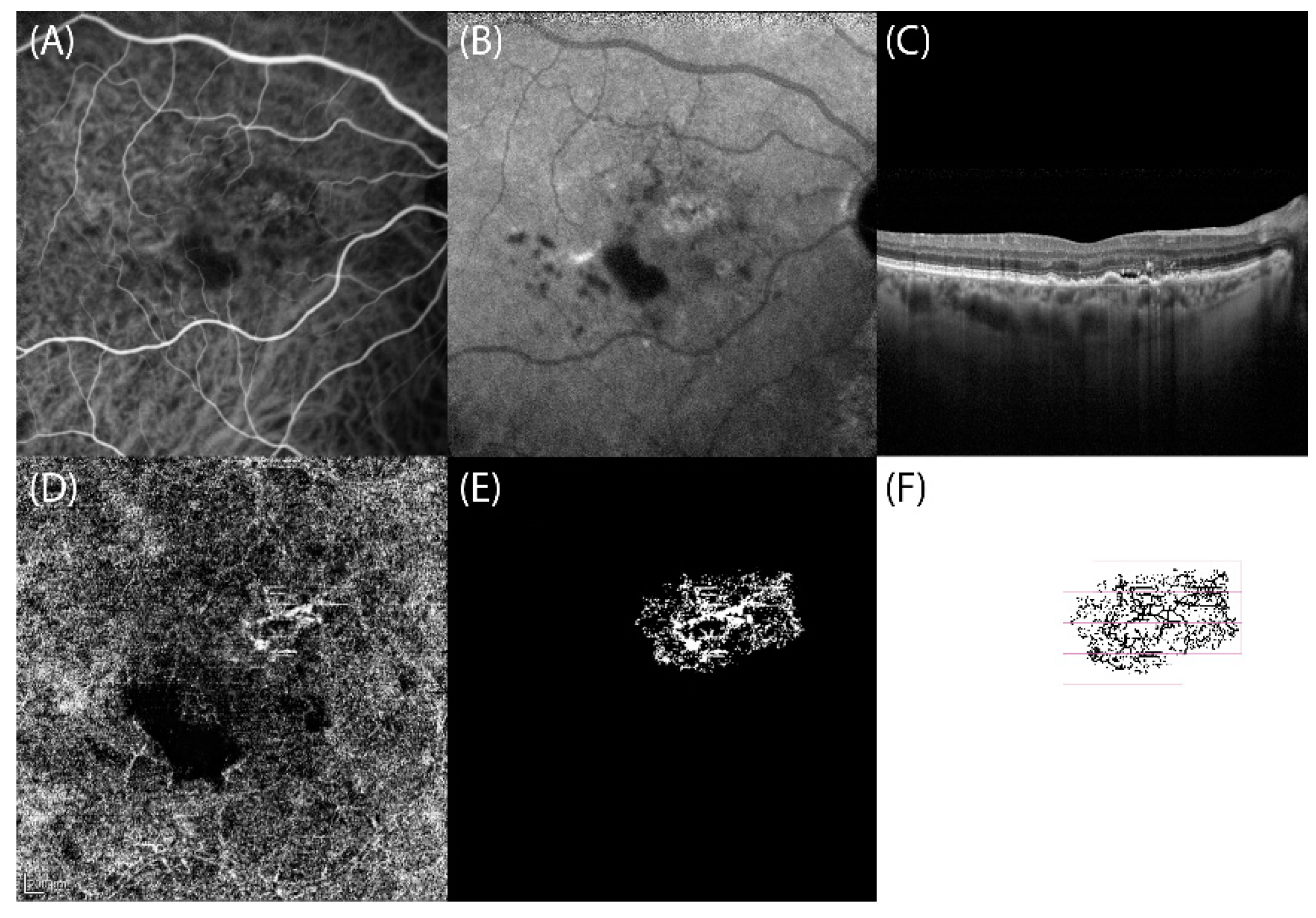
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dansingani, K.K.; Balaratnasingam, C.; Naysan, J.; Freund, K.B. En face imaging of pachychoroid spectrum disorders with swept-source optical coherence tomography. Retina 2016, 36, 499–516. [Google Scholar] [CrossRef]
- Savastano, M.C.; Rispoli, M.; Lumbroso, B. The incidence of neovascularization in central serous chorioretinopathy by optical coherence tomography angiography. Retina 2021, 41, 302–308. [Google Scholar] [CrossRef]
- Spaide, R.F.; Campeas, L.; Haas, A.; Yannuzzi, L.A.; Fisher, Y.L.; Guyer, D.R.; Slakter, J.S.; Sorenson, J.A.; Orlock, D.A. Central Serous Chorioretinopathy in Younger and Older Adults. Ophthalmology 1996, 103, 2070–2080. [Google Scholar] [CrossRef]
- Haimovici, R.; Koh, S.; Gagnon, D.; Lehrfeld, T.; Wellik, S. Risk factors for central serous chorioretinopathy: A case–control study. Ophthalmology 2004, 111, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Piccolino, F.C.; De La Longrais, R.R.; Manea, M.; Cicinelli, S. Posterior Cystoid Retinal Degeneration in Central Serous Chorioretinopathy. Retina 2008, 28, 1008–1012. [Google Scholar] [CrossRef] [PubMed]
- Chhablani, J.; Behar-Cohen, F.; Central Serous Chorioretinopathy International Group. Validation of central serous chorioretinopathy multimodal imaging-based classification system. Graefes Arch. Clin. Exp. Ophthalmol. 2021; online ahead of print. [Google Scholar] [CrossRef]
- Lee, G.-I.; Kim, A.Y.; Kang, S.W.; Cho, S.C.; Park, K.H.; Kim, S.J.; Kim, K.T. Risk Factors and Outcomes of Choroidal Neovascularization Secondary to Central Serous Chorioretinopathy. Sci. Rep. 2019, 9, 3927. [Google Scholar] [CrossRef] [Green Version]
- Fung, A.T.; Yannuzzi, L.A.; Freund, K. Type 1 (sub-retinal pigment epithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degeneration. Retina 2012, 32, 1829–1837. [Google Scholar] [CrossRef]
- Biçer, Ö; Batıoğlu, F.; Demirel, S.; Özmert, E. Multimodal Imaging in Pachychoroid Neovasculopathy: A Case Report. Turk. J. Ophthalmol. 2018, 48, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, R.M.; Downes, S.M.; Stanga, P.E.; Koh, A.H.; Vingerling, J.R.; Bird, A.C. Polypoidal choroidal vasculopathy and central serous chorioretinopathy. Ophthalmology 2001, 108, 1009–1010. [Google Scholar] [CrossRef]
- Guyer, D.R.; Yannuzzi, L.A.; Slakter, J.S.; Sorenson, J.A.; Ho, A.; Orlock, D. Digital Indocyanine Green Videoangiography of Central Serous Chorioretinopathy. Arch. Ophthalmol. 1994, 112, 1057–1062. [Google Scholar] [CrossRef]
- Anantharaman, G.; Sheth, J.; Bhende, M.; Narayanan, R.; Natarajan, S.; Rajendran, A.; Manayath, G.; Sen, P.; Biswas, R.; Banker, A.; et al. Polypoidal choroidal vasculopathy: Pearls in diagnosis and management. Indian J. Ophthalmol. 2018, 66, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Yannuzzi, L.A.; Sorenson, J.; Spaide, R.F.; Lipson, B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.W.; Wong, T.Y.; Cheung, C.M.G. Polypoidal Choroidal Vasculopathy in Asians. J. Clin. Med. 2015, 4, 782–821. [Google Scholar] [CrossRef]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus Nomenclature for reporting neovascular age-related macular degeneration data: Consensus on neovascular age related macular degeneration nomenclature study group. Ophthalmology 2020, 127, 616–636. [Google Scholar] [CrossRef]
- Serra, R.; Coscas, F.; Pinna, A.; Cabral, D.; Coscas, G.; Souied, E.H. Quantitative optical coherence tomography angiography features of inactive macular neovascularization in age-related macular degeneration. Retina 2021, 41, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Coscas, F.; Pinna, A.; Cabral, D.; Coscas, G.; Souied, E.H. Fractal analysis of polypoidal choroidal neovascularisation in age-related macular degeneration. Br. J. Ophthalmol. 2021, 105, 1421–1426. [Google Scholar] [CrossRef]
- Romdhane, K.; Zola, M.; Matet, A.; Daruich, A.; Elalouf, M.; Behar-Cohen, F.; Mantel, I. Predictors of treatment response to intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy for choroidal neovascularisation secondary to chronic central serous chorioretinopathy. Br. J. Ophthalmol. 2020, 104, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Romdhane, K.; Mantel, I. Choroidal Neovascularisation Complicating Chronic Central Serous Chorioretinopathy: The Discovery Rate on Multimodal Imaging. Klin. Mon. Für Augenheilkd. 2019, 236, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheikh, M.; Iafe, N.A.; Phasukkjiwatana, N.; Sadda, S.R.; Sarraf, D. Biomarkers of neovascular activity in age-related macular degeneration using optical coherence tomography angiography. Retina 2018, 38, 220–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forte, R.; Coscas, F.; Serra, R.; Cabral, D.; Colantuono, D.; Souied, E.H. Long-term follow-up of quiescent choroidal neovascularisation associated with age-related macular degeneration or pachychoroid disease. Br. J. Ophthalmol. 2020, 104, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Garrity, S.T.; Sarraf, D.; Freund, K.B.; Sadda, S.R. Multimodal Imaging of Nonneovascular Age-Related Macular Degeneration. Investig. Opthalmol. Vis. Sci. 2018, 59, AMD48–AMD64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pece, A.; Bolognesi, G.; Introini, U.; Pacelli, G.; Calori, G.; Brancato, R. Indocyanine green angiography of well-defined plaque choroidal neovascularization in age-related macular degeneration. Arch. Ophthalmol. 2000, 118, 630–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man. Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Maftouhi, M.Q.-E.; El Maftouhi, A.; Eandi, C.M. Chronic Central Serous Chorioretinopathy Imaged by Optical Coherence Tomographic Angiography. Am. J. Ophthalmol. 2015, 160, 581.e1–587.e1. [Google Scholar] [CrossRef]
- Manayath, G.J.; Ranjan, R.; Shah, V.S.; Karandikar, S.S.; Saravanan, V.R.; Narendran, V. Central serous chorioretinopathy: Current update on pathophysiology and multimodal imaging. Oman J. Ophthalmol. 2018, 11, 103–112. [Google Scholar] [CrossRef]
- Sartini, F.; Figus, M.; Casini, G.; Nardi, M.; Posarelli, C. Pachychoroid neovasculopathy: A type-1 choroidal neovascularization belonging to the pachychoroid spectrum—Pathogenesis, imaging and available treatment options. Int. Ophthalmol. 2020, 40, 3577–3589. [Google Scholar] [CrossRef]
- Spaide, R.F.; Cheung, C.M.G.; Matsumoto, H.; Kishi, S.; Boon, C.J.; van Dijk, E.H.; Mauget-Faysse, M.; Behar-Cohen, F.; Hartnett, M.E.; Sivaprasad, S.; et al. Venous overload choroidopathy: A hypothetical framework for central serous chorioretinopathy and allied disorders. Prog. Retin. Eye Res. 2022, 86, 100973. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, E.; Provost, J.; Zola, M.; Spaide, R.F.; Mehanna, C.; Behar-Cohen, F. Mid-Phase Hyperfluorescent Plaques Seen on Indocyanine Green Angiography in Patients with Central Serous Chorioretinopathy. J. Clin. Med. 2021, 10, 4525. [Google Scholar] [CrossRef] [PubMed]
- Chhablani, J.; Kozak, I.; Pichi, F.; Chenworth, M.; Berrocal, M.H.; Bedi, R.; Singh, R.P.; Wu, L.; Meyerle, C.; Casella, A.M.; et al. Outcomes of treatment of choroidal neovascularization associated with central serous chorioretinopathy with intravitreal antiangiogenic agents. Retina 2015, 35, 2489–2497. [Google Scholar] [CrossRef]
- Lafaut, B.A.; Leys, A.M.; Snyers, B.; Rasquin, F.; De Laey, J.J. Polypoidal choroidal vasculopathy in Caucasians. Graefe’s Arch. Clin. Exp. Ophthalmol. 2000, 238, 752–759. [Google Scholar] [CrossRef]
- Scassellati-Sforzolini, B.; Mariotti, C.; Bryan, R.; Yannuzzi, L.A.; Giuliani, M.; Giovannini, A. Polypoidal choroidal vasculopathy in Italy. Retina 2001, 21, 121–125. [Google Scholar] [CrossRef]
- Lee, W.K.; Baek, J.; Dansingani, K.K.; Lee, J.H.; Freund, K.B. Choroidal morphology in eyes with polypoidal choroidal vasculopathy and normal or subnormal subfoveal choroidal thickness. Retina 2016, 36 (Suppl. 1), S73–S82. [Google Scholar] [CrossRef] [PubMed]
- Coscas, F.; Lupidi, M.; Boulet, J.F.; Sellam, A.; Cabral, D.R.; Serra, R.; Français, C.; Souied, E.H.; Coscas, G. Optical coherence tomography angiography in exudative age-related macular degeneration: A predictive model for treatment decisions. Br. J. Ophthalmol. 2019, 103, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki, J.; Schworm, B.; Priglinger, S.G. The Pachychoroid Disease Spectrum—And the Need for a Uniform Classification System. Ophthalmol. Retin. 2019, 3, 1013–1015. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.; Duan, J.; Zhang, M. Pachychoroid Spectrum Disease: Underlying Pathology, Classification, and Phenotypes. Curr. Eye Res. 2021, 46, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Coscas, F.; Boulet, J.F.; Cabral, D.R.; Lupidi, M.; Coscas, G.J.; Souied, E.H. Predictive activation biomarkers of treatment-naive asymptomatic choroidal neovascularization in age-related macular degeneration. Retina 2020, 40, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Rebhun, C.B.; Moult, E.M.; Novais, E.A.; Moreira-Neto, C.; Ploner, S.B.; Louzada, R.N.; Lee, B.; Baumal, C.R.; Fujimoto, J.G.; Duker, J.S.; et al. Polypoidal Choroidal Vasculopathy on Swept-Source Optical Coherence Tomography Angiography with Variable Interscan Time Analysis. Transl. Vis. Sci. Technol. 2017, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Munk, M.R.; Giannakaki-Zimmermann, H.; Berger, L.; Huf, W.; Ebneter, A.; Wolf, S.; Zinkernagel, M.S. OCT-angiography: A qualitative and quantitative comparison of 4 OCT-A devices. PLoS ONE 2017, 12, e0177059. [Google Scholar] [CrossRef]
- Mastropasqua, R.; Evangelista, F.; Amodei, F.; D’Aloisio, R.; Pinto, F.; Doronzo, E.; Viggiano, P.; Porreca, A.; Di Nicola, M.; Parravano, M.; et al. Optical Coherence Tomography Angiography in Macular Neovascularization: A Comparison Between Different OCTA Devices. Transl. Vis. Sci. Technol. 2020, 9, 6. [Google Scholar] [CrossRef]
- Corvi, F.; Cozzi, M.; Barbolini, E.; Nizza, D.; Belotti, M.; Staurenghi, G.; Giani, A. Comparison between several optical coherence tomography angiography devices and indocyanine green angiography of choroidal neovascularization. Retina 2020, 40, 873–880. [Google Scholar] [CrossRef]
| Acute CSCR | Complex CSCR | p Value | |
|---|---|---|---|
| Total eyes, n (%) | 40 (39.21%) | 62 (60.79%) | - |
| Sex | |||
| - Male, n (%) | 32 (80%) | 40 (64.51%) | - |
| - Female, n (%) | 8 (20%) | 22 (35.49%) | - |
| Age, mean ± SD (years) | 52.20 ± 9.52 | 68.27 ± 10 | <0.0001 |
| BCVA, mean ± SD (ETDRS letters) | 87.06 ± 18.58 | 79.61 ± 23.32 | 0.03 |
| Type 1 CNV | PCV | p Value | |
|---|---|---|---|
| VPD, mean ± SD (%) | 0.53 ± 0.23 | 0.52 ± 0.19 | 0.98 |
| FD, mean ± SD | 1.46 ± 0.15 | 1.43 ± 0.10 | 0.61 |
| LAC, mean ± SD | 2.10 ± 0.49 | 2.53 ± 1.26 | 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, R.; Pinna, A.; Behar-Cohen, F.; Coscas, F. OCT Angiography Fractal Analysis of Choroidal Neovessels Secondary to Central Serous Chorioretinopathy, in a Caucasian Cohort. J. Clin. Med. 2022, 11, 1443. https://doi.org/10.3390/jcm11051443
Serra R, Pinna A, Behar-Cohen F, Coscas F. OCT Angiography Fractal Analysis of Choroidal Neovessels Secondary to Central Serous Chorioretinopathy, in a Caucasian Cohort. Journal of Clinical Medicine. 2022; 11(5):1443. https://doi.org/10.3390/jcm11051443
Chicago/Turabian StyleSerra, Rita, Antonio Pinna, Francine Behar-Cohen, and Florence Coscas. 2022. "OCT Angiography Fractal Analysis of Choroidal Neovessels Secondary to Central Serous Chorioretinopathy, in a Caucasian Cohort" Journal of Clinical Medicine 11, no. 5: 1443. https://doi.org/10.3390/jcm11051443
APA StyleSerra, R., Pinna, A., Behar-Cohen, F., & Coscas, F. (2022). OCT Angiography Fractal Analysis of Choroidal Neovessels Secondary to Central Serous Chorioretinopathy, in a Caucasian Cohort. Journal of Clinical Medicine, 11(5), 1443. https://doi.org/10.3390/jcm11051443






