Expression of Macrophage Scavenger Receptor (MSR1) in Peripheral Blood Cells from Patients with Different Respiratory Diseases: Beyond Monocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design: Subjects
2.2. Isolation of PBMCs and RNA Extraction
2.3. Differential Gene Expression Analysis
2.4. MSR1 Expression in Peripheral Samples by Confocal Microscopy and Flow Cytometry
2.5. Statistical Analysis
3. Results
3.1. Subjects
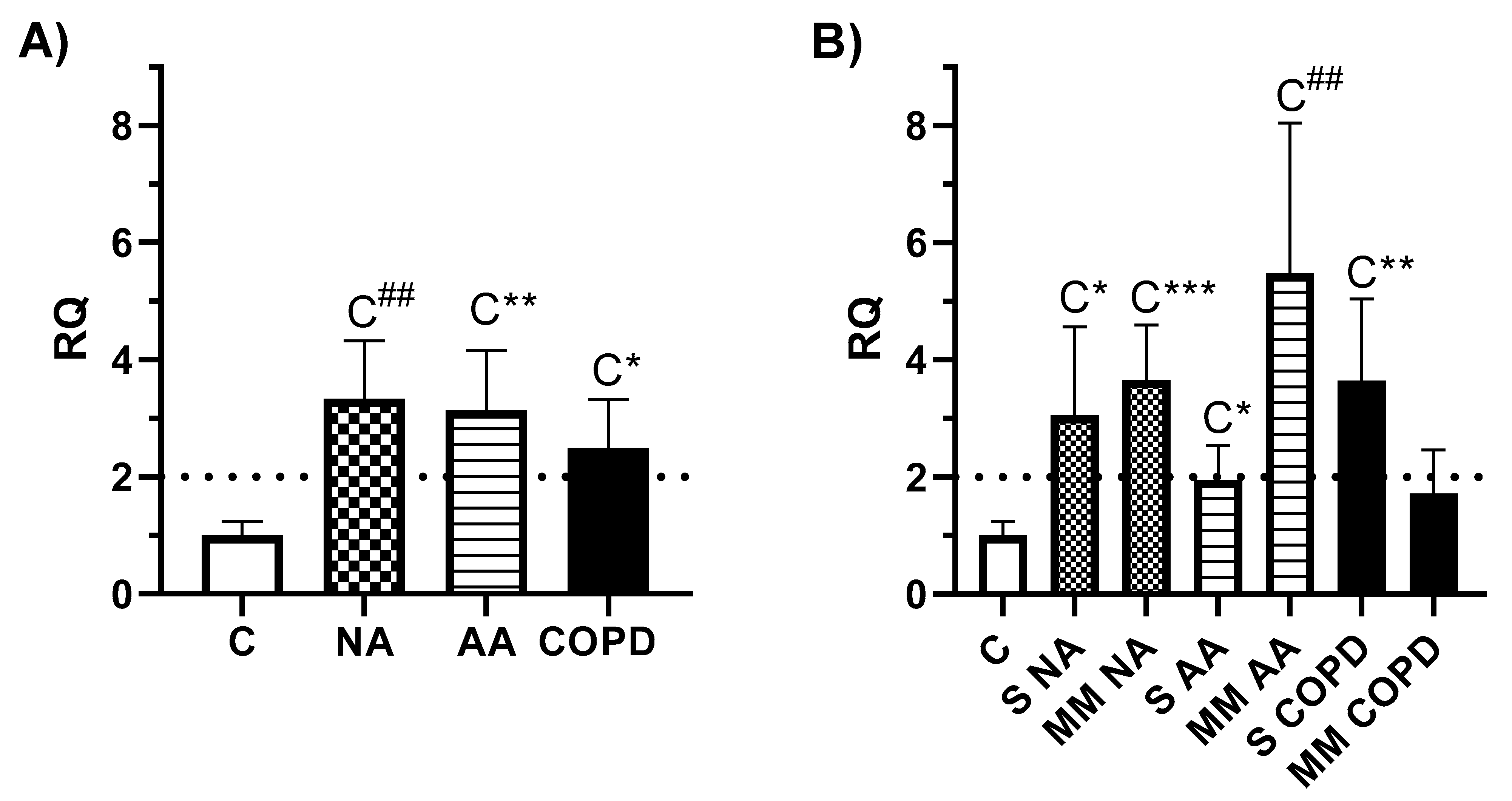
3.2. Gene expression Analysis
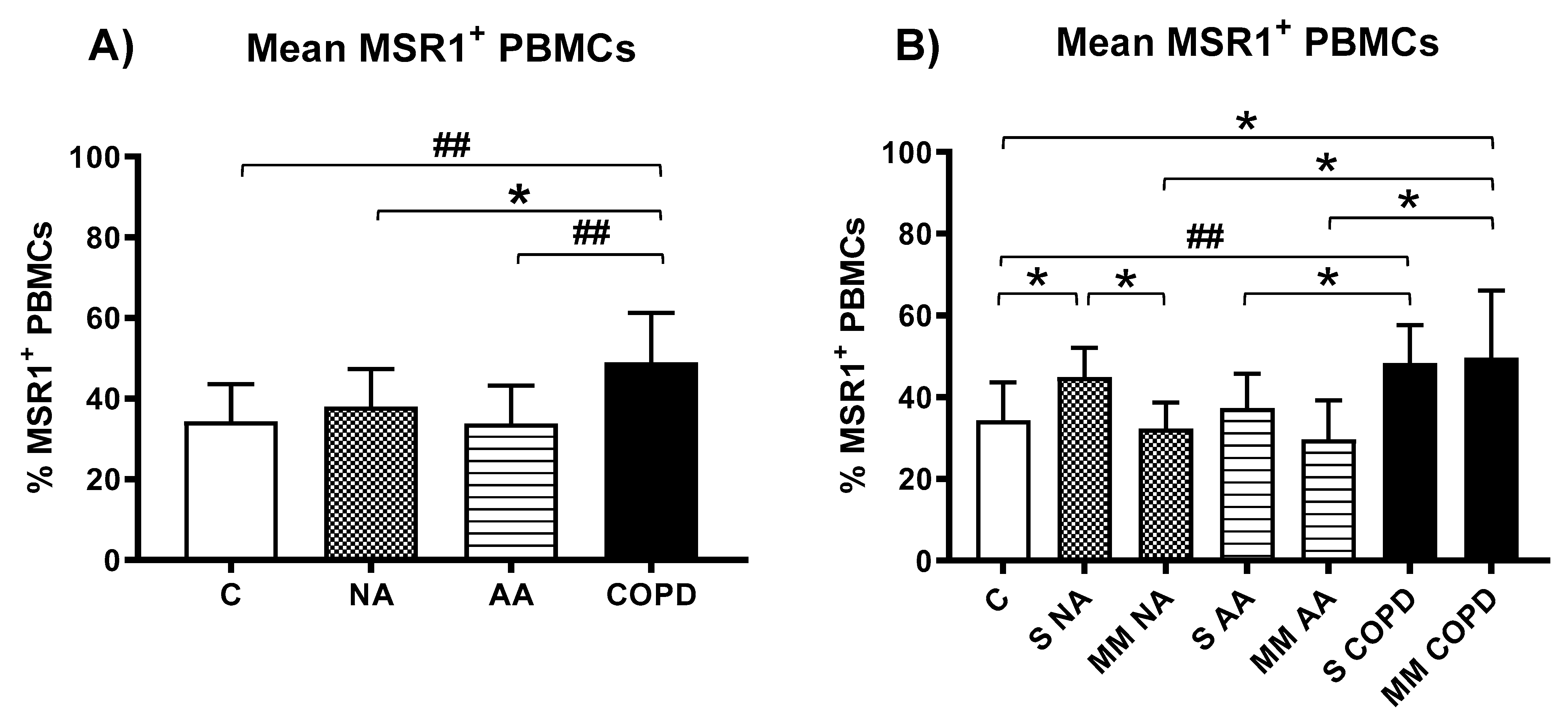
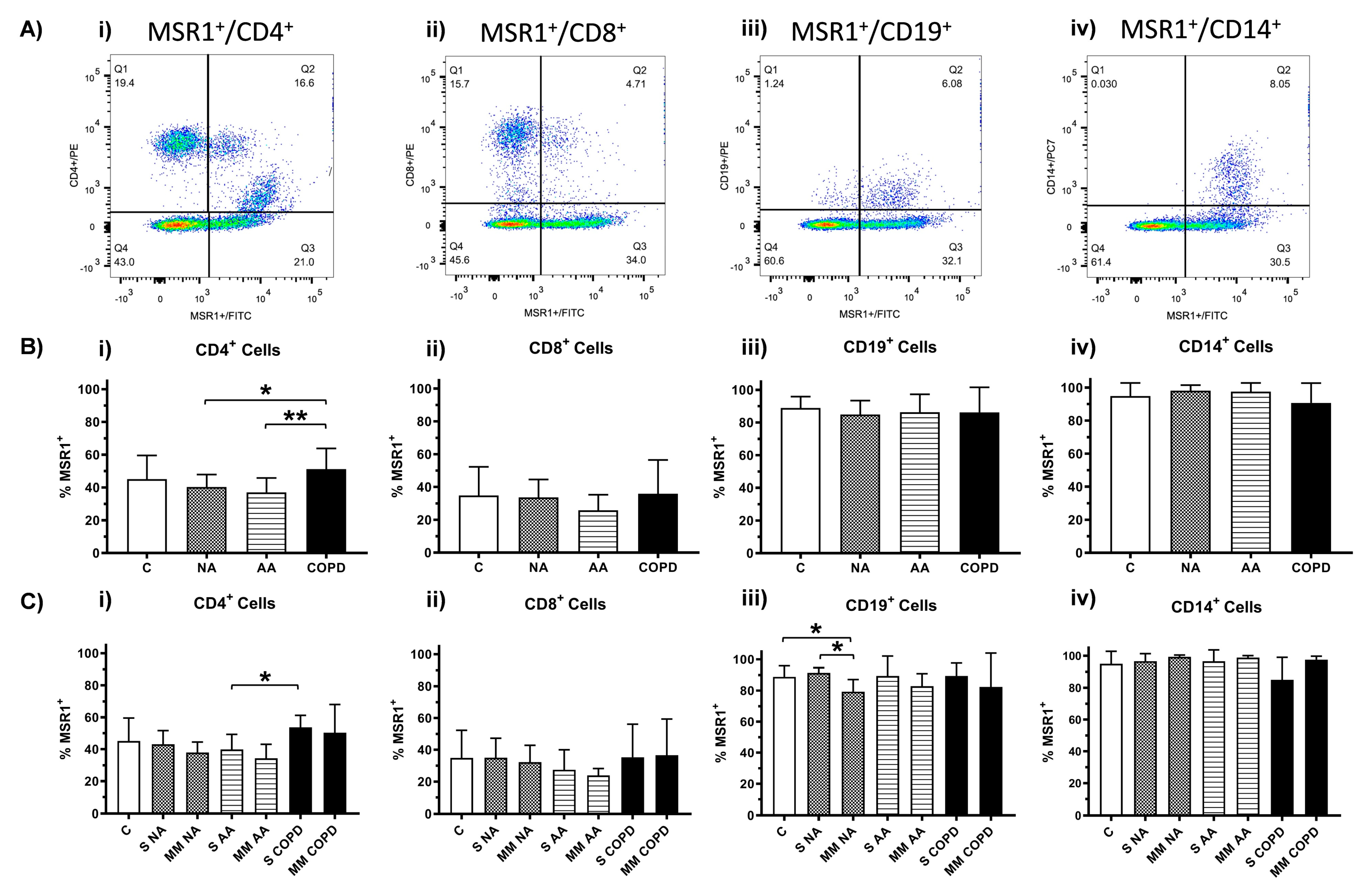
3.3. Expression of MSR1 on Different Subpopulations of PBMCs
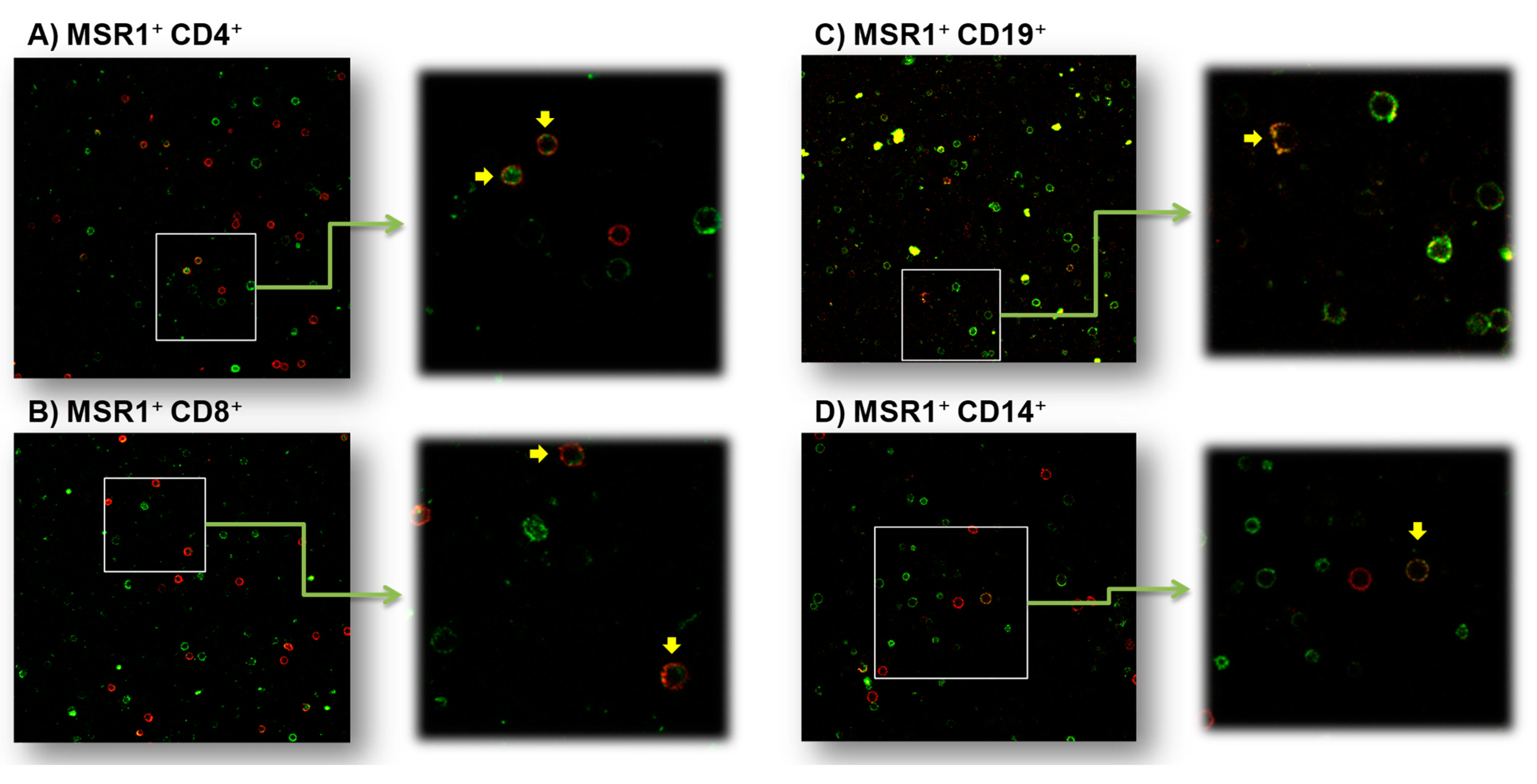
3.3.1. MSR1 Expression in PBMCs by Confocal Microscopy
3.3.2. MSR1 Expression in PBMCs by Flow Cytometry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldstein, J.L.; Ho, Y.K.; Basu, S.K.; Brown, M.S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc. Natl. Acad. Sci. USA 1979, 76, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Naito, M.; Kodama, T.; Matsumoto, A.; Doi, T.; Takahashi, K. Tissue distribution, intracellular localization, and in vitro expression of bovine macrophage scavenger receptors. Am. J. Pathol. 1991, 139, 1411–1423. [Google Scholar] [PubMed]
- Takahashi, K.; Takeya, M.; Sakashita, N. Multifunctional roles of macrophages in the development and progression of atherosclerosis in humans and experimental animals. Med. Electron. Microsc. 2002, 35, 179–203. [Google Scholar] [CrossRef]
- Tomokiyo, R.; Jinnouchi, K.; Honda, M.; Wada, Y.; Hanada, N.; Hiraoka, T.; Suzuki, H.; Kodama, T.; Takahashi, K.; Takeya, M. Production, characterization, and interspecies reactivities of monoclonal antibodies against human class A macrophage scavenger receptors. Atherosclerosis 2002, 161, 123–132. [Google Scholar] [CrossRef]
- Nakayama, M.; Kudoh, T.; Kaikita, K.; Yoshimura, M.; Oshima, S.; Miyamoto, Y.; Takeya, M.; Ogawa, H. Class A macrophage scavenger receptor gene expression levels in peripheral blood mononuclear cells specifically increase in patients with acute coronary syndrome. Atherosclerosis 2008, 198, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Piechota, M.; Banaszewska, A.; Dudziak, J.; Slomczynski, M.; Plewa, R. Highly upregulated expression of CD36 and MSR1 in circulating monocytes of patients with acute coronary syndromes. Protein. J. 2012, 31, 511–518. [Google Scholar] [CrossRef]
- Liu, H.; Ding, J.; Ma, Z.; Zhu, Z.; Shankowsky, H.A.; Tredget, E.E. A novel subpopulation of peripheral blood mononuclear cells presents in major burn patients. Burns 2015, 41, 998–1007. [Google Scholar] [CrossRef]
- Higashi-Kuwata, N.; Jinnin, M.; Makino, T.; Fukushima, S.; Inoue, Y.; Muchemwa, F.C.; Yonemura, Y.; Komohara, Y.; Takeya, M.; Mitsuya, H.; et al. Characterization of monocyte/macrophage subsets in the skin and peripheral blood derived from patients with systemic sclerosis. Arthritis Res. Ther. 2010, 12, R128. [Google Scholar] [CrossRef]
- Kelley, J.L.; Ozment, T.R.; Li, C.; Schweitzer, J.B.; Williams, D.L. Scavenger receptor-A (CD204): A two-edged sword in health and disease. Crit. Rev. Immunol. 2014, 34, 241–261. [Google Scholar] [CrossRef]
- Krieger, M. The other side of scavenger receptors: Pattern recognition for host defense. Curr. Opin. Lipidol. 1997, 8, 275–280. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Plüddemann, A.; Gordon, S. Macrophage pattern recognition in immunity, homeostasis and self tolerance. Adv. Exp. Med. Biol. 2009, 653, 1–14. [Google Scholar] [PubMed]
- Canton, J.; Neculai, D.; Grinstein, S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 2013, 13, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Todt, J.C.; Hu, B.; Curtis, J.L. The scavenger receptor SR-A I/II (CD204) signals via the receptor tyrosine kinase Mertk during apoptotic cell uptake by murine macrophages. J. Leukoc. Biol. 2008, 84, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ha, T.; Liu, L.; Wang, X.; Gao, M.; Kelley, J.; Kao, R.; Williams, D.; Lia, C. Scavenger receptor A (SR-A) is required for LPS-induced TLR4 mediated NF-κB activation in macrophages. Biochim. Biophys. Acta 2012, 1823, 1192–1198. [Google Scholar] [CrossRef]
- Haworth, R.; Platt, N.; Kesjav, S.; Hughes, D.; Darley, E.; Suzuki, H.; Kurihara, Y.; Kodama, T.; Gordon, S. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J Exp. Med. 1997, 186, 1431–1439. [Google Scholar] [CrossRef]
- Ohnishi, K.; Komohara, Y.; Fujiwara, Y.; Takemura, K.; Lei, X.; Nakagawa, T.; Sakashita, N.; Takeya, M. Suppression of TLR4-mediated inflammatory response by macrophage class A scavenger receptor (CD204). Biochem. Biophys. Res. Commun. 2011, 411, 516–522. [Google Scholar] [CrossRef]
- Yu, X.; Yi, H.; Guo, C.; Zuo, D.; Wang, Y.; Kim, H.L.; Subjeck, J.R.; Wang, X.-Y. Pattern recognition scavenger receptor CD204 attenuates Toll-like receptor 4-induced NFkappaB activation by directly inhibiting ubiquitination of tumor necrosis factor (TNF) receptor-associated factor 6. J. Biol. Chem. 2011, 286, 18795–18806. [Google Scholar] [CrossRef]
- Ozment, T.R.; Ha, T.; Breuel, K.F.; Ford, T.R.; Ferguson, D.A.; Kalbfleisch, J.; Schweitzer, J.B.; Kelley, J.L.; Li, C.; Williams, D.L. Scavenger receptor class a plays a central role in mediating mortality and the development of the proinflammatory phenotype in polymicrobial sepsis. PLoS Pathog. 2012, 8, e1002967. [Google Scholar] [CrossRef]
- Drummond, R.; Cauvi, D.M.; Hawisher, D.; Song, D.; Nino, D.F.; Coimbra, R.; Bickler, S.; De Maio, A. Deletion of scavenger receptor A gene in mice resulted in protection from septic shock and modulation of TLR4 signaling in isolated peritoneal macrophages. Innate Immun. 2013, 19, 30–41. [Google Scholar] [CrossRef]
- Tsujita, K.; Kaita, K.; Hayasaki, T.; Honda, T.; Kobayashi, H.; Sakashita, N.; Suzuki, H.; Kodama, T.; Ogawa, H.; Takeya, M. Targeted deletion of class A macrophage scavenger receptor increases the risk of cardiac rupture after experimental myocardial infarction. Circulation 2007, 115, 1904–1911. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, H.; Lu, Y.; Bai, H.; Xu, Y.; Zhu, X.; Zhou, R.; Ben, J.; Xu, Y.; Chen, Q. Class A scavenger receptor attenuates myocardial infarction-induced cardiomyocyte necrosis through suppressing M1 macrophage subset polarization. Basic Res. Cardiol. 2011, 106, 1311–1328. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Wang, X.; Ha, T.; Liu, L.; Kalbfleisch, J.; Gao, X.; Williams, D.; Li, C. SR-A deficiency reduces myocardial ischemia/reperfusion injury; involvement of increased microRNA-125b expression in macrophages. Biochim. Biophys. Acta 2013, 1832, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, K.; El Khoury, J. Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer’s disease. Int. J. Alzheimers Dis. 2012, 489456. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, J.; Hickman, S.E.; Thomas, C.A.; Cao, L.; Silverstein, S.C.; Loike, J.D. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature 1996, 382, 716–719. [Google Scholar] [CrossRef]
- Xu, Y.; Qian, L.; Zong, G.; Ma, K.; Zhu, X.; Zhang, H.; Li, N.; Yang, Q.; Bai, H.; Ben, J.; et al. Class A scavenger receptor promotes cerebral ischemic injury by pivoting microglia/macrophage polarization. Neuroscience 2012, 218, 35–48. [Google Scholar] [CrossRef]
- Lu, C.; Hua, F.; Liu, L.; Ha, T.; Kalbfleisch, J.; Schweitzer, J.; Kelley, J.; Kao, R.; Williams, D.; Li, C. Scavenger receptor class-A has a central role in cerebral ischemia-reperfusion injury. J. Cereb. Blood Flow Metab. 2010, 30, 1972–1981. [Google Scholar] [CrossRef]
- Thomsen, M.; Nordestgaard, B.G.; Tybjaerg-Hansen, A.; Dahl, M. Scavenger receptor AI/II truncation, lung function and COPD: A large population-based study. J. Intern. Med. 2011, 269, 340–348. [Google Scholar] [CrossRef]
- Ohar, J.A.; Hamilton Jr, R.F.; Zheng, S.; Sadeghnejad, A.; Sterling, D.A.; Xu, J.; Meyers, D.A.; Bleecker, E.R.; Holian, A. COPD is associated with a macrophage scavenger receptor-1 gene sequence variation. Chest 2010, 137, 1098–1107. [Google Scholar] [CrossRef][Green Version]
- Kaku, Y.; Imaoka, H.; Morimatsu, Y.; Komohara, Y.; Ohnishi, K.; Oda, H.; Takenaka, S.; Matsuoka, M.; Kawayama, T.; Takeya, M.; et al. Overexpression of CD163, CD204 and CD206 on Alveolar Macrophages in the Lungs of Patients with Severe Chronic Obstructive Pulmonary Disease. PLoS ONE 2014, 9, e87400. [Google Scholar] [CrossRef]
- Kobayashi, H.; Sakashita, N.; Okuma, T.; Terasaki, Y.; Tsujita, K.; Suzuki, H.; Kodama, T.; Nomori, H.; Kawasuji, M.; Takeya, M. Class A scavenger receptor (CD204) attenuates hyperoxia-induced lung injury by reducing oxidative stress. J. Pathol. 2007, 212, 38–46. [Google Scholar] [CrossRef]
- Arredouani, M.S.; Franco, F.; Imrich, A.; Fedulov, A.; Lu, X.; Perkins, D.; Soininen, R.; Tryggvason, K.; Shapiro, S.D.; Kobzik, L. Scavenger Receptors SR-AI/II and MARCO limit pulmonary dendritic cell migration and allergic airway inflammation. J. Immunol. 2007, 178, 5912–5920. [Google Scholar] [CrossRef]
- Baos, S.; Calzada, D.; Cremades, L.; Sastre, J.; Quiralte, J.; Florido, F.; Lahoz, C.; Cárdaba, B. Biomarkers associated with disease severity in allergic and nonallergic asthma. Mol. Immunol. 2017, 82, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Baos, S.; Calzada, D.; Cremades-Jimeno, L.; Sastre, J.; Picado, C.; Quiralte, J.; Florido, F.; Lahoz, C.; Cárdaba, B. Nonallergic asthma and its severity: Biomarkers for its discrimination in peripheral samples. Front. Immunol. 2018, 9, 1416. [Google Scholar] [CrossRef] [PubMed]
- Baos, S.; Calzada, D.; Cremades-Jimeno, L.; de Pedro, M.A.; Sastre, J.; Picado, C.; Quiralte, J.; Florido, F.; Lahoz, C.; Cárdaba, B. Discriminatory molecular biomarkers of allergic and nonallergic asthma and its severity. Front. Immunol. 2019, 10, 1051. [Google Scholar] [CrossRef] [PubMed]
- Baos, S.; Calzada, D.; Cremades, L.; Sastre, J.; Quiralte, J.; Florido, F.; Lahoz, C.; Cárdabaab, B. Data set on a study of gene expression in peripheral simples to identify biomarkers of severity of allergic and nonallergic asthma. Data Brief. 2016, 10, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Plaza Moral, V. Comité Ejecutivo de GEMA. [GEMA (4.0) Guidelines for asthma management]. Arch. Bronconeumol. 2015, 51, 2–54. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martínez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Arch. Bronconeumol. 2017, 53, 128–149. [Google Scholar] [CrossRef]
- Desai, M.; Oppenheimer, J. Elucidating asthma phenotypes and endotypes: Progress towards personalized medicine. Ann. Allergy Asthma Immunol. 2016, 116, 394–401. [Google Scholar] [CrossRef]
- Muraro, A.; Lemanske Jr, R.F.; Hellings, P.W.; Akdis, C.A.; Bieber, T.; Casale, T.B.; Jutel, M.; Ong, P.Y.; Poulsen, L.K.; Schmid-Grendelmeier, P.; et al. Precision medicine in patients with allergic diseases: Airway diseases and atopic dermatitis-PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2016, 137, 1347–1358. [Google Scholar]
- Pavord, I.D.; Beasley, R.; Agusti, A.; Anderson, G.P.; Bel, E.; Brusselle, G.; Cullinan, P.; Custovic, A.; Ducharme, F.M.; Fahy, J.V.; et al. After asthma: Redefining airways diseases. Lancet 2018, 391, 350–400. [Google Scholar] [CrossRef]
- Agache, I.; Strasser, D.S.; Pierlot, G.M.; Farine, H.; Izuhara, K.; Akdis, C.A. Monitoring inflammatory heterogeneity with multiple biomarkers for multidimensional endotyping of asthma. J. Allergy Clin. Immunol. 2018, 141, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Aguerri, M.; Calzada, D.; Montaner, D.; Mata, M.; Florido, F.; Quiralte, J.; Dopazo, J.; Lahoz, C.; Cárdaba, B. Differential gene-expression analysis defines a molecular pattern related to olive pollen allergy. J. Biol. Regul. Homeost. Agents. 2013, 27, 337–350. [Google Scholar] [PubMed]
- Kong, S.L.; Chui, P.; Lim, B.; Salto-Tellez, M. Elucidating the molecular physiopathology of acute respiratory distress syndrome in severe acute respiratory syndrome patients. Virus Res. 2009, 145, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, L.; Barczyk, M.; Cardoso, J.; Schmidt, M.; Bellini, A.; Mattoli, S. Extracellular matrix remodelling properties of human fibrocytes. J. Cell. Mol. Med. 2012, 16, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, L.; Isgrò, M.; Marini, M.A.; Bellini, A.; Schmidt, M.; Mattoli, S. Enumeration of circulating fibrocytes for clinical use in asthma by an optimized single-platform flow cytometry assay. BBA Clin. 2014, 1, 52–58. [Google Scholar] [CrossRef]
- Shipe, R.; Burdick, M.D.; Strieter, B.A.; Liu, L.; Shim, Y.M.; Sung, S.; Teague, W.G.; Mehrad, B.; Strieter, R.M.; Rose, E., Jr. Number, activation, and differentiation of circulating fibrocytes correlate with asthma severity. J. Allergy Clin. Immunol. 2016, 137, 750–757.e3. [Google Scholar] [CrossRef]
- Wang, C.H.; Huang, C.D.; Lin, H.C.; Huang, T.T.; Lee, K.Y.; Lo, Y.L.; Lin, S.-M.; Chung, K.F.; Kuo, H.-P. Increased activation of fibrocytes in patients with chronic obstructive asthma through an epidermal growth factor receptor-dependent pathway. J. Allergy Clin. Immunol. 2012, 129, 1367–1376. [Google Scholar] [CrossRef]
- Lo, C.Y.; Michaeloudes, C.; Bhavsar, P.K.; Huang, C.D.; Wang, C.H.; Kuo, H.P.; Chung, K.F. Increased phenotypic differentiation and reduced corticosteroid sensitivity of fibrocytes in severe asthma. J. Allergy Clin. Immunol. 2015, 135, 1186–1195. [Google Scholar] [CrossRef]
| N | Gender | Age | Smoking Habit | Clinical Diagnosis | %FVC | %FEV1 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F (%) | M (%) | NS (%) | S (%) | ES (%) | Asthma/COPD | Allergy | |||||
| Control (C) subjects | 11 | 81.8 | 18.2 | 49.1 ± 14.5 * | 91 | 9.0 | 0 | 100% healthy | - | - | |
| Nonallergic asthmatic (NA) subjects | 11 | 81.8 | 18.2 | 56.2 ± 16.8 * | 63.6 | 9.1 | 27.3 | 45.5% SA | No-allergic symptoms | 98.1 ± 14.4 | 86.6 ± 23 * |
| 54.5% MMA | Negative-skin prick test | ||||||||||
| Asthmatic allergic (AA) subjects | 13 | 92.3 | 7.7 | 41.2 ± 13.5 #,† | 76.9 | 15.4 | 7.7 | 53.8% SA | 92.3%pollen allergy | 91.3 ± 24.8 | 75.85 ± 24.27 * |
| 46.2% MMA | 7.7% no pollen allergy | ||||||||||
| COPD subjects | 11 | 0 | 100 | 70.4 ± 7.9 | 9.1 | 9.1 | 81.8 | 54.5% SC | 9.1% pollen allergy | 76.2 ± 31.8 | 52 ± 22.1 |
| 45.5% MMC | 90.9% no allergies | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baos, S.; Cremades-Jimeno, L.; López-Ramos, M.; de Pedro, M.Á.; Uriarte, S.A.; Sastre, J.; González-Mangado, N.; Rodríguez-Nieto, M.J.; Peces-Barba, G.; Cárdaba, B. Expression of Macrophage Scavenger Receptor (MSR1) in Peripheral Blood Cells from Patients with Different Respiratory Diseases: Beyond Monocytes. J. Clin. Med. 2022, 11, 1439. https://doi.org/10.3390/jcm11051439
Baos S, Cremades-Jimeno L, López-Ramos M, de Pedro MÁ, Uriarte SA, Sastre J, González-Mangado N, Rodríguez-Nieto MJ, Peces-Barba G, Cárdaba B. Expression of Macrophage Scavenger Receptor (MSR1) in Peripheral Blood Cells from Patients with Different Respiratory Diseases: Beyond Monocytes. Journal of Clinical Medicine. 2022; 11(5):1439. https://doi.org/10.3390/jcm11051439
Chicago/Turabian StyleBaos, Selene, Lucía Cremades-Jimeno, María López-Ramos, María Ángeles de Pedro, Silvia A. Uriarte, Joaquín Sastre, Nicolás González-Mangado, María Jesús Rodríguez-Nieto, Germán Peces-Barba, and Blanca Cárdaba. 2022. "Expression of Macrophage Scavenger Receptor (MSR1) in Peripheral Blood Cells from Patients with Different Respiratory Diseases: Beyond Monocytes" Journal of Clinical Medicine 11, no. 5: 1439. https://doi.org/10.3390/jcm11051439
APA StyleBaos, S., Cremades-Jimeno, L., López-Ramos, M., de Pedro, M. Á., Uriarte, S. A., Sastre, J., González-Mangado, N., Rodríguez-Nieto, M. J., Peces-Barba, G., & Cárdaba, B. (2022). Expression of Macrophage Scavenger Receptor (MSR1) in Peripheral Blood Cells from Patients with Different Respiratory Diseases: Beyond Monocytes. Journal of Clinical Medicine, 11(5), 1439. https://doi.org/10.3390/jcm11051439






