Adverse Events Associated with BNT162b2 and AZD1222 Vaccines in the Real World: Surveillance Report in a Single Italian Vaccine Center
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Measurements
2.3. Statistical Analysis
3. Results
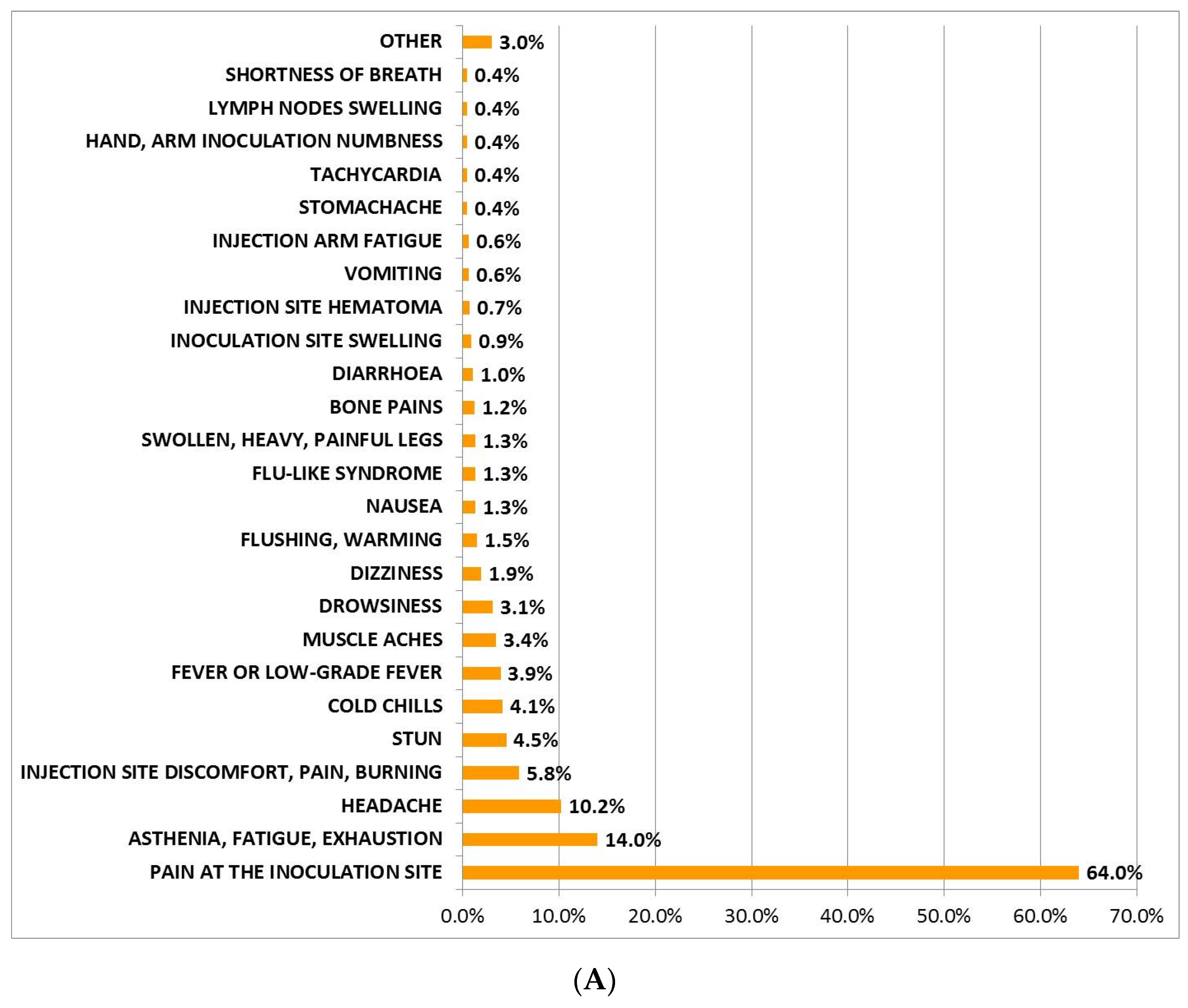
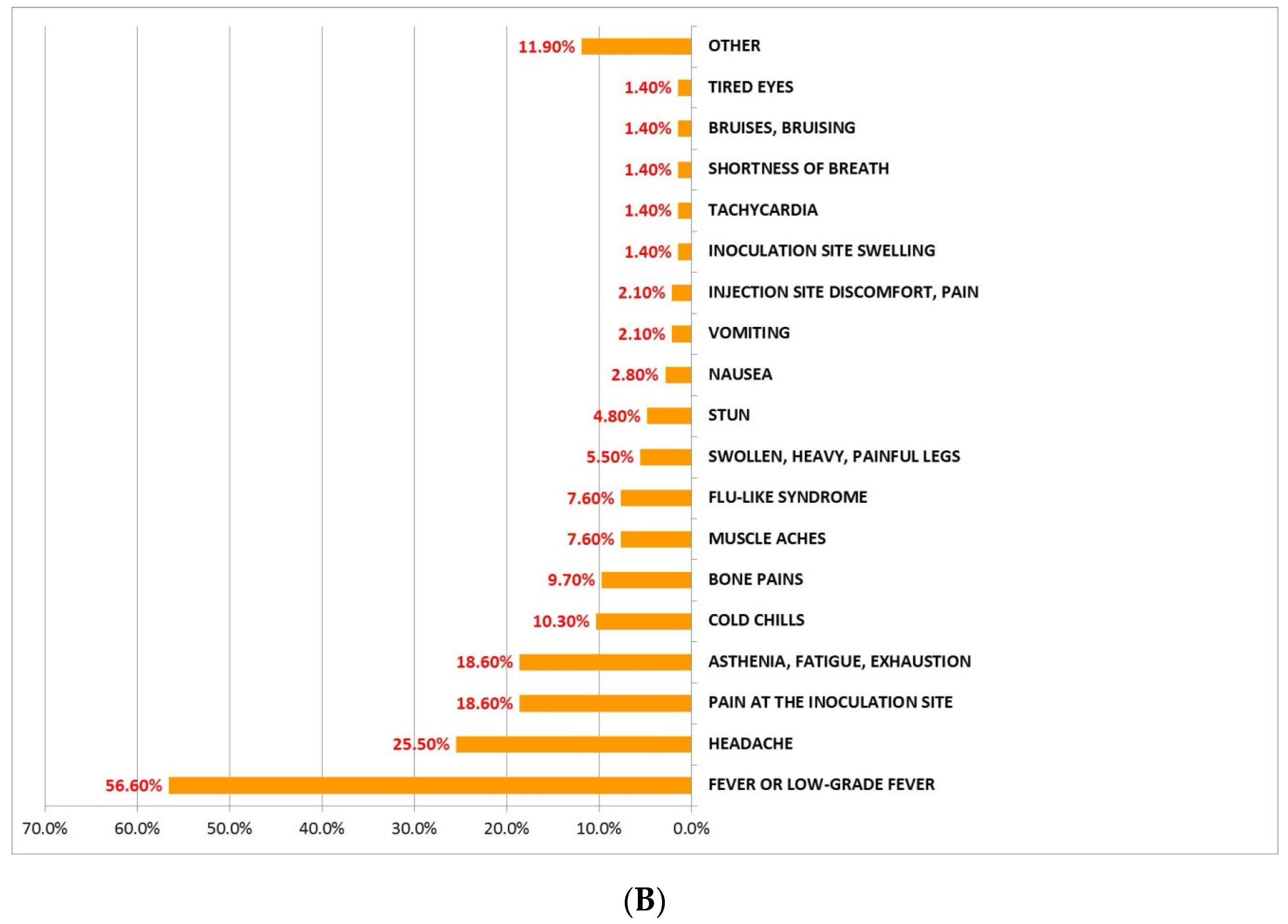
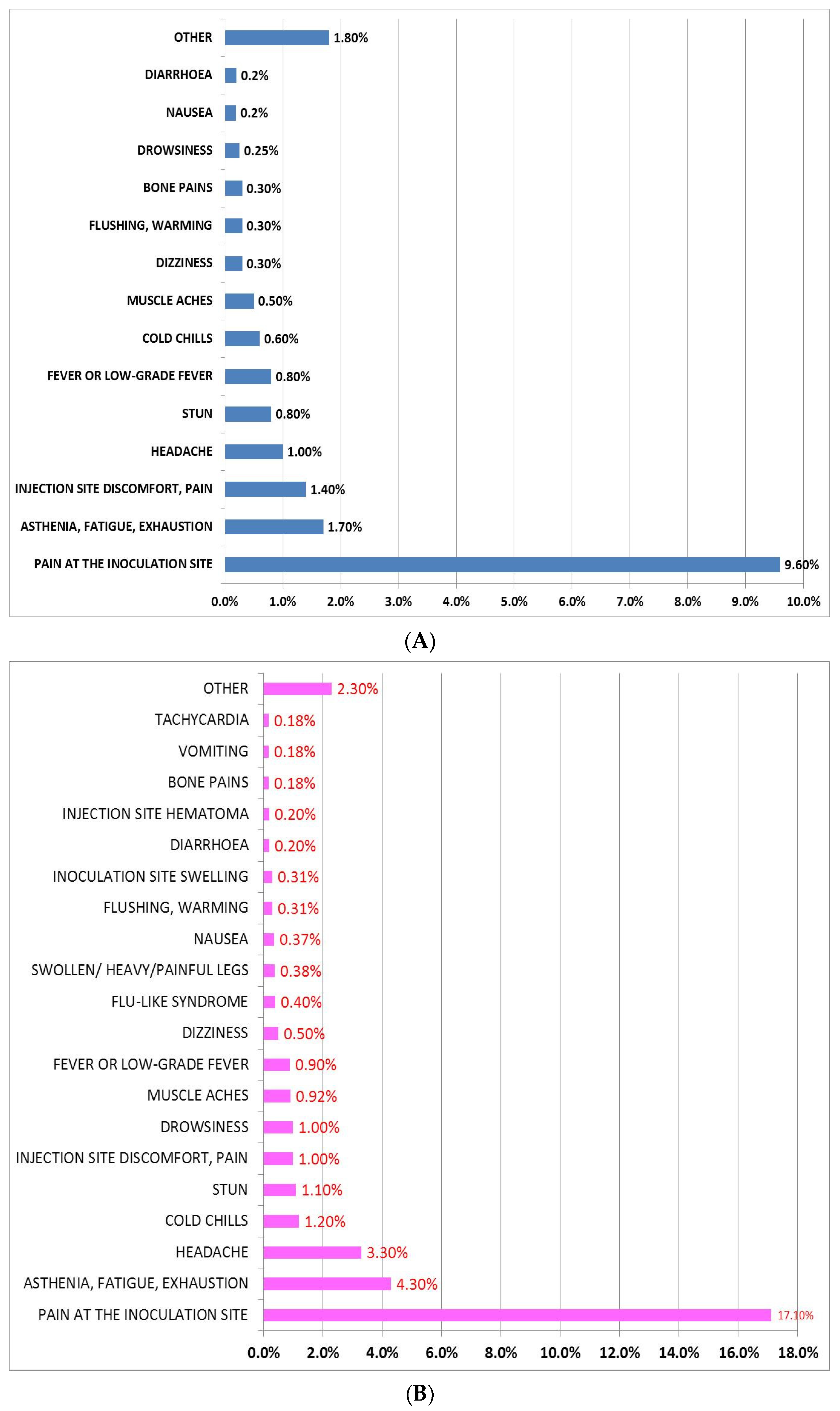
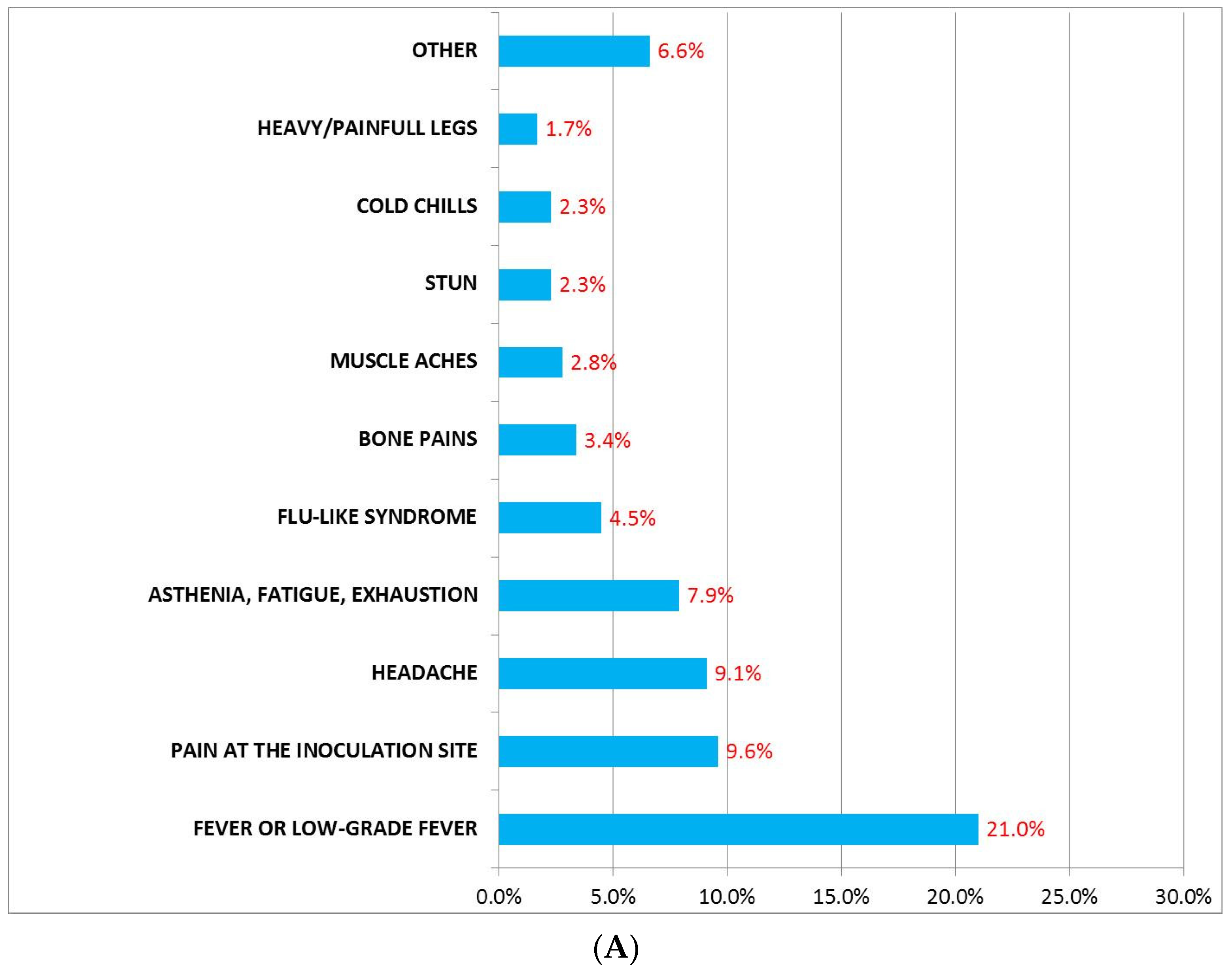
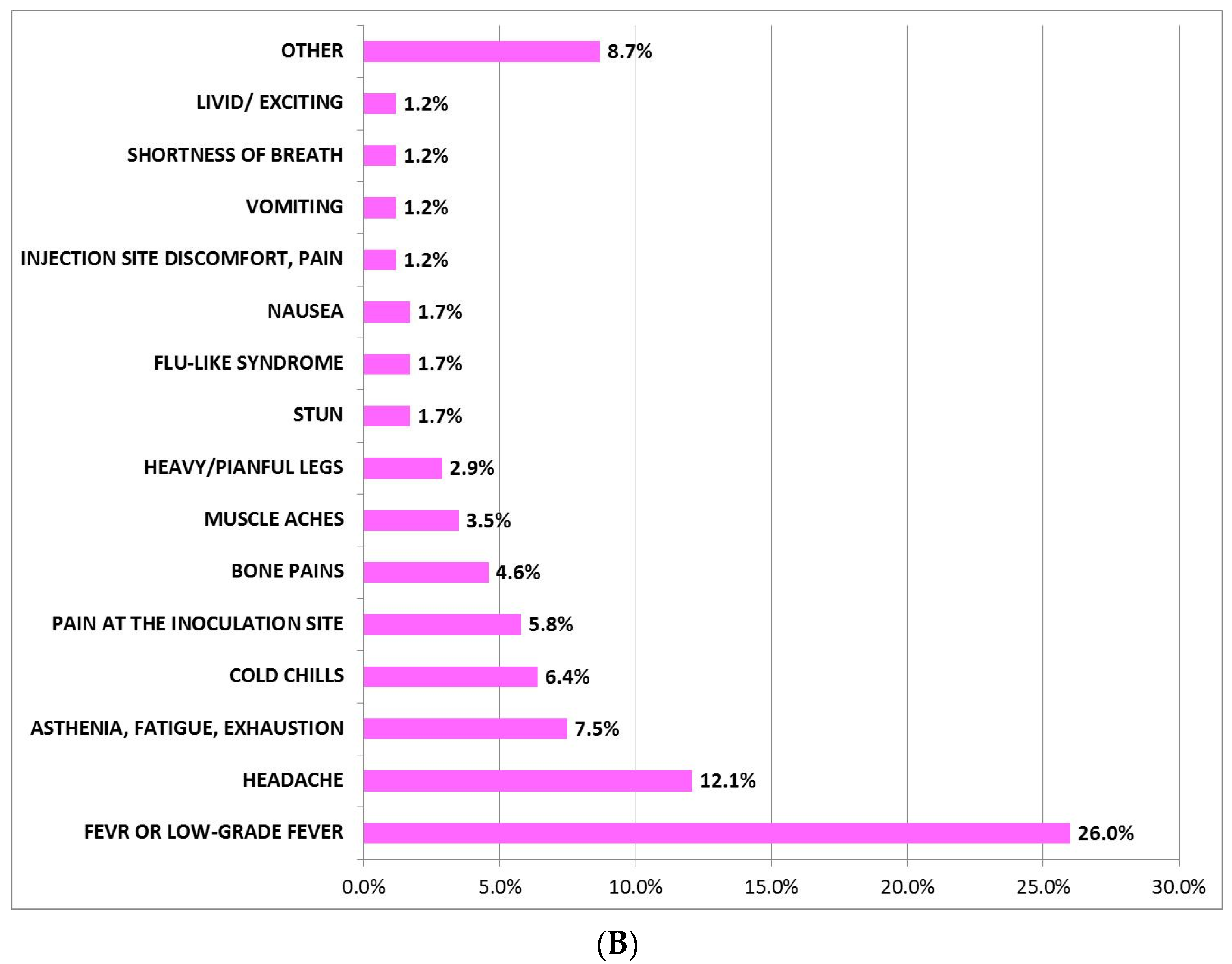
3.1. Incidence, Description, and Mean Duration of AEFI after Administration of BNT162b2 and AZD1222 First Dose
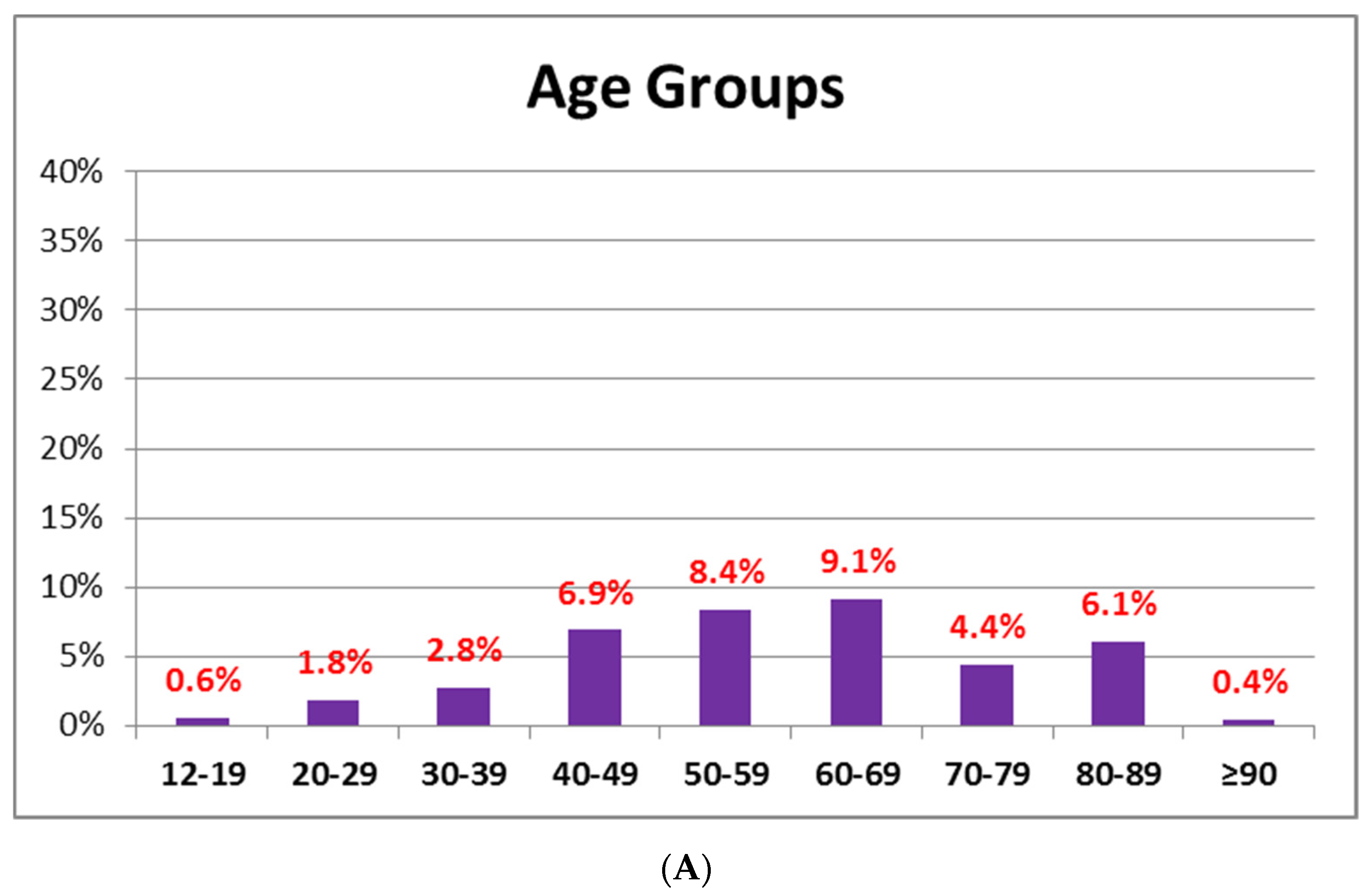
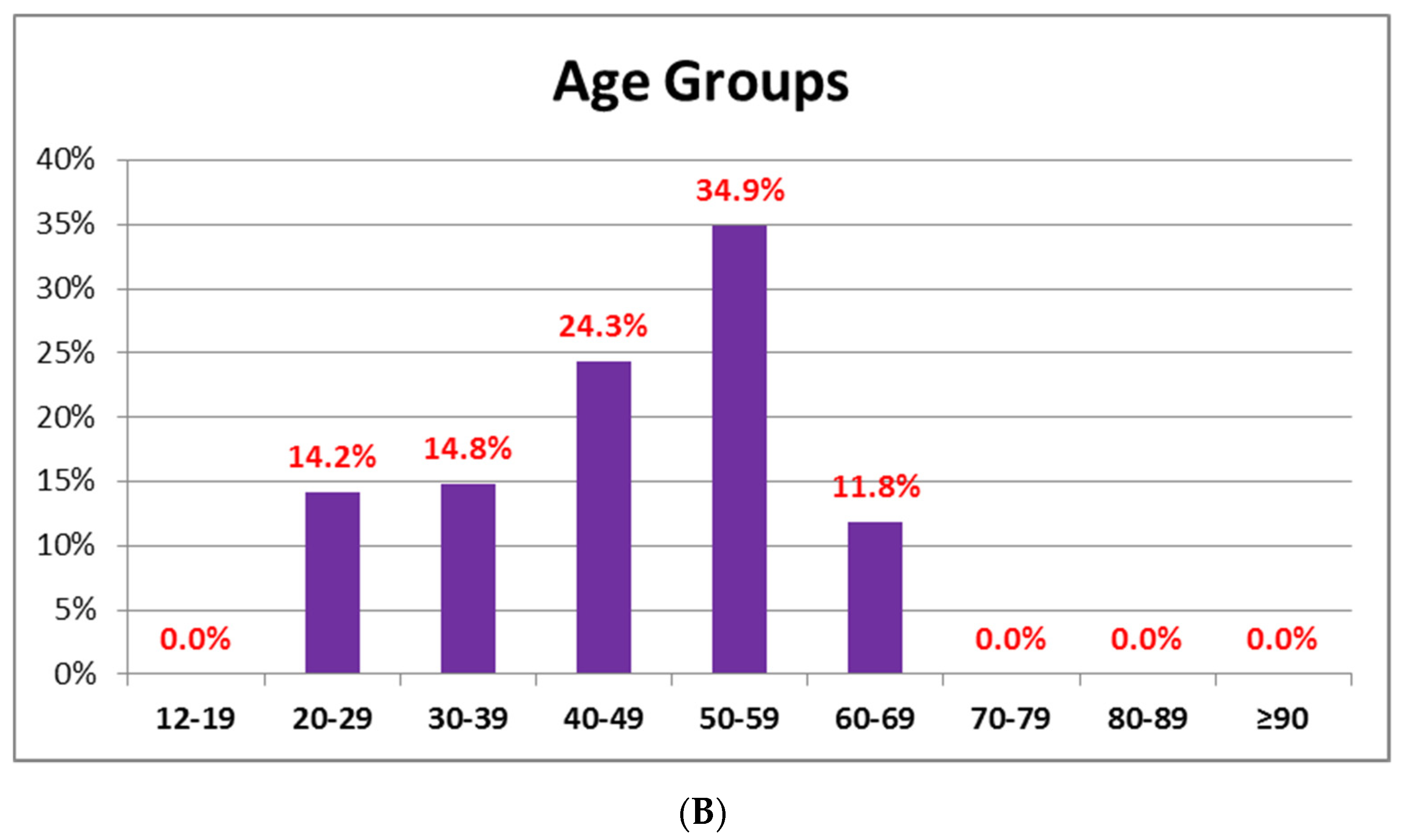
3.2. AEFI after Administration of BNT162B2 or AZD1222 Based on Age
3.3. AEFI after Administration of BNT162b2 or AZD1222 Based on Gender
3.4. Differences of AEFI between BNT162b2 and AZD1222
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 1 February 2022).
- Casillo, M.; Castiglione, A.; Colace, F.; De Santo, M.; Marongiu, F.; Santaniello, D. COVID-19 data sharing and organization through blockchain and decentralized models. In Proceedings of the 2021 Joint Business Informatics Research Workshops and Doctoral Consortium, BIR-WS 2021, Vienna, Austria, 22–24 September 2021; Volume 2991, pp. 128–140. [Google Scholar]
- Pagliano, P.; Sellitto, C.; Conti, V.; Ascione, T.; Esposito, S. Characteristics of viral pneumonia in the COVID-19 era: An update. Infection 2021, 49, 607–616. [Google Scholar] [CrossRef]
- Pagliano, P.; Scarpati, G.; Sellitto, C.; Conti, V.; Spera, A.M.; Ascione, T.; Piazza, O.; Filippelli, A. Experimental Pharmacotherapy for COVID-19: The Latest Advances. J. Exp. Pharmacol. 2021, 13, 1–13. [Google Scholar] [CrossRef]
- Conti, V.; Corbi, G.; Sellitto, C.; Sabbatino, F.; Maci, C.; Bertini, N.; De Bellis, E.; Iuliano, A.; Davinelli, S.; Pagliano, P.; et al. Effect of Tocilizumab in Reducing the Mortality Rate in COVID-19 Patients: A Systematic Review with Meta-Analysis. J. Pers. Med. 2021, 11, 628. [Google Scholar] [CrossRef]
- Evrard, B.; Coudeyre, E.; Rochette, C.; Meriade, L.; Blavignac, C.; Fournier, A.C.; Bignon, Y.J.; Dutheil, F.; Duclos, F.; Thivel, D. Health management of patients with COVID-19: Is there a room for hydrotherapeutic approaches? Int. J. Biometeorol. 2022, 1–8. [Google Scholar] [CrossRef]
- Costantino, M. The rhinogenic deafness and SPA therapy: Clinical-experimental study. Clin. Ter. 2008, 159, 311–315. [Google Scholar]
- Costantino, M.; Izzo, V.; Conti, V.; Manzo, V.; Guida, A.; Filippelli, A. Sulphate mineral waters: A medical resource in several disorders. JTCM 2020, 10, 320–326. [Google Scholar] [CrossRef]
- Costantino, M.; Rossi, F.; Lampa, E. Inhalant therapy with sulphur water in ORL: Clinical-experimental study. Clin. Ter. 2003, 154, 395–400. [Google Scholar]
- Costantino, M.; Conti, V.; Corbi, G.; Filippelli, A. Hydropinotherapy with Sulphurous Mineral Water as Comple-mentary Treatment to Improve Glucose Metabolism, Oxidative Status, and Quality of Life. Antioxidants 2021, 10, 1773. [Google Scholar] [CrossRef]
- Costantino, M.; Giampaolo, C.; Filippelli, A. Effects of drinking SPA therapy on oxidative stress. Clin. Ter. 2013, 163, e13–e17. [Google Scholar]
- Costantino, M.; Lampa, E. Psoriasis and mud-bath therapy: Clinical-experimental study. Clin. Ter. 2005, 156, 145–149. [Google Scholar]
- COVID-19 Vaccines: Development, Evaluation, Approval and Monitoring. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-development-evaluation-approval-monitoring#fast-track-vaccine-development (accessed on 1 February 2022).
- Forni, G.; Mantovani, A. COVID-19 Commission of Accademia Nazionale dei Lincei, Rome. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef]
- Plotkin, S. Hystory of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef] [Green Version]
- Wallinga, J.; Teunis, P. Different Epidemic Curves for Severe Acute Respiratory Syndrome Reveal Similar Impacts of Control Measures. Am. J. Epidemiol. 2004, 160, 509–516. [Google Scholar] [CrossRef] [Green Version]
- AIFA. Annual Report on Safety of COVID-19 Vaccine (Period 2-12-2020/26-12-2021); AIFA: Rome, Italy, 2022. [Google Scholar]
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A., Jr.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E.; et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 1423–1437. [Google Scholar] [CrossRef]
- Ambrosino, I.; Barbagelata, E.; Corbi, G.; Ciarambino, T.; Politi, C.; Moretti, A.M. Gender differences in treatment of Coronavirus Disease-2019. Monaldi Arch. Chest Dis. 2020, 90, 646–656. [Google Scholar] [CrossRef]
- Demographic Breakdown, Updated Weekly. Available online: https://covid19-vaccine-report.ecdc.europa.eu/ (accessed on 1 February 2022).
- Liu, Y.; Shao, Z.; Wang, H. SARS-CoV-2 vaccine-induced immune thrombotic thrombocytopenia. Thromb. Res. 2022, 209, 75–79. [Google Scholar] [CrossRef]
- Italy Hits Target of Fully Vaccinating 80% of People Over-12s. Available online: https://www.reuters.com/world/europe/italy-hits-target-fully-vaccinating-80-people-over-12s-2021–10–10/ (accessed on 1 February 2022).
- Statement for Healthcare Professionals: How COVID-19 Vaccines Are Regulated for Safety and Effectiveness. Available online: https://www.who.int/news/item/11-06-2021-statement-for-healthcare-professionals-how-covid-19-vaccines-are-regulated-for-safety-and-effectiveness (accessed on 1 February 2022).
- Pharmacovigilance Plan of the EU Regulatory Network for COVID-19 Vaccines. Available online: https://www.ema.europa.eu/en/documents/other/pharmacovigilance-plan-eu-regulatory-network-covid-19-vaccines_en.pdf (accessed on 1 February 2022).
- Moccia, G.; Carpinelli, L.; Savarese, G.; Borrelli, A.; Boccia, G.; Motta, O.; Capunzo, M.; De Caro, F. Perception of Health, Mistrust, Anxiety, and Indecision in a Group of Italians Vaccinated against COVID-19. Vaccines 2021, 9, 612. [Google Scholar] [CrossRef]
- Adverse Event Following Immunization. Available online: https://www.who.int/vaccine_safety/publications/AEFI_aide_memoire.pdf (accessed on 1 February 2022).
- Hartwig, S.; Siegel, J.; Schneider, P.J. Preventability and severity assessment in reporting adverse drug reactions. Am. J. Hosp. Pharm. 1992, 49, 2229–2232. [Google Scholar] [CrossRef]
- AIFA: Annual Report on Safety of COVID-19 Vaccine (Period 27-12-2020/26-7-2021). Available online: https://www.aifa.gov.it/documents/20142/1315190/Rapporto_sorveglianza_vaccini_COVID-19_7_EN.pdf (accessed on 1 February 2022).
- Pagliano, P.; Sellitto, C.; Scarpati, G.; Ascione, T.; Conti, V.; Franci, G.; Piazza, O.; Filippelli, A. An overview of the preclinical discovery and development of remdesivir for the treatment of coronavirus disease 2019 (COVID-19). Expert Opin. Drug Discov. 2021, 27, 1–10. [Google Scholar] [CrossRef]
- Rivera-Izquierdo, M.; Soler-Iborte, E.; de Rojas, J.P.; Pegalajar-García, M.D.; Gil-Villalba, A.; Ruiz-Villaverde, R.; Valero-Ubierna, M.D.C. Factors Associated with Adverse Reactions to BNT162b2 COVID-19 Vaccine in a Cohort of 3969 Hospital Workers. Vaccines 2021, 10, 15. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Gee, J.; Marquez, P.; Su, J.; Calvert, G.M.; Liu, R.; Myers, T.; Nair, N.; Martin, S.; Clark, T.; Markowitz, L.; et al. First Month of COVID-19 Vaccine Safety Monitoring—United States, 14 December 2020–13 January 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 283–288. [Google Scholar] [CrossRef]
- Thompson, M.G.; Stenehjem, E.; Grannis, S.; Ball, S.W.; Naleway, A.L.; Ong, T.C.; DeSilva, M.B.; Natarajan, K.; Bozio, C.H.; Lewis, N.; et al. Effectiveness of COVID-19 Vaccines in Ambulatory and Inpatient Care Settings. N. Engl. J. Med. 2021, 385, 1355–1371. [Google Scholar] [CrossRef] [PubMed]
- AIFA: COVID-19 Vaccine Surveillance Report (Period 27/12/2020–26/08/2021). Available online: https://www.aifa.gov.it/documents/20142/1315190/Rapporto_sorveglianza_vaccini_COVID-19_8_EN.pdf (accessed on 1 February 2022).
- Straus, W.; Urdaneta, V.; Esposito, D.B.; Mansi, J.A.; Rodriguez, C.S.; Burton, P.; Vega, J.M. Myocarditis After mRNA-1273 Vaccination: A Population-Based Analysis of 151 million Vaccine Recipients Worldwide. medRxiv 2021. [Google Scholar] [CrossRef]
- Perry, R.J.; Tamborska, A.; Singh, B.; Craven, B.; Marigold, R.; Arthur-Farraj, P.; Yeo, J.M.; Zhang, L.; Hassan-Smith, G.; Jones, M.; et al. Cerebral venous thrombosis after vaccination against COVID-19 in the UK: A multicentre cohort study. Lancet 2021, 398, 1147–1156. [Google Scholar] [CrossRef]
- Chan, E.W.W.; Leung, M.T.Y.; Lau, L.K.W.; Leung, J.; Lum, D.; Wong, R.S.; Li, X.; Chui, C.S.L.; Wan, E.Y.F.; Wong, C.K.H.; et al. Comparing self-reported reactogenicity between adolescents and adults following the use of BNT162b2 (Pfizer-BioNTech) messenger RNA COVID-19 vaccine: A prospective cohort study. Int. J. Infect. Dis. 2021, 116, 47–50. [Google Scholar] [CrossRef]
- Saita, M.; Yan, Y.; Ito, K.; Sasano, H.; Seyama, K.; Naito, T. Reactogenicity following two doses of the BNT162b2 mRNA COVID-19 vaccine: Real-world evidence from healthcare workers in Japan. J. Infect. Chemother. 2022, 28, 116–119. [Google Scholar] [CrossRef]
- Potluri, T.; Fink, A.L.; Sylvia, K.E.; Dhakal, S.; Vermillion, M.S.; vom Steeg, L.; Deshpande, S.; Narasimhan, H.; Klein, S.L. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccines 2019, 4, 29. [Google Scholar] [CrossRef]
- Carrel, L.; Willard, H. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 2005, 434, 400–404. [Google Scholar] [CrossRef]
| Total n = 1.788 | BNT-Vac Group n = 1.613 | AZD-Vac Group n = 175 | p Value | |
|---|---|---|---|---|
| Age, years | ||||
| mean ± SD | 62.5 ± 18.2 | 64 ± 17.9 | 46 ± 11.9 | 0.001 |
| median [range] | 63 [12–99] | 41 [12–99] | 49 [23–69] | |
| GENDER, n (%) | ||||
| Men | 817 (46) | 728 (45) | 89 (51) | 0.39 |
| Women | 971 (54) | 885 (55) | 86 (49) | 0.425 |
| Self-reported Allergy to food or drugs, n (%) | 329 (18) | 274 (28) | 55 (31) | 0.001 |
| Distribution by age, n (%) | ||||
| 12–19 | 16 (0.9) | 16 (1) | 0 (0) | 0.188 |
| 20–29 | 78 (4.4) | 54 (3.4) | 24 (14.2) | 0.001 |
| 30–39 | 99 (5.5) | 74 (4.7) | 25 (14.8) | 0.001 |
| 40–49 | 227 (12.7) | 186 (11.9) | 41 (24.3) | 0.001 |
| 50–59 | 325 (18.2) | 266 (17.0) | 59 (34.9) | 0.001 |
| 60–69 | 332 (18.6) | 312 (19.9) | 20 (11.8) | 0.03 |
| 70–79 | 200 (11.2) | 200 (12.8) | 0 (0) | 0.001 |
| 80–89 | 419 (23.4) | 419 (26.7) | 0 (0) | 0.001 |
| ≥90 | 40 (2.2) | 40 (2.6) | 0 (0) | 0.038 |
| Total n = 684 | Men | Women | p | |
|---|---|---|---|---|
| Age, years | ||||
| mean ± SD | 58.5 ± 17.3 | 59.0 ± 16 | 58.0 ± 18 | 0.464 |
| median [range] | 59 [12–94] | 59 [16–92] | 60 [12–94] | |
| AEFI, n (%) | 684 (42) | 256 (15.8) | 428 (26.2) | 0.01 |
| AEFI duration (hours) | ||||
| mean ± SD | 32.6 ± 34.6 | 29.6 ± 25.3 | 33.9 ± 37.8 | 0.107 |
| Range in hours | 0.25–504 | 0.25–168 | 0.5–504 | |
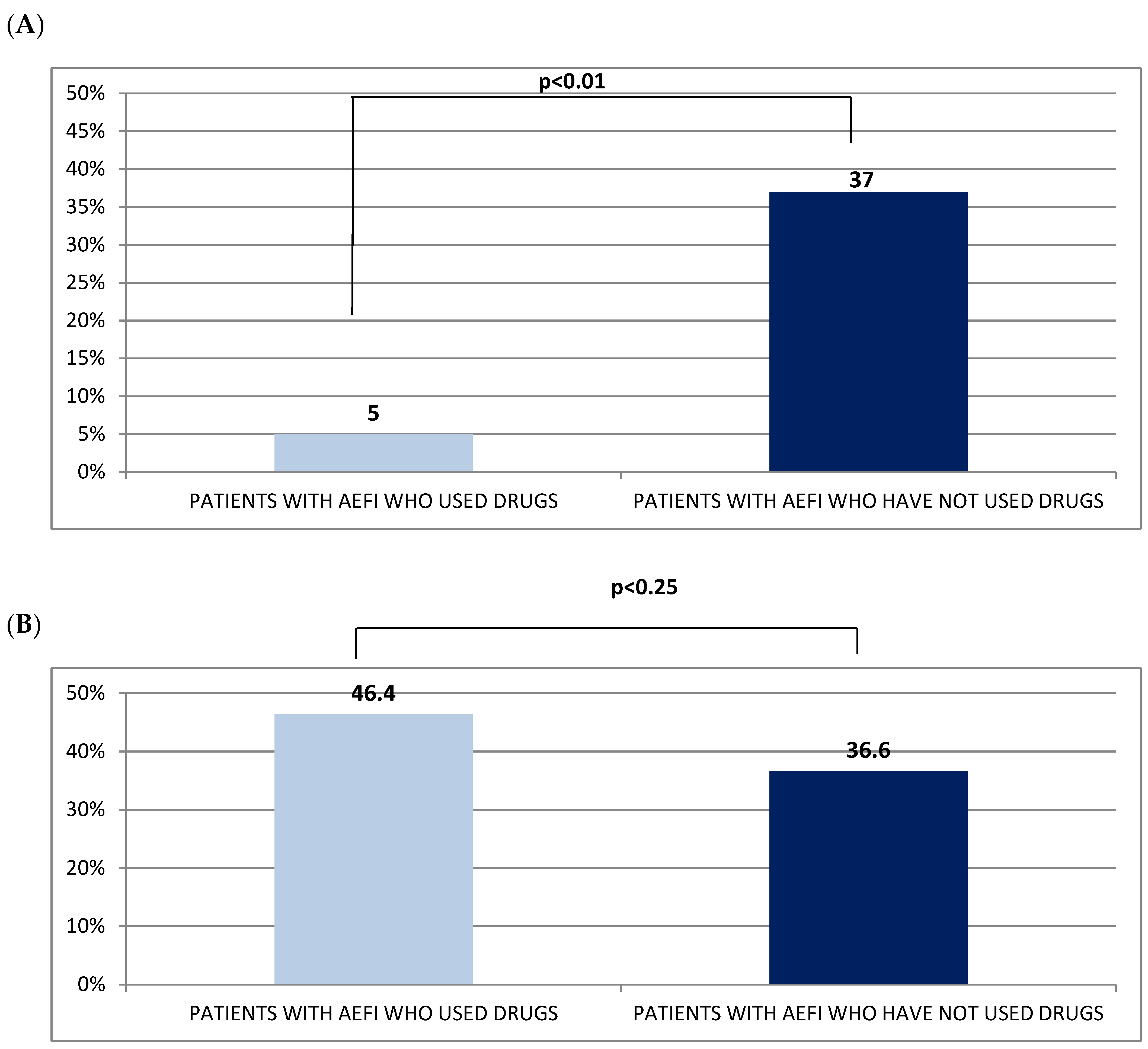
| N subjects with drugs after AEFI, n (%) | 83 (12.1) | 23 (1.4) | 60 (3.6) | 0.01 |
| Drugs after AEFI, n (%) | ||||
| Paracetamol | 68 (4.14) | 19 (1.20) | 49 (2.94) | 0.01 |
| Nimesulide | 4 (0.22) | 2 (0.10) | 2 (0.12) | 0.96 |
| Ketoprofen | 4 (0.22) | 2 (0,10) | 2 (0.12) | 0.96 |
| ASA | 1 (0.06) | 0 (0) | 1 (0.06) | 0.30 |
| Diosmin | 1 (0.06) | 0 (0) | 1 (0.06) | 0.30 |
| Lichtena cream | 1 (0.06) | 0 (0) | 1 (0.06) | 0.30 |
| Disinfectants + vitamins | 1 (0.06) | 0 (0) | 1 (0.06) | 0.30 |
| Paracetamol + ibuprofen | 1 (0.06) | 0 (0) | 1 (0.06) | 0.30 |
| Paracetamol + ketoprofen | 1 (0.06) | 0 (0) | 1 (0.06) | 0.30 |
| alcohol compressed | 1 (0.06) | 0 (0) | 1 (0.06) | 0.30 |
| Total | Men | Women | p | |
|---|---|---|---|---|
| Age, years | ||||
| mean ± SD | 45.0 ± 11.9 | 46.5 ± 11.6 | 43.3 ± 11.7 | 0.100 |
| median [range] | 47 [23–69] | 48 [26–67] | 45 [23–69] | |
| AEFI, n (%) | 145 (83) | 74 (42.0) | 71 (41.0) | 0.697 |
| AEFI duration (hours) | ||||
| mean ± SD | 37.1 ± 90.6 | 30.0 ± 23.3 | 44.3 ± 127.1 | 0.343 |
| Range in hours | 0.5–1080 | 0.5–168 | 0.5–1080 | |
| N subjects with drugs after AEFI, n (%) | 81 (46.4) | 35 (20.0) | 46 (26.4) | 0.041 |
| Drugs after AEFI, n (%) | ||||
| Paracetamol | 73 (41.7) | 32 (18.2) | 41 (23.5) | 0.07 |
| Ketoprofen | 1 (0.06) | 1 (0.6) | 0 (0 | 0.20 |
| ASA | 1 (0.06) | 1 (0.6) | 0 (0) | 0.20 |
| Nimesulide+ASA | 1 (0.57) | 0 (0) | 1 (0.57) | 0.20 |
| Ibuprofen | 2 (1.17) | 1 (0.6) | 1 (0.57) | 0.97 |
| Bilastine | 1 (0.57) | 0 (0) | 1 (0.57) | 0.21 |
| Indomethacin + caffeine + Prochlorperazine | 1 (0.57) | 0 (0) | 1 (0.57) | 0.21 |
| Paracetamol + ibuprofen + metoclopramide | 1 (0.57) | 0 (0) | 1 (0.57) | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costantino, M.; Sellitto, C.; Conti, V.; Corbi, G.; Marongiu, F.; Genovese, G.; Moccia, G.; Capunzo, M.; Borrelli, A.; Pagliano, P.; et al. Adverse Events Associated with BNT162b2 and AZD1222 Vaccines in the Real World: Surveillance Report in a Single Italian Vaccine Center. J. Clin. Med. 2022, 11, 1408. https://doi.org/10.3390/jcm11051408
Costantino M, Sellitto C, Conti V, Corbi G, Marongiu F, Genovese G, Moccia G, Capunzo M, Borrelli A, Pagliano P, et al. Adverse Events Associated with BNT162b2 and AZD1222 Vaccines in the Real World: Surveillance Report in a Single Italian Vaccine Center. Journal of Clinical Medicine. 2022; 11(5):1408. https://doi.org/10.3390/jcm11051408
Chicago/Turabian StyleCostantino, Maria, Carmine Sellitto, Valeria Conti, Graziamaria Corbi, Francesco Marongiu, Giovanni Genovese, Giuseppina Moccia, Mario Capunzo, Anna Borrelli, Pasquale Pagliano, and et al. 2022. "Adverse Events Associated with BNT162b2 and AZD1222 Vaccines in the Real World: Surveillance Report in a Single Italian Vaccine Center" Journal of Clinical Medicine 11, no. 5: 1408. https://doi.org/10.3390/jcm11051408
APA StyleCostantino, M., Sellitto, C., Conti, V., Corbi, G., Marongiu, F., Genovese, G., Moccia, G., Capunzo, M., Borrelli, A., Pagliano, P., Farroni, M., Lombardi, G. M., Elberti, M. G., Filippelli, A., & De Caro, F. (2022). Adverse Events Associated with BNT162b2 and AZD1222 Vaccines in the Real World: Surveillance Report in a Single Italian Vaccine Center. Journal of Clinical Medicine, 11(5), 1408. https://doi.org/10.3390/jcm11051408







