Colchicine for Prevention of Atrial Fibrillation after Cardiac Surgery in the Early Postoperative Period
Abstract
:1. Introduction
2. Materials and Method
2.1. Study Design
2.2. Inclusion Criteria
2.3. Exclusion Criteria
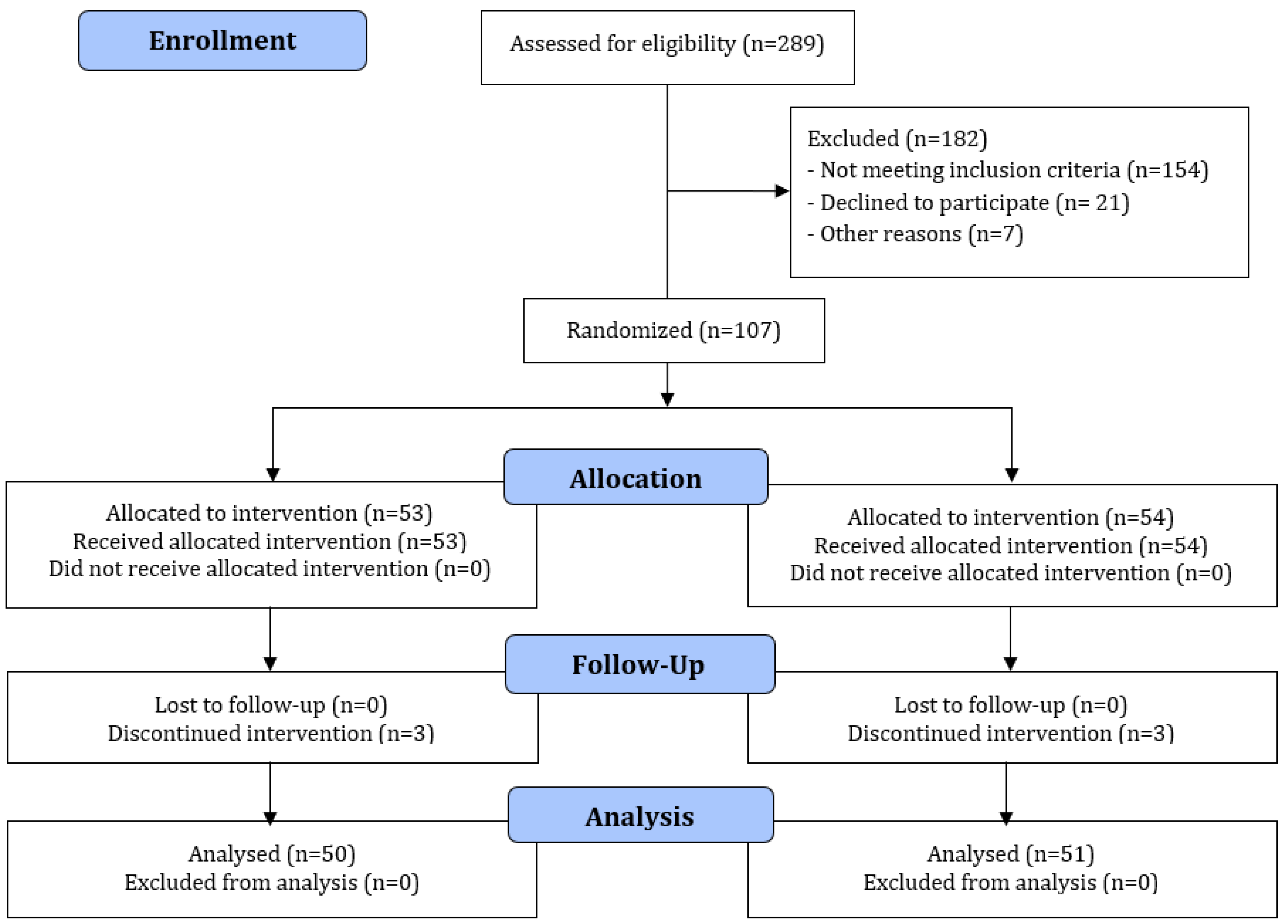
2.4. Randomization
2.5. Statistical Analysis
2.6. Endpoints: Measuring Primary Outcome
2.7. Endpoints: Measuring Secondary Outcomes
2.8. Surgery
2.9. Monitoring
3. Results
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rezaei, Y.; Peighambari, M.M.; Naghshbandi, S.; Samiei, N.; Ghavidel, A.A.; Dehghani, M.R.; Haghjoo, M.; Hosseini, S. Postoperative Atrial Fibrillation Following Cardiac Surgery: From Pathogenesis to Potential Therapies. Am. J. Cardiovasc. Drugs 2020, 20, 19–49. [Google Scholar] [CrossRef] [PubMed]
- Gudbjartsson, T.; Helgadottir, S.; Sigurdsson, M.I.; Taha, A.; Jeppsson, A.; Christensen, T.D.; Riber, L.P.S. New-onset postoperative atrial fibrillation after heart surgery. Acta Anaesthesiol. Scand. 2020, 64, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Ronsoni, R.M.; Souza, A.Z.M.; Leiria, T.L.L.; Lima, G.G. Update on Management of Postoperative Atrial Fibrillation after Cardiac Surgery. Braz. J. Cardiovasc. Surg. 2020, 35, 206–210. [Google Scholar] [PubMed]
- Eikelboom, R.; Sanjanwala, R.; Le, M.L.; Yamashita, M.H.; Arora, R.C. Postoperative Atrial Fibrillation After Cardiac Surgery: A Systematic Review and Meta-Analysis. Ann. Thorac. Surg. 2021, 111, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Dobrev, D.; Aguilar, M.; Heijman, J.; Guichard, J.B.; Nattel, S. Postoperative atrial fibrillation: Mechanisms, manifestations and management. Nat. Rev. Cardiol. 2019, 16, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Gillinov, A.M.; Bagiella, E.; Moskowitz, A.J.; Raiten, J.M.; Groh, M.A.; Bowdish, M.E. Rate control versus rhythm control for atrial fbrillation after cardiac surgery. N. Engl. J. Med. 2016, 374, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, K.A.; Yusuf, A.M.; Crystal, E.; Healey, J.S.; Morillo, C.A.; Nair, G.M.; Whitlock, R.P. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane. Datab. Syst. Rev. 2013, 2013, Cd003611. [Google Scholar]
- Dvirnik, N.; Belley-Cote, E.P.; Hanif, H.; Devereaux, P.J.; Lamy, A.; Dieleman, J.M.; Vincent, J.; Whitlock, R.P. Steroids in cardiac surgery: A systematic review and meta-analysis. Br. J. Anaesth. 2018, 120, 657–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezaei, Y.; Gholami-Fesharaki, M.; Dehghani, M.R.; Arya, A.; Haghjoo, M.; Arjmand, N. Statin antiarrhythmic effect on atrial fibrillation in statin-naive patients undergoing cardiac surgery: A meta-analysis of randomized controlled trials. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Liang, W.; Gu, J.; Zhao, X.; Zhang, T.; Qin, X.; Zhu, G.; Wu, Z. Comparative transcriptome analysis to elucidate the therapeutic mechanism of colchicine against atrial fibrillation. Biomed. Pharmacother. 2019, 119, 109422. [Google Scholar] [CrossRef] [PubMed]
- Nomani, H.; Saei, S.; Johnston, T.P.; Sahebkar, A.; Mohammadpour, A.H. The Efficacy of Anti-inflammatory Agents in the Prevention of Atrial Fibrillation Recurrences. Curr. Med. Chem. 2021, 28, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Brucato, A.; Ferrazzi, P.; Rovere, M.E.; Gandino, A.; Cemin, R.; Ferrua, S.; Belli, R.; Maestroni, S.; Simon, C. Colchicine reduces postoperative atrial fibrillation: Results of the Colchicine for the Prevention of the Postpericardiotomy Syndrome (COPPS) atrial fibrillation substudy. Circulation 2011, 124, 2290–2295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imazio, M.; Brucato, A.; Ferrazzi, P.; Pullara, A.; Adler, Y.; Barosi, A.; Caforio, A.L.; Cemin, R.; Chirillo, F.; Comoglio, C. Colchicine for prevention of postpericardiotomy syndrome and postoperative atrial fibrillation: The COPPS-2 randomized clinical trial. JAMA 2014, 312, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Heijman, J.; Muna, A.P.; Veleva, T.; Molina, C.E.; Sutanto, H.; Tekook, M.; Wang, Q.; Abu-Taha, I.H.; Gorka, M.; Künzel, S.; et al. Atrial Myocyte NLRP3/CaMKII Nexus Forms a Substrate for Postoperative Atrial Fibrillation. Circ. Res. 2020, 127, 1036–1055. [Google Scholar] [CrossRef] [PubMed]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C., Jr.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014, 130, 2071–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lennerz, C.; Barman, M.; Tantawy, M.; Sopher, M.; Whittaker, P. Colchicine for primary prevention of atrial fibrillation after open heart surgery: Systematic review and meta-analysis. Int. J. Cardiol. 2017, 249, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Nomani, H.; Mohammadpour, A.H.; Moallem, S.M.H.; Sahebkar, A. Anti-inflammatory drugs in the prevention of post-operative atrial fibrillation: A literature review. Inflammopharmacology 2020, 28, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Tabbalat, R.A.; Alhaddad, I.; Hammoudeh, A.; Khader, Y.S.; Khalaf, H.A.; Obaidat, M.; Barakat, J. Effect of Low-dose ColchiciNe on the InciDence of Atrial Fibrillation in Open Heart Surgery Patients: END-AF Low Dose Trial. J. Int. Med. Res. 2020, 48, 300060520939832. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, R.; Amir, T.; Gupta, S.; Whitlock, R.P. Optimal medical therapy after coronary artery bypass grafting: A primer for surgeons. Curr. Opin. Cardiol. 2021, 36, 609–615. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Colchicine (n = 50) | Placebo (n = 51) | p |
|---|---|---|---|
| Clinical parameters of patients | |||
| Age, y | 63 (56; 68) | 60 (55; 67) | 0.420 |
| Male, n (%) | 41 (82) | 41 (80,4) | 1.000 |
| BSA, m2 | 2.01 (1.9; 2.18) | 2.04 (1.87; 2.14) | 0.922 |
| Weight, kg | 85 (74; 94.3) | 87 (75; 95) | 0.984 |
| BMI, kg/m2 | 29.3 (26.6; 32.1) | 28.4 (24.7; 32.4) | 0.561 |
| Angina pectoris, n (%) | 47 (94) | 47 (92.2) | 1.000 |
| Angina pectoris class III-IV, n (%) | 30 (60) | 28 (54.9) | 0.604 |
| Diabetes, n (%) | 14 (28) | 9 (17.6) | 0.215 |
| COPD, n (%) | 2 (4) | 2 (3.9) | 1.000 |
| Hypertension, n (%) | 47 (94) | 48 (94.1) | 1.000 |
| Prior AMI, n (%) | 21 (42) | 24 (47.1) | 0.609 |
| Stroke, n (%) | 2 (4) | 1 (2) | 0.617 |
| Smoking, n (%) | 10 (20) | 14 (27.5) | 0.379 |
| Echocardiographic parameters | |||
| LVEF, % | 59.5 (56; 64) | 60 (55; 67) | 0.276 |
| LVESD/BSA, cm/m2 | 16.5 (15.7; 18.4) | 16 (14.6; 17.6) | 0.138 |
| LVEDD/BSA, cm/m2 | 24.8 (23.2; 26.7) | 23.6 (21.8; 26.1) | 0.135 |
| LVESV/BSA, mL/m2 | 22.8 (19.2; 28.6) | 20.4 (16.3; 26.1) | 0.058 |
| LVEDV/BSA, mL/m2 | 56.3 (48.8; 69.2) | 53.3 (44.2; 62.4) | 0.079 |
| AV peak gradient, mm Hg | 9 (7; 47.3) | 9.5 (5.2; 44.8) | 0.711 |
| AV mean gradient, mm H g | 5 (3.4; 31.5) | 10.5 (3.9; 55) | 0.171 |
| MR, degree | 1.5 (1; 2) | 1.5 (1; 1.5) | 0.483 |
| AR, degree | 1 (0; 1,5) | 1.5 (1; 1.5) | 0.122 |
| LV PWth, mm | 9 (9; 14.3) | 13 (11.5; 15) | 0.211 |
| IVS, mm | 13 (12; 15) | 13 (12; 14.3) | 0.935 |
| LA size, cm | 4.2 (3.8; 4.6) | 4.1 (3.7; 4.5) | 0.596 |
| Laboratory data | |||
| WBC, 109/L | 7.9 (6.8; 8.7) | 7.5 (6.6; 9.1) | 0.973 |
| Neutrophils, 109/L | 4.6 (3.8; 5.2) | 4.7 (3.5; 5.3) | 0.863 |
| Neutrophils, % | 60.7 (54; 65.1) | 58.8 (52.5; 63) | 0.532 |
| Platelets,109/L | 247.5 (191.7; 295) | 253 (205; 292) | 0.519 |
| Creatinine, mkmol/L | 85 (73; 94.3) | 80 (74; 95) | 0.732 |
| eGFR mL/min per 1.73 m2 (MDRD) | 93.5 (79.8; 107.3) | 90 (77; 109) | 0.954 |
| Glucose, mmol/L | 5.6 (5; 6.5) | 5.5 (5; 6.1) | 0.530 |
| AST, IU/L | 20.5 (17; 30.8) | 21 (17.8; 28.5) | 0.921 |
| ALT, IU/L | 23 (18; 33) | 27 (17.8; 34) | 0.444 |
| Potassium, mmol/L | 4.4 (4.1; 5) | 4.6 (4.2; 4.8) | 0.488 |
| Drug therapy | |||
| Beta-blockers, % | 41 (82) | 37 (72.5) | 0.257 |
| ACE inhibitors, % | 31 (62) | 29 (56.9) | 0.599 |
| Calcium antagonists, % | 17 (34) | 16 (31.4) | 0.778 |
| Thiazide diuretics,% | 8 (16) | 3 (5.9) | 0.122 |
| Loop diuretics, % | 7 (14) | 2 (3.9) | 0.092 |
| Potassium-sparing diuretics, % | 8 (16) | 8 (15.7) | 1.000 |
| NSAIDs, n (%) | 0 (0) | 0 (0) | |
| Acetylsalicylic acid, n (%) | 11 (22) | 8 (15.7) | 0.455 |
| Other antiaggregant, n (%) | 3 (6) | 3 (5.9) | 1.000 |
| Nitrates, % | 14 (28) | 12 (23.5) | 0.607 |
| Statins, % | 35 (70) | 35 (68.6) | 0.881 |
| LMWHs/UFH, n (%) | 14 (28) | 15 (29.4) | 0.875 |
| Parameters | Colchicine (n = 50) | Placebo (n = 51) | p |
|---|---|---|---|
| Extracorporeal circulation, n (%) | 34 (68) | 35 (68.6) | 0.946 |
| CPB time, min | 120 (89; 138) | 120 (90; 142) | 0.736 |
| Cardioplegia, n (%) | 16 (32) | 14 (27.5) | 0.617 |
| ACC time, min | 63.5 (59.3; 71.5) | 67.5 (53.8; 80) | 0.755 |
| CABG, n (%) | 40 (80) | 45 (88.2) | 0.288 |
| AV repair, n (%) | 16 (32) | 13 (25.5) | 0.470 |
| Cardiotonic support in ICU, n (%) | 19 (38) | 15 (29.4) | 0.361 |
| Lung ventilation time, h | 8 (5.8; 13.3) | 8.2 (5.2; 14.4) | 0.911 |
| Parameters | Colchicine (n = 50) | Placebo (n = 51) | OR | 95% CI | p |
|---|---|---|---|---|---|
| POAF, n (%) | 9 (18) | 15 (29.4) | 0.527 | 0.206–1.349 | 0.178 |
| Effective management of POAF (AAT, cardioversion), n (%) | 9 (18) | 15 (29.4) | 0.527 | 0.206–1.349 | 0.178 |
| Ineffective management of POAF, n (%) | 0 (0) | 0 (0) | |||
| Hospital mortality, n (%) | 0 (0) | 0 (0) | |||
| Complications in the department: | |||||
| Respiratory failure, n (%) | 0 (0) | 0 (0) | |||
| TIA, n (%) | 0 (0) | 0 (0) | |||
| Bleeding, n (%) | 0 (0) | 0 (0) | |||
| General infectious complications, n (%) | 0 (0) | 0 (0) | |||
| Infectious complications of postoperative wound, n (%) | 0 (0) | 0 (0) | |||
| Acute renal failure (3 postoperative day) | 1 (2) | 3 (5.9) | 0.327 | 0033–3.250 | 0.618 |
| Acute renal failure (5 postoperative day) | 1 (2) | 0 (0) | 1.000 | ||
| Arrhythmias, except AF, n (%) | 4 (8) | 3 (5.9) | 1.524 | 0.322–7.202 | 0.715 |
| PVC, n (%) | 0 (0) | 0 (0) | |||
| SVESs, n (%) | 0 (0) | 1 (2) | 1.000 | ||
| AV-block, n (%) | 2 (4) | 0 (0) | 0.243 | ||
| Others, n (%) | 2 (4) | 2 (3.9) | 1.021 | 0.138–7.543 | 1.000 |
| Pacemaker implantation, n (%) | 1 (2) | 0 (0) | 0.495 | ||
| Parameters | Colchicine (n = 50) | Placebo (n = 51) | p |
|---|---|---|---|
| postoperative day 3 | |||
| LVEF, % | 54 (52; 56) | 55 (53; 56) | 0.221 |
| LVESV/BSA mL/m2 | 21.5 (17.6; 29.4) | 18.7 (16.2; 23.1) | 0.051 |
| LVEDV/BSA mL/m2 | 47.7 (43.1; 57.6) | 43.8 (35.8; 52.6) | 0.032 |
| Pericardial effusion, n (%) | 3 (6) | 8 (15.7) | 0.204 |
| Pericardial effusion, mm | 5 (4; 5) | 5 (5; 10.8) | 0.220 |
| Pericardial effusion, n (%) | 22 (44) | 16 (31.4) | 0.139 |
| Pericardial effusion, mm | 19 (11; 21.5) | 19 (10; 27) | 0.811 |
| postoperative day 5 | |||
| LVEF, % | 55 (51; 56) | 55 (54.2; 57) | 0.098 |
| LVESV/BSA mL/m2 | 21.5 (17.9; 26.6) | 18.5 (16.3; 23.9) | 0.075 |
| LVEDV/BSA mL/m2 | 49.3 (42.3; 57.6) | 42.8 (37.2; 53.3) | 0.027 |
| Pericardial effusion, n (%) | 8 (16) | 15 (29,4) | 0.094 |
| Pericardial effusion, mm | 5.5 (4.25; 6) | 5 (4; 5.5) | 0.427 |
| Pericardial effusion, n (%) | 19 (38) | 21 (41.2) | 0.809 |
| Pericardial effusion, mm | 15 (11.5; 22) | 20 (12.3; 33.5) | 0.225 |
| Parameters | Colchicine (n = 50) | Placebo (n = 51) | p |
|---|---|---|---|
| postoperative day 3 | |||
| WBC, 109/L | 12 (9.5; 14.4) | 11.8 (10.1; 14) | 0.869 |
| Neutrophils, 109/L | 10.4 (6.5; 12) | 8.8 (7.2; 13) | 0.965 |
| Neutrophils, % | 80 (68; 85.3) | 77.5 (70.6; 84.8) | 0.811 |
| Platelets, 109/L | 201.7 (143; 251.5) | 206.5 (171.9; 277.9) | 0.324 |
| Creatinine, mkmol/L | 75.7 (67; 85.5) | 75.9 (68,3; 90.5) | 0.415 |
| eGFR mL/min per 1.73 m2 (MDRD) | 106 (87.8; 123.3) | 98.5 (85.8; 120.8) | 0.318 |
| Glucose, mmol/L | 7.4 (5.4; 8.6) | 7 (5.7; 8.2) | 0.771 |
| AST, IU/L | 31 (24; 43) | 28 (20.5; 39) | 0.407 |
| ALT, IU/L | 20 (15; 30) | 23 (15; 30.5) | 0.812 |
| Potassium, mmol/L | 4 (3.9; 4.5) | 4.3 (3.9; 4.7) | 0.070 |
| postoperative day 5 | |||
| WBC, 109/L | 9.1 (8.1; 10.5) | 9.7 (8.1; 11) | 0.417 |
| Neutrophils, 109/L | 5.8 (4.8; 7) | 6.3 (5; 7.2) | 0.559 |
| Neutrophils, % | 61.5 (58.2; 67.2) | 63.4 (56.5; 66) | 1.000 |
| Platelets, 109/L | 255 (194.8; 315) | 262 (213; 307) | 0.573 |
| Creatinine, mkmol/L | 76 (71; 86.4) | 73.4 (67.6; 92.1) | 0.607 |
| eGFR mL/min per 1.73 m2 (MDRD) | 100 (83; 111) | 98 (79.8; 129.8) | 0.889 |
| Glucose, mmol/L | 6.6 (5.3; 7.5) | 6.1 (5.6; 8.6) | 0.820 |
| AST, IU/L | 26 (21; 31) | 30 (20; 46) | 0.215 |
| ALT, IU/L | 26 (17; 37) | 28 (21; 45) | 0.218 |
| Potassium, mmol/L | 4.1 (3.8; 4.4) | 4.3 (4; 4.8) | 0.248 |
| Parameters | Colchicine (n = 50) | Placebo (n = 51) | p |
|---|---|---|---|
| postoperative day 1 | |||
| Beta-blockers, n (%) | 46 (92) | 44 (86.3) | 0.525 |
| Statins, n (%) | 35 (70) | 35 (68.6) | 0.881 |
| ACE inhibitors, n (%) | 30 (60) | 31 (60.8) | 0.936 |
| Calcium antagonists, n (%) | 15 (30) | 12 (23.5) | 0.463 |
| Thiazide diuretics, n (%) | 5 (10) | 4 (7.8) | 0.741 |
| Loop diuretics, n (%) | 3 (6) | 2 (3.9) | 0.678 |
| Potassium-sparing diuretics, n (%) | 22 (44) | 11 (21.6) | 0.016 |
| NSAIDs, n (%) | 20 (40) | 15 (29.4) | 0.264 |
| Acetylsalicylic acid, n (%) | 41 (82) | 40 (78.4) | 0.804 |
| Other antiaggregant, n (%) | 35 (70) | 41 (80.4) | 0.226 |
| Nitrates, n (%) | 3 (6) | 8 (15.7) | 0.200 |
| Antiarrhythmic drugs, n (%) | 3 (6) | 4 (7.8) | 1.000 |
| Cardiotonic support, n (%) | 28 (56) | 29 (56.9) | 0.930 |
| Adrenaline, n (%) | 3 (6) | 2 (3.9) | 0.678 |
| Norepinephrine, n (%) | 14 (28) | 11(21.6) | 0.454 |
| Dopamine, n (%) | 19 (38) | 25 (49) | 0.264 |
| Dobutamine, n (%) | 0 (0) | 0 (0) | |
| LMWHs/UFH, n (%) | 50 (100) | 51 (100) | |
| Warfarin, n (%) | 16 (32) | 12 (23.5) | 0.342 |
| Antibiotics, n (%) | 49 (98) | 47 (92.2) | 0.362 |
| Steroids, n (%) | 38 (76) | 41 (80.4) | 0.593 |
| postoperative day 3 | |||
| Beta-blockers, n (%) | 44 (88) | 45 (88.2) | 1.000 |
| Statins, n (%) | 36 (72) | 36 (70.6) | 0.875 |
| ACE inhibitors, n (%) | 29 (58) | 32 (62.7) | 0.626 |
| Calcium antagonists, n (%) | 14 (28) | 10 (19.6) | 0.322 |
| Thiazide diuretics, n (%) | 4 (8) | 4 (7.8) | 1.000 |
| Loop diuretics, n (%) | 5 (10) | 5 (9.8) | 1.000 |
| Potassium-sparing diuretics, n (%) | 24 (48) | 17 (33.3) | 0.133 |
| NSAIDs, n (%) | 20 (40) | 16 (31.4) | 0.365 |
| Acetylsalicylic acid, n (%) | 39 (78) | 41 (80.4) | 0.767 |
| Other antiaggregant, n (%) | 35 (70) | 40 (78.4) | 0.333 |
| Nitrates, n (%) | 1 (2) | 2 (3.9) | 1.000 |
| Antiarrhythmic drugs, n (%) | 4 (8) | 6 (11.8) | 0.741 |
| Cardiotonic support, n (%) | 14 (28) | 14 (27.5) | 0.951 |
| Adrenaline, n (%) | 1 (2) | 2 (3.9) | 1.000 |
| Norepinephrine, n (%) | 9 (18) | 5 (9.8) | 0.263 |
| Dopamine, n (%) | 8 (16) | 11 (216) | 0.612 |
| Dobutamine, n (%) | 0 (0) | 0 (0) | |
| LMWHs/UFH, n (%) | 48 (96) | 45 (88.2) | 0.269 |
| Warfarin, n (%) | 16 (32) | 14 (27.5) | 0.617 |
| Antibiotics, n (%) | 36 (72) | 39 (76.5) | 0.607 |
| Steroids, n (%) | 17 (34) | 18 (35.3) | 0.891 |
| postoperative day 5 | |||
| Beta-blockers, n (%) | 44 (88) | 43 (84.3) | 0.775 |
| Statins, n (%) | 36 (72) | 36 (70.6) | 0.875 |
| ACE inhibitors, n (%) | 30 (60) | 31 (60.8) | 0.936 |
| Calcium antagonists, n (%) | 13 (26) | 10 (19.6) | 0.444 |
| Thiazide diuretics, n (%) | 4 (8) | 3 (5.9) | 0.715 |
| Loop diuretics, n (%) | 3 (6) | 6 (11.8) | 0.487 |
| Potassium-sparing diuretics, n (%) | 24 (48) | 17 (33.3) | 0.133 |
| NSAIDs, n (%) | 15 (30) | 7 (13.7) | 0.048 |
| Acetylsalicylic acid, n (%) | 42 (84) | 42 (82.4) | 1.000 |
| Other antiaggregant, n (%) | 36 (72) | 41 (80.4) | 0.322 |
| Nitrates, n (%) | 0 (0) | 2 (3.9) | 0.495 |
| Antiarrhythmic drugs, n (%) | 6 (12) | 8 (15,7) | 0.775 |
| Cardiotonic support, n (%) | 2 (4) | 1 (2) | 0.617 |
| Adrenaline, n (%) | 1 (2) | 0 (0) | 0.495 |
| Norepinephrine, n (%) | 0 (0) | 0 (0) | |
| Dopamine, n (%) | 1 (2) | 1 (2) | 1.000 |
| Dobutamine, n (%) | 0 (0) | 0 (0) | |
| LMWHs/UFH, n (%) | 40 (80) | 38 (74.5) | 0.511 |
| Warfarin, n (%) | 16 (32) | 15 (29.4) | 0.778 |
| Antibiotics, n (%) | 23 (46) | 32 (62.7) | 0.091 |
| Steroids, n (%) | 4 (8) | 6 (11.8) | 0.741 |
| Parameters | Colchicine (n = 50) | Placebo (n = 51) | OR | 95% Cl | p |
|---|---|---|---|---|---|
| Nausea, n (%) | 6 (12) | 5 (9.8) | 1.255 | 0.357–4.408 | 0.755 |
| Vomiting, n (%) | 1 (2) | 2 (3.9) | 0.500 | 0.044–5.696 | 1.000 |
| Lack of appetite, n (%) | 11 (22) | 14 (27.5) | 0.745 | 0.3–1.85 | 0.605 |
| Diarrhea, n (%) | 16 (32) | 6 (11.8) | 3.529 | 1.249–9.972 | 0.010 |
| Abdominal pain, n (%) | 6 (12) | 2 (3.9) | 3.341 | 0.641–17.419 | 0.151 |
| Convulsions, n (%) | 1 (2) | 4 (7.8) | 0.24 | 0.026–2.225 | 0.363 |
| Tingling in the extremities, n (%) | 5 (10) | 7 (13.7) | 0.698 | 0.206–2.367 | 0.761 |
| Skin rashes, n (%) | 0 (0) | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shvartz, V.; Le, T.; Kryukov, Y.; Sokolskaya, M.; Ispiryan, A.; Khugaeva, E.; Yurkulieva, G.; Shvartz, E.; Petrosyan, A.; Bockeria, L.; et al. Colchicine for Prevention of Atrial Fibrillation after Cardiac Surgery in the Early Postoperative Period. J. Clin. Med. 2022, 11, 1387. https://doi.org/10.3390/jcm11051387
Shvartz V, Le T, Kryukov Y, Sokolskaya M, Ispiryan A, Khugaeva E, Yurkulieva G, Shvartz E, Petrosyan A, Bockeria L, et al. Colchicine for Prevention of Atrial Fibrillation after Cardiac Surgery in the Early Postoperative Period. Journal of Clinical Medicine. 2022; 11(5):1387. https://doi.org/10.3390/jcm11051387
Chicago/Turabian StyleShvartz, Vladimir, Tatyana Le, Yuri Kryukov, Maria Sokolskaya, Artak Ispiryan, Eleonora Khugaeva, Gulsuna Yurkulieva, Elena Shvartz, Andrey Petrosyan, Leo Bockeria, and et al. 2022. "Colchicine for Prevention of Atrial Fibrillation after Cardiac Surgery in the Early Postoperative Period" Journal of Clinical Medicine 11, no. 5: 1387. https://doi.org/10.3390/jcm11051387
APA StyleShvartz, V., Le, T., Kryukov, Y., Sokolskaya, M., Ispiryan, A., Khugaeva, E., Yurkulieva, G., Shvartz, E., Petrosyan, A., Bockeria, L., & Bockeria, O. (2022). Colchicine for Prevention of Atrial Fibrillation after Cardiac Surgery in the Early Postoperative Period. Journal of Clinical Medicine, 11(5), 1387. https://doi.org/10.3390/jcm11051387








