Differences in Atrial Remodeling in Hypertrophic Cardiomyopathy Compared to Hypertensive Heart Disease and Athletes’ Hearts
Abstract
:1. Introduction
2. Methods
2.1. Hypertrophic Cardiomyopathy Patient Population
2.2. Hypertensive Heart Disease Patient Population
2.3. Endurance Athlete Population
2.4. Patient Work-Up
3. Statistical Analysis
4. Results
4.1. Electrical Markers of Atrial Cardiomyopathy
4.2. Echocardiographic Markers of Atrial Cardiomyopathy
4.3. Biochemical Markers of Atrial Cardiomyopathy
4.4. Differentiation of HCM Patients from HHD Patients with IVS13
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclosures
References
- Elliott, P.M.; Anastasakis, A.; Borger, M.A.; Borggrefe, M.; Cecchi, F.; Charron, P.; Hagege, A.A.; Lafont, A.; Limongelli, G.; Mahrholdt, H.; et al. 2014 Esc Guidelines on Diagnosis and Management of Hypertrophic Cardiomyopathy: The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (Esc). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar] [PubMed]
- Brito, D.; Miltenberger-Miltenyi, G.; Vale Pereira, S.; Silva, D.; Diogo, A.N.; Madeira, H. Sarcomeric Hypertrophic Cardiomyopathy: Genetic Profile in a Portuguese Population. Rev. Port. Cardiol. 2012, 31, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Ingles, J.; Burns, C.; Barratt, A.; Semsarian, C.; Couns, G.D.G. Application of Genetic Testing in Hypertrophic Cardiomyopathy for Preclinical Disease Detection. Circ. Cardiovasc. Genet. 2015, 8, 852–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassem, H.S.; Azer, R.S.; Saber-Ayad, M.; Moharem-Elgamal, S.; Magdy, G.; Elguindy, A.; Cecchi, F.; Olivotto, I.; Yacoub, M.H. Early Results of Sarcomeric Gene Screening from the Egyptian National BA-HCM Program. J. Cardiovasc. Transl. Res. 2012, 6, 65–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maron, B.J.; Casey, S.A.; Poliac, L.C.; Gohman, T.E.; Almquist, A.K.; Aeppli, D.M. Clinical Course of Hypertrophic Cardiomyopathy in a Regional United States Cohort. JAMA J. Am. Med. Assoc. 1999, 281, 650–655. [Google Scholar] [CrossRef]
- Morita, H.; Rehm, H.L.; Menesses, A.; McDonough, B.; Roberts, A.E.; Kucherlapati, R.; Towbin, J.A.; Seidman, J.; Seidman, C.E. Shared Genetic Causes of Cardiac Hypertrophy in Children and Adults. N. Engl. J. Med. 2008, 358, 1899–1908. [Google Scholar] [CrossRef] [Green Version]
- Van Driest, S.L.; Ommen, S.R.; Tajik, A.J.; Gersh, B.J.; Ackerman, M.J. Sarcomeric genotyping in hypertrophic cardiomyopathy. Mayo Clin. Proc. 2005, 80, 463–469. [Google Scholar] [CrossRef]
- Semsarian, C.; Ingles, J.; Maron, M.S.; Maron, B.J. New Perspectives on the Prevalence of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, 1249–1254. [Google Scholar] [CrossRef] [Green Version]
- McLeod, C.J.; Ackerman, M.J.; Nishimura, R.A.; Tajik, A.J.; Gersh, B.J.; Ommen, S.R. Outcome of Patients With Hypertrophic Cardiomyopathy and a Normal Electrocardiogram. J. Am. Coll. Cardiol. 2009, 54, 229–233. [Google Scholar] [CrossRef] [Green Version]
- Rowin, E.J.; Maron, B.J.; Appelbaum, E.; Link, M.S.; Gibson, C.M.; Lesser, J.R.; Haas, T.S.; Udelson, J.E.; Manning, W.J.; Maron, M.S. Significance of False Negative Electrocardiograms in Preparticipation Screening of Athletes for Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2012, 110, 1027–1032. [Google Scholar] [CrossRef]
- Shirani, J.; Pick, R.; Roberts, W.C.; Maron, B.J. Morphology and significance of the left ventricular collagen network in young patients with hypertrophic cardiomyopathy and sudden cardiac death. J. Am. Coll. Cardiol. 2000, 35, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Charron, P.; Forissier, J.; Amara, M.; Dubourg, O.; Desnos, M.; Bouhour, J.-B.; Isnard, R.; Hagege, A.; Bénaıche, A.; Richard, P.; et al. Accuracy of European diagnostic criteria for familial hypertrophic cardiomyopathy in a genotyped population. Int. J. Cardiol. 2003, 90, 33–38. [Google Scholar] [CrossRef]
- Guttmann, O.P.; Rahman, M.S.; O’Mahony, C.; Anastasakis, A.; Elliott, P.M. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: Systematic review. Heart 2013, 100, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Lip, G.Y.; Van Gelder, I.C.; Bax, J.; Hylek, E.; Kaab, S.; Schotten, U.; Wegscheider, K.; Boriani, G.; Brandes, A.; et al. Comprehensive risk reduction in patients with atrial fibrillation: Emerging diagnostic and therapeutic options—A report from the 3rd Atrial Fibrillation Competence NETwork/European Heart Rhythm Association consensus conference. Europace 2012, 14, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. Ehra/Hrs/Aphrs/Solaece Expert Consensus on Atrial Cardiomyopathies: Definition, Characterization, and Clinical Implication. Europace 2016, 18, 1455–1490. [Google Scholar] [CrossRef]
- Farhad, H.; Seidelmann, S.B.; Vigneault, D.; Abbasi, S.A.; Yang, E.; Day, S.M.; Colan, S.D.; Russell, M.W.; Towbin, J.; Sherrid, M.V.; et al. Left Atrial structure and function in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. J. Cardiovasc. Magn. Reson. 2017, 19, 107. [Google Scholar] [CrossRef] [Green Version]
- Latif, S.R.; Nguyen, V.Q.; Peters, D.C.; Soufer, A.; Henry, M.L.; Grunseich, K.; Testani, J.; Hur, D.; Huber, S.; Mojibian, H.; et al. Left atrial fibrosis correlates with extent of left ventricular myocardial delayed enhancement and left ventricular strain in hypertrophic cardiomyopathy. Int. J. Cardiovasc. Imaging 2019, 35, 1309–1318. [Google Scholar] [CrossRef]
- Wilhelm, M.; Nuoffer, J.-M.; Schmid, J.-P.; Wilhelm, I.; Saner, H. Comparison of Pro-Atrial Natriuretic Peptide and Atrial Remodeling in Marathon Versus Non-Marathon Runners. Am. J. Cardiol. 2012, 109, 1060–1065. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef] [Green Version]
- Nozica, N.; Lam, A.; Goulouti, E.; Elchinova, E.; Spirito, A.; Branca, M.; Servatius, H.; Noti, F.; Seiler, J.; Baldinger, S.H.; et al. The SilenT AtRial FIBrillation (STAR-FIB) study programme—Design and rationale. Swiss Med. Wkly. 2021, 151, w20421. [Google Scholar] [CrossRef]
- Roten, L.; Goulouti, E.; Lam, A.; Elchinova, E.; Nozica, N.; Spirito, A.; Wittmer, S.; Branca, M.; Servatius, H.; Noti, F.; et al. Age and Sex Specific Prevalence of Clinical and Screen-Detected Atrial Fibrillation in Hospitalized Patients. J. Clin. Med. 2021, 10, 4871. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Roten, L.; Tanner, H.; Schmid, J.-P.; Wilhelm, I.; Saner, H. Long-Term Cardiac Remodeling and Arrhythmias in Nonelite Marathon Runners. Am. J. Cardiol. 2012, 110, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Brugger, N.; Krause, R.; Carlen, F.; Rimensberger, C.; Hille, R.; Steck, H.; Wilhelm, M.; Seiler, C. Effect of lifetime endurance training on left atrial mechanical function and on the risk of atrial fibrillation. Int. J. Cardiol. 2014, 170, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Darbar, D.; Jahangir, A.; Hammill, S.C.; Gersh, B.J. P Wave Signal-Averaged Electrocardiography to Identify Risk for Atrial Fibrillation. Pacing Clin. Electrophysiol. 2002, 25, 1447–1453. [Google Scholar] [CrossRef]
- Dhala, A.; Underwood, D.; Leman, R.; Madu, E.; Baugh, D.; Ozawa, Y.; Kasamaki, Y.; Xue, Q.; Reddy, S. Signal-Averaged P-Wave Analysis of Normal Controls and Patients with Paroxysmal Atrial Fibrillation: A Study in Gender Differences, Age Dependence, and Reproducibility. Clin. Cardiol. 2002, 25, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A.; Flachskampf, F.; Lancellotti, P.; Badano, L.; Aguilar, R.; Monaghan, M.; Zamorano, J.; Nihoyannopoulos, P.; on behalf of the European Association of Echocardiography. European Association of Echocardiography recommendations for standardization of performance, digital storage and reporting of echocardiographic studies. Eur. J. Echocardiogr. 2008, 9, 438–448. [Google Scholar] [CrossRef] [Green Version]
- Magnani, J.W.; Williamson, M.A.; Ellinor, P.T.; Monahan, K.M.; Benjamin, E.J. P Wave Indices: Current Status and Future Directions in Epidemiology, Clinical, and Research Applications. Circ. Arrhythm. Electrophysiol. 2009, 2, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Kornej, J.; Magnani, J.W.; Preis, S.R.; Soliman, E.Z.; Trinquart, L.; Ko, D.; Benjamin, E.J.; Lin, H. P-wave signal-averaged electrocardiography: Reference values, clinical correlates, and heritability in the Framingham Heart Study. Heart Rhythm 2021, 18, 1500–1507. [Google Scholar] [CrossRef]
- Aytemir, K.; Amasyali, B.; Abali, G.; Kose, S.; Kilic, A.; Onalan, O.; Tokgozoglu, L.; Kabakci, G.; Ozkutlu, H.; Nazli, N.; et al. The signal-averaged P-wave duration is longer in hypertensive patients with history of paroxysmal atrial fibrillation as compared to those without. Int. J. Cardiol. 2005, 103, 37–40. [Google Scholar] [CrossRef]
- Militaru, C.; Donoiu, I.; Ionescu, D.-D. P Wave Signal-Averaged ECG in Normal Population and in Patients with Converted Atrial Fibrillation. Ann. Noninvasive Electrocardiol. 2011, 16, 351–356. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Sullivan, L.; Levy, D.; Pencina, M.J.; Massaro, J.; D’Agostino, R.B.; Newton-Cheh, C.; Yamamoto, J.F.; Magnani, J.W.; Tadros, T.M.; et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): A community-based cohort study. Lancet 2009, 373, 739–745. [Google Scholar] [CrossRef] [Green Version]
- Dewland, T.A.; Vittinghoff, E.; Mandyam, M.C.; Heckbert, S.R.; Siscovick, D.S.; Stein, P.K.; Psaty, B.M.; Sotoodehnia, N.; Gottdiener, J.S.; Marcus, G.M. Atrial Ectopy as a Predictor of Incident Atrial Fibrillation: A Cohort Study. Ann. Intern. Med. 2013, 159, 721–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaaf, M.; Andre, P.; Altman, M.; Maucort-Boulch, D.; Placide, J.; Chevalier, P.; Bergerot, C.; Thibault, H. Left atrial remodelling assessed by 2D and 3D echocardiography identifies paroxysmal atrial fibrillation. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finocchiaro, G.; Sheikh, N.; Biagini, E.; Papadakis, M.; Maurizi, N.; Sinagra, G.; Pelliccia, A.; Rapezzi, C.; Sharma, S.; Olivotto, I. The electrocardiogram in the diagnosis and management of patients with hypertrophic cardiomyopathy. Heart Rhythm 2020, 17, 142–151. [Google Scholar] [CrossRef]
- Basavarajaiah, S.; Wilson, M.; Whyte, G.; Shah, A.; McKenna, W.; Sharma, S. Prevalence of Hypertrophic Cardiomyopathy in Highly Trained Athletes: Relevance to Pre-Participation Screening. J. Am. Coll. Cardiol. 2008, 51, 1033–1039. [Google Scholar] [CrossRef] [Green Version]
- Caselli, S.; Maron, M.S.; Urbano-Moral, J.A.; Pandian, N.G.; Maron, B.J.; Pelliccia, A. Differentiating Left Ventricular Hypertrophy in Athletes from That in Patients with Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2014, 114, 1383–1389. [Google Scholar] [CrossRef]
- Cheng, T.O. Hypertrophic Cardiomyopathy Vs Athlete’s Heart. Int. J. Cardiol. 2009, 131, 151–155. [Google Scholar] [CrossRef]
| HCM n = 27 | HHD n = 324 | p Value * | Athletes n = 215 | p Value ** | |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| Age, years | 50 ± 14 | 75 ± 5.5 | <0.001 | 42 ± 7.5 | <0.001 |
| Sex, female | 8 (30%) | 155 (48%) | 0.074 | 61 (28%) | 1.000 |
| BMI, kg/m2 | 27 ± 4.8 | 28 ± 4.4 | 0.667 | 22 ± 2.2 | <0.001 |
| Arterial hypertension | 10 (37%) | 324 (100%) | <0.001 | - | - |
| Diabetes mellitus | 4 (15%) | 85 (26%) | 0.012 | - | - |
| Dyslipidemia | 11 (41%) | 209 (65%) | 0.269 | - | - |
| Coronary artery disease | 2 (7%) | 134 (41%) | <0.001 | - | - |
| Congestive heart failure | - | 9 (3%) | 1.000 | - | - |
| Previous thrombotic event | 2 (7%) | 57 (18%) | 0.282 | - | - |
| Medication | |||||
| Betablocker | 14 (52%) | 155 (48%) | 0.842 | - | - |
| Calcium channel blocker | 9 (33%) | 99 (31%) | 0.831 | - | - |
| ACE inhibitors | 5 (19%) | 97 (30%) | 0.272 | - | - |
| ARB | 3 (11%) | 133 (41%) | 0.002 | - | - |
| Aldactone | 1 (4%) | 10 (3%) | 0.602 | - | - |
| Diuretics | 4 (15%) | 95 (29%) | 0.121 | - | - |
| Statins | 10 (37%) | 200 (62%) | 0.013 | - | - |
| ECG | |||||
| Heart rate, bpm | 63 ± 11 | 67 ± 11 | 0.042 | 55 ± 7.8 | <0.001 |
| PR interval, ms | 191 ± 48 | 178 ± 31 | 0.034 | 165 ± 26 | <0.001 |
| QRS width, ms | 110 ± 27 | 97 ± 20 | 0.002 | 95 ± 10 | <0.001 |
| QTc, ms | 448 ± 27 | 439 ± 24 | 0.059 | 414 ± 23 | <0.001 |
| 24 h Holter ECG | |||||
| Minimal heart rate, beats per minute | 54 ± 8.4 | 54 ± 7.6 | 0.570 | 45 ± 6.3 | <0.001 |
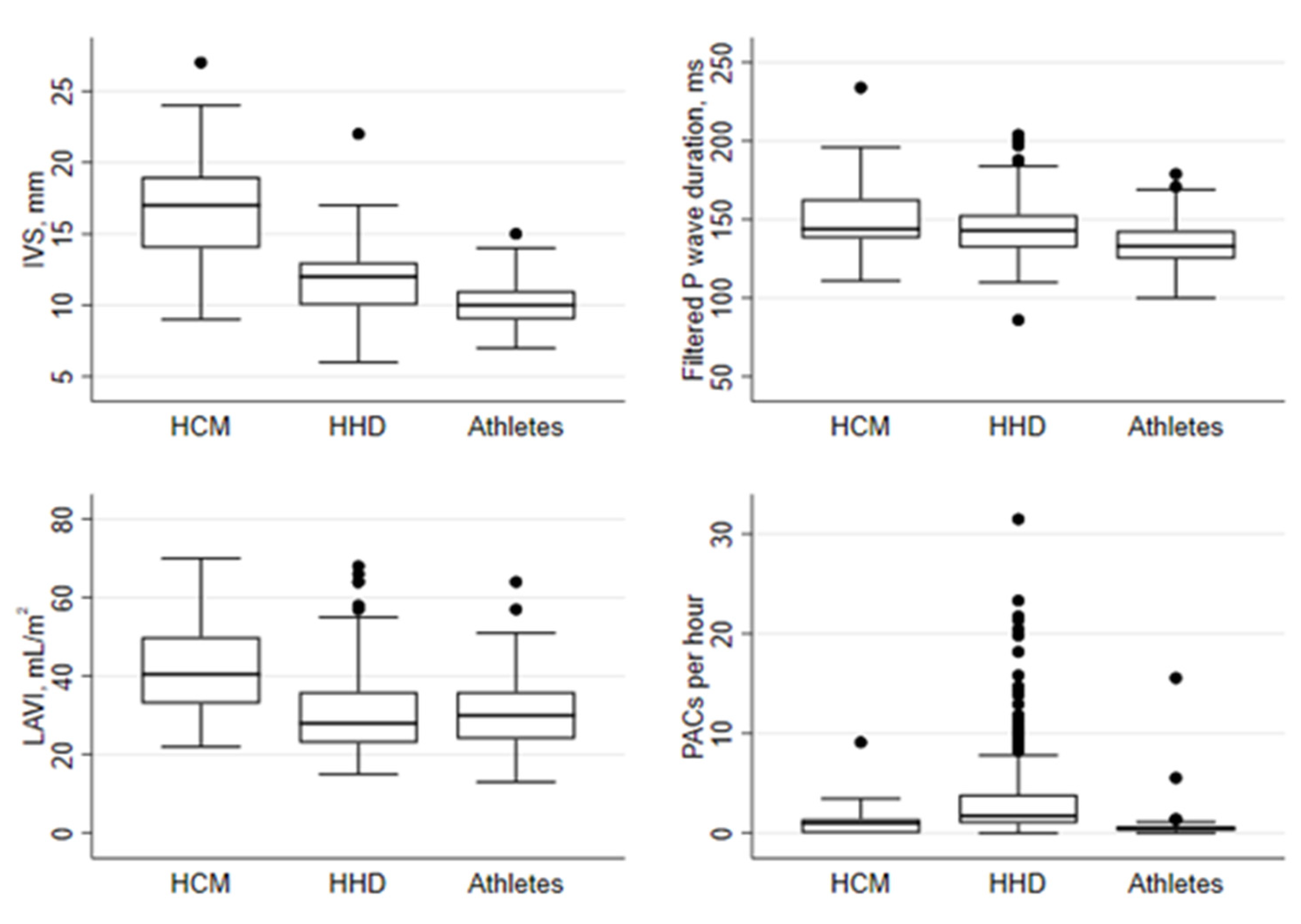
| Number of PACs per hour | 4.9 ± 16 | 27 ± 86 | <0.001 | 2.7 ± 23 | 0.639 |
| SAECG | |||||
| Filtered P-wave duration, ms | 153 ± 26 | 144 ± 16 | 0.012 | 134 ± 14 | <0.001 |
| RMS voltage of P wave, μV | 7.5 ± 2.4 | 6.4 ± 2.3 | 0.018 | 7.7 ± 2.6 | 0.648 |
| P-wave integral, μVs | 850 ± 272 | 672 ± 235 | <0.001 | 773 ± 260 | 0.153 |
| RMS voltage of terminal 20 ms, μV | 3.8 ± 1.4 | 4.3 ± 2.4 | 0.320 | 4.6 ± 2.2 | 0.073 |
| RMS voltage of terminal 30 ms, μV | 3.9 ± 1.3 | 4.3 ± 2.4 | 0.332 | 4.7 ± 2.3 | 0.073 |
| RMS voltage of terminal 40 ms, μV | 5.2 ± 1.8 | 5.5 ± 8.6 | 0.878 | 5.7 ± 2.7 | 0.330 |
| Laboratory | |||||
| BNP, pg/mL | 142 ± 126 | 84 ± 93 | 0.002 | - | - |
| hsTNT, μg/L | 0.02 ± 0.02 | 0.01 ± 0.02 | 0.391 | - | - |
| hsCRP, mg/L | 2.8 ± 4.0 | 3.1 ± 3.9 | 0.726 | - | - |
| Echocardiography | |||||
| LVEF, % | 66 ± 7.0 | 61 ± 6.9 | <0.001 | 65 ± 5.3 | 0.475 |
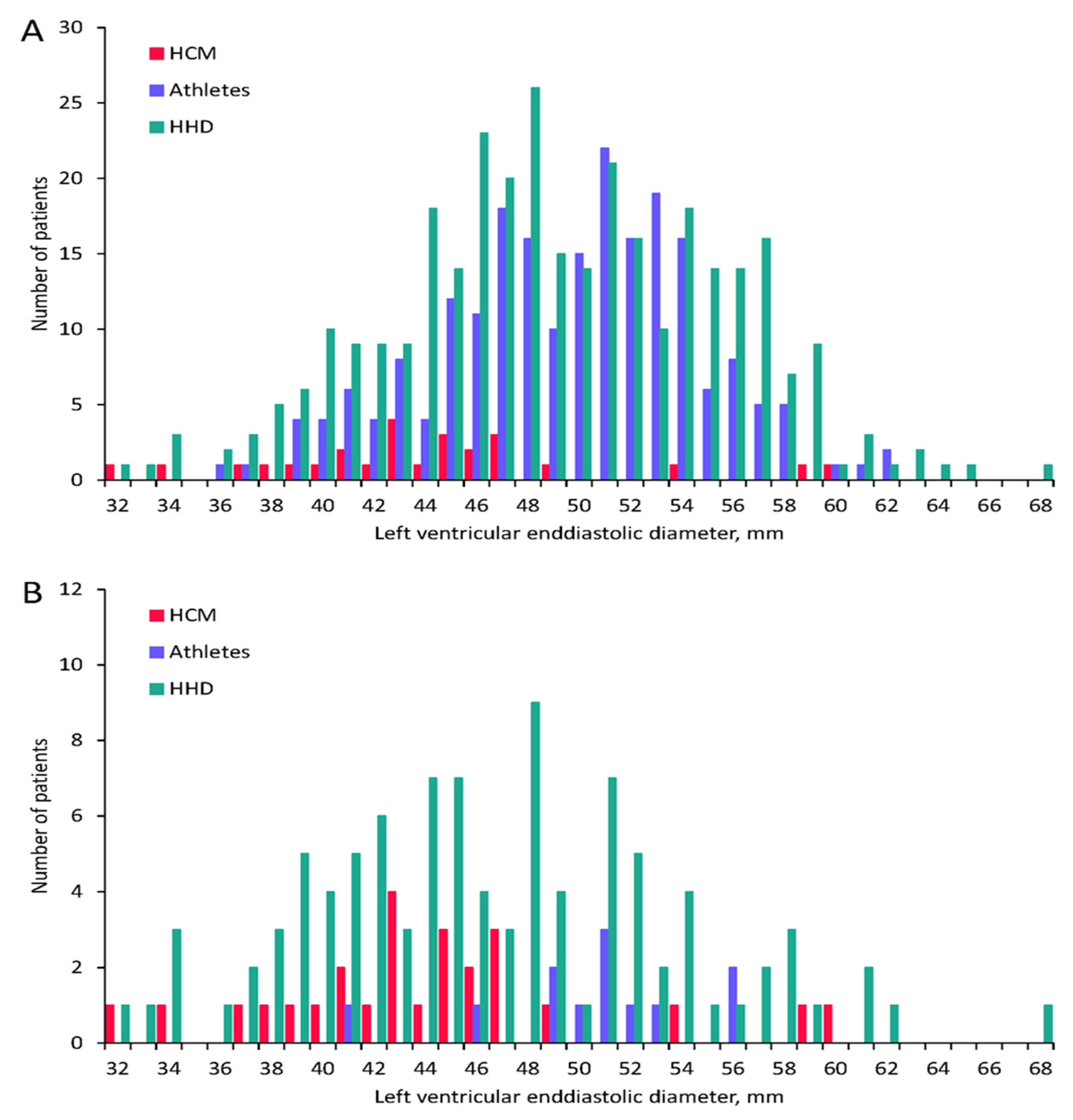
| LVEDD, mm | 44 ± 6.4 | 49 ± 6.3 | <0.001 | 50 ± 4.9 | <0.001 |
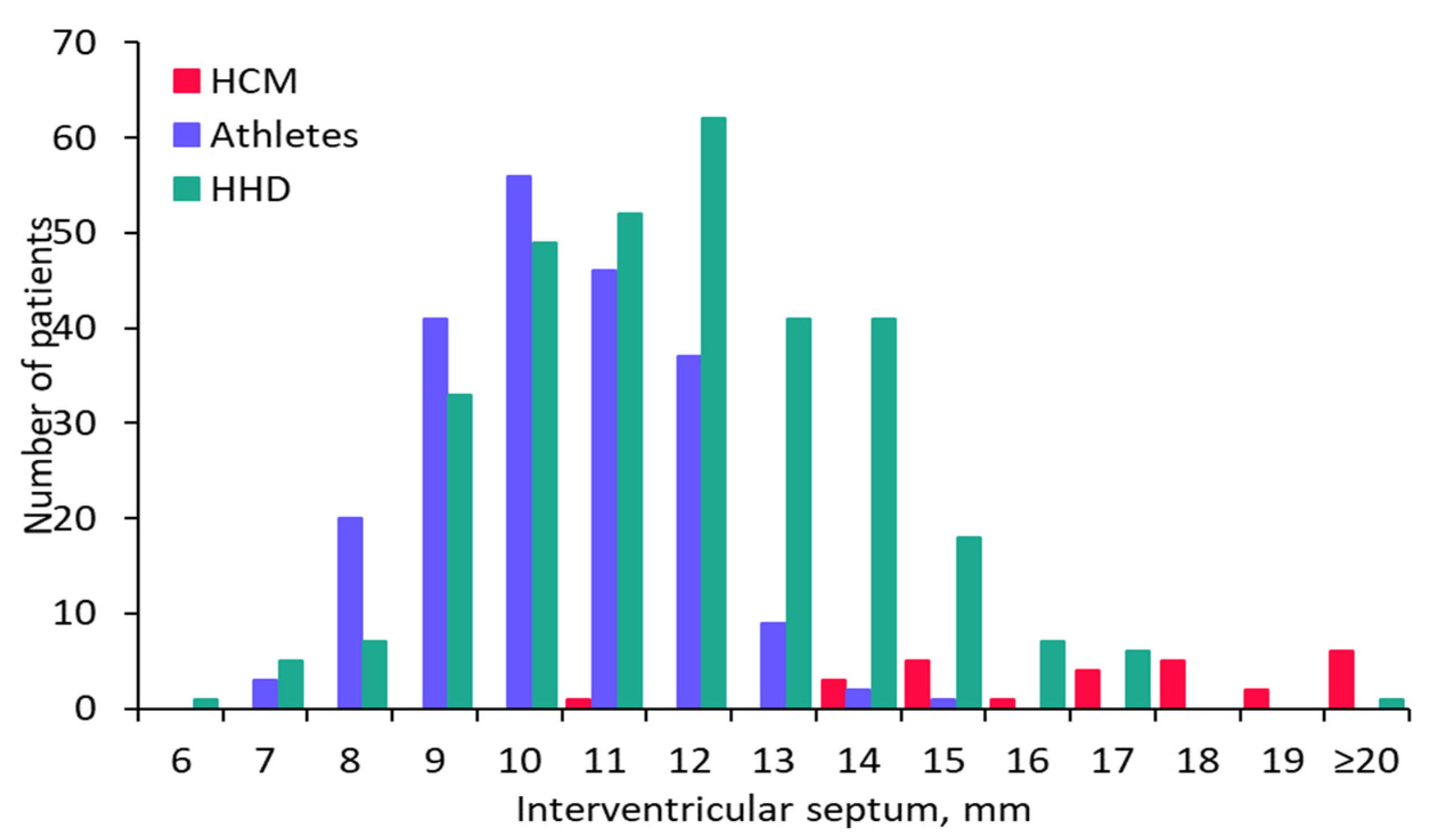
| IVS, mm | 18 ± 3.4 | 12 ± 2.2 | <0.001 | 10 ± 1.5 | <0.001 |
| PW, mm | 11 ± 3.3 | 11 ± 1.9 | 0.318 | 10 ± 1.3 | <0.001 |
| LVMI, g/m2 | 154 ± 56 | 133 ± 30 | 0.003 | 99 ± 20 | <0.001 |
| RVD, mm | 32 ± 5.7 | 32 ± 4.5 | 0.947 | 36 ± 3.8 | <0.001 |
| RV TDI S, cm/s | 13 ± 2.8 | 13 ± 3.1 | 0.230 | 14 ± 2.1 | 0.001 |
| RV diastolic area, cm2 | 18 ± 3.5 | 17 ± 4.6 | 0.280 | 23 ± 3.6 | <0.001 |
| LAVI, ml/m2 | 43 ± 14 | 30 ± 10 | <0.001 | 31 ± 9.5 | <0.001 |
| E wave, cm/s | 71 ± 20 | 67 ± 20 | 0.353 | 77 ± 13 | 0.042 |
| A wave, cm/s | 62 ± 25 | 87 ± 20 | <0.001 | 51 ± 12 | <0.001 |
| E wave/A wave ratio | 1.4 ± 1.0 | 0.8 ± 0.3 | <0.001 | 1.6 ± 0.4 | 0.123 |
| Isovolumetric relaxation time, ms | 101 ± 13 | 97 ± 23 | 0.501 | 84 ± 13 | <0.001 |
| E deceleration time, ms | 222 ± 60 | 259 ± 66 | 0.006 | 175 ± 29 | <0.001 |
| E wave TDI, cm/s | 5.8 ± 1.8 | 5.7 ± 1.6 | 0.722 | 10 ± 1.8 | <0.001 |
| E wave/E wave TDI ratio | 14 ± 10 | 12 ± 4.5 | 0.144 | 7.5 ± 1.5 | <0.001 |
| A wave TDI, cm/s | 7.2 ± 2.1 | 10 ± 2.2 | <0.001 | 8.7 ± 1.7 | <0.001 |
| Age | p Value | Sex (Female) | p Value | HCM vs. HHD | p Value | HCM vs. Athletes | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Univariable | ||||||||
| fPWD, ms | 0.3 (0.2 to 0.4) | <0.001 | −7.7 (−10.6 to −4.7) | <0.001 | −8.8 (−18.6 to 1.0) | 0.079 | −19.2 (−29.0 to −9.3) | <0.001 |
| PACs per hour, sqrt | 0.3 (0.3 to 0.4) | <0.001 | −3.2 (−6.4 to 0.0) | 0.053 | 8.8 (5.0 to 12.6) | <0.001 | −3.9 (−7.3 to −0.5) | 0.026 |
| PR interval, ms | 0.5 (0.3 to 0.6) | <0.001 | −7.6 (−12.6 to −2.5) | 0.003 | −13.7 (−31.7 to 4.3) | 0.135 | −26.3 (−44.3 to −8.2) | 0.004 |
| LAVI, mL/m2 | −0.0 (−0.1 to 0.0) | 0.219 | 0.6 (−1.3 to 2.5) | 0.537 | −12.8 (−18.1 to −7.4) | <0.001 | −12.5 (−18.0 to −6.9) | <0.001 |
| BNP, pg/mL | 0.3 (−1.3 to 1.8) | 0.738 | 15.4 (−5.1 to 35.8) | 0.140 | −59 (−107 to −11) | 0.017 | - | - |
| Multivariable | ||||||||
| fPWD, ms | 0.4 (0.2 to 0.6) | <0.001 | −9.4 (−12.0 to −6.7) | <0.001 | −16.9 (−26.3 to −7.5) | <0.001 | −16.3 (−26.2 to −6.3) | 0.001 |
| PACs per hour, sqrt | 0.0 (−0.2 to 0.3) | 0.804 | −3.1 (−6.2 to 0.0) | 0.053 | 8.5 (0.8 to 16.1) | 0.029 | −2.9 (−7.8 to 1.9) | 0.238 |
| PR interval, ms | 1.1 (0.7 to 1.5) | <0.001 | −10.1 (−14.9 to −5.2) | <0.001 | −38.5 (−59.2 to −17.7) | <0.001 | −18.4 (−33.8 to −3.0) | 0.020 |
| LAVI, mL/m2 | 0.1 (−0.1 to 0.2) | 0.395 | 1.0 (−0.8 to 2.9) | 0.253 | −14.6 (−21.0 to −8.2) | <0.001 | −12.3 (−18.0 to −6.5) | <0.001 |
| BNP, pg/mL | 2.9 (1.3 to 4.6) | 0.001 | 18.9 (−0.6 to 38.5) | 0.058 | −135.2 (−190.7 to −79.8) | <0.001 | - | - |
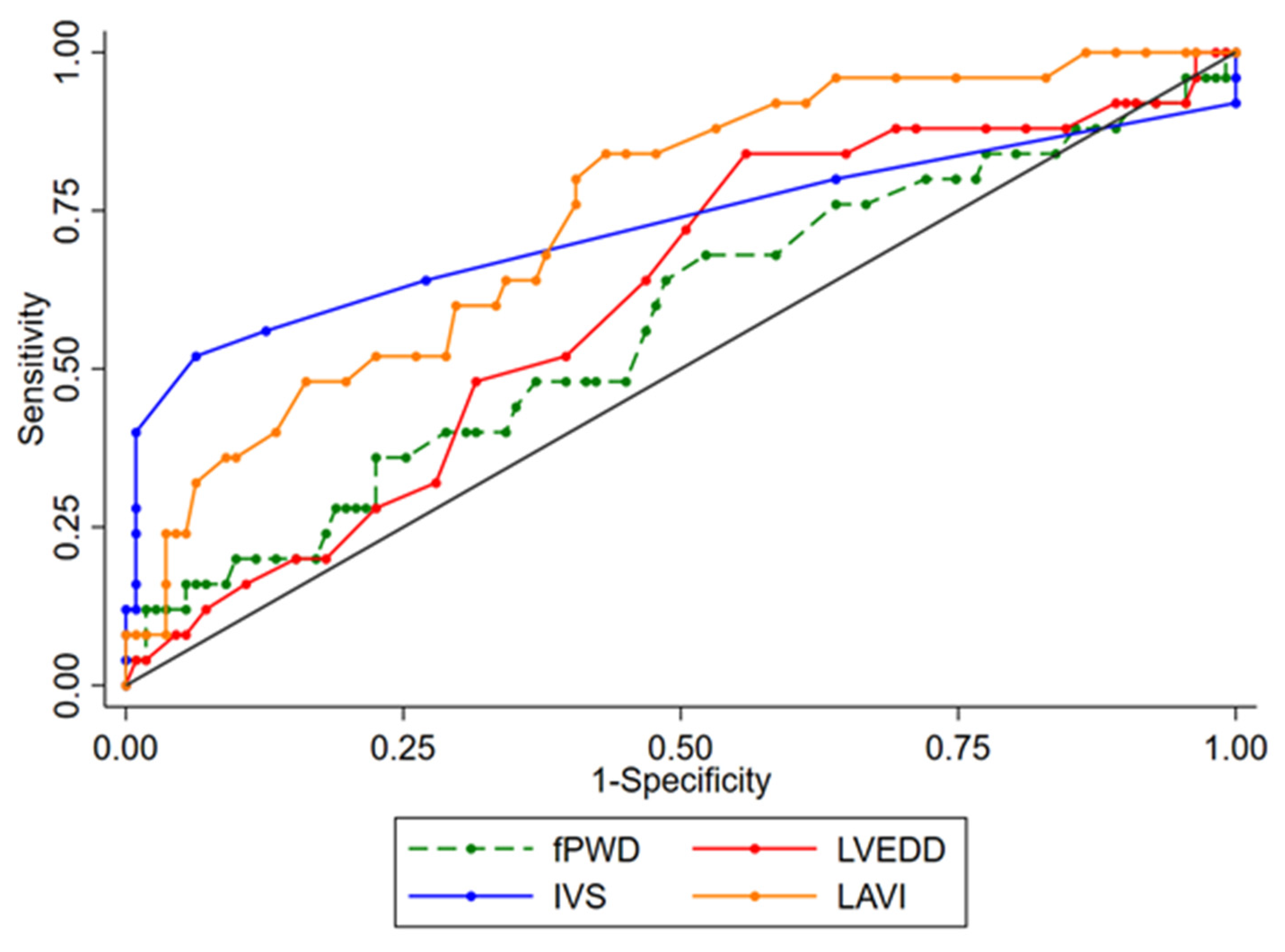
| Variable | AUC (95%-CI) | Cut-Off Point | Youden Index | Sensitivity | Specificity | p-Value | |
|---|---|---|---|---|---|---|---|
| HCM vs. HHD | fPWD, ms | 0.587 (0.461,0.712) | 139 | 0.177 | 0.704 | 0.474 | 0.176 |
| HCM vs. HHD | LVEDD, mm * | 0.610 (0.494,0.727) | 48 | 0.285 | 0.846 | 0.439 | 0.088 |
| HCM vs. HHD | IVS, mm | 0.863 (0.773,0.952) | 14 | 0.571 | 0.852 | 0.719 | <0.001 |
| HCM vs. HHD | LAVI, mL/m2 | 0.733 (0.633,0.832) | 31 | 0.382 | 0.815 | 0.568 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Servatius, H.; Raab, S.; Asatryan, B.; Haeberlin, A.; Branca, M.; de Marchi, S.; Brugger, N.; Nozica, N.; Goulouti, E.; Elchinova, E.; et al. Differences in Atrial Remodeling in Hypertrophic Cardiomyopathy Compared to Hypertensive Heart Disease and Athletes’ Hearts. J. Clin. Med. 2022, 11, 1316. https://doi.org/10.3390/jcm11051316
Servatius H, Raab S, Asatryan B, Haeberlin A, Branca M, de Marchi S, Brugger N, Nozica N, Goulouti E, Elchinova E, et al. Differences in Atrial Remodeling in Hypertrophic Cardiomyopathy Compared to Hypertensive Heart Disease and Athletes’ Hearts. Journal of Clinical Medicine. 2022; 11(5):1316. https://doi.org/10.3390/jcm11051316
Chicago/Turabian StyleServatius, Helge, Simon Raab, Babken Asatryan, Andreas Haeberlin, Mattia Branca, Stefano de Marchi, Nicolas Brugger, Nikolas Nozica, Eleni Goulouti, Elena Elchinova, and et al. 2022. "Differences in Atrial Remodeling in Hypertrophic Cardiomyopathy Compared to Hypertensive Heart Disease and Athletes’ Hearts" Journal of Clinical Medicine 11, no. 5: 1316. https://doi.org/10.3390/jcm11051316
APA StyleServatius, H., Raab, S., Asatryan, B., Haeberlin, A., Branca, M., de Marchi, S., Brugger, N., Nozica, N., Goulouti, E., Elchinova, E., Lam, A., Seiler, J., Noti, F., Madaffari, A., Tanner, H., Baldinger, S. H., Reichlin, T., Wilhelm, M., & Roten, L. (2022). Differences in Atrial Remodeling in Hypertrophic Cardiomyopathy Compared to Hypertensive Heart Disease and Athletes’ Hearts. Journal of Clinical Medicine, 11(5), 1316. https://doi.org/10.3390/jcm11051316






