The Influence of Surgical Correction of Idiopathic Scoliosis on the Function of Respiratory Muscles
Abstract
1. Introduction
- Curvatures larger than 50 degrees progress even after skeletal maturity;
- Curvatures of greater magnitude cause loss of pulmonary function, and much larger ones cause respiratory failure;
- The more progressed the curvature is, the more difficult its surgical treatment [9].
- Does the size of the curvature of the spine affect initial values of strength of respiratory muscles?
- Does the type of surgery performed affect the strength of respiratory muscles?
- What is the strength of respiratory muscles before the procedure, 7 days after the procedure and 3 months after the procedure?
2. Materials and Methods
2.1. Intervention
2.2. Outcome Measures
2.3. Statistical Analysis
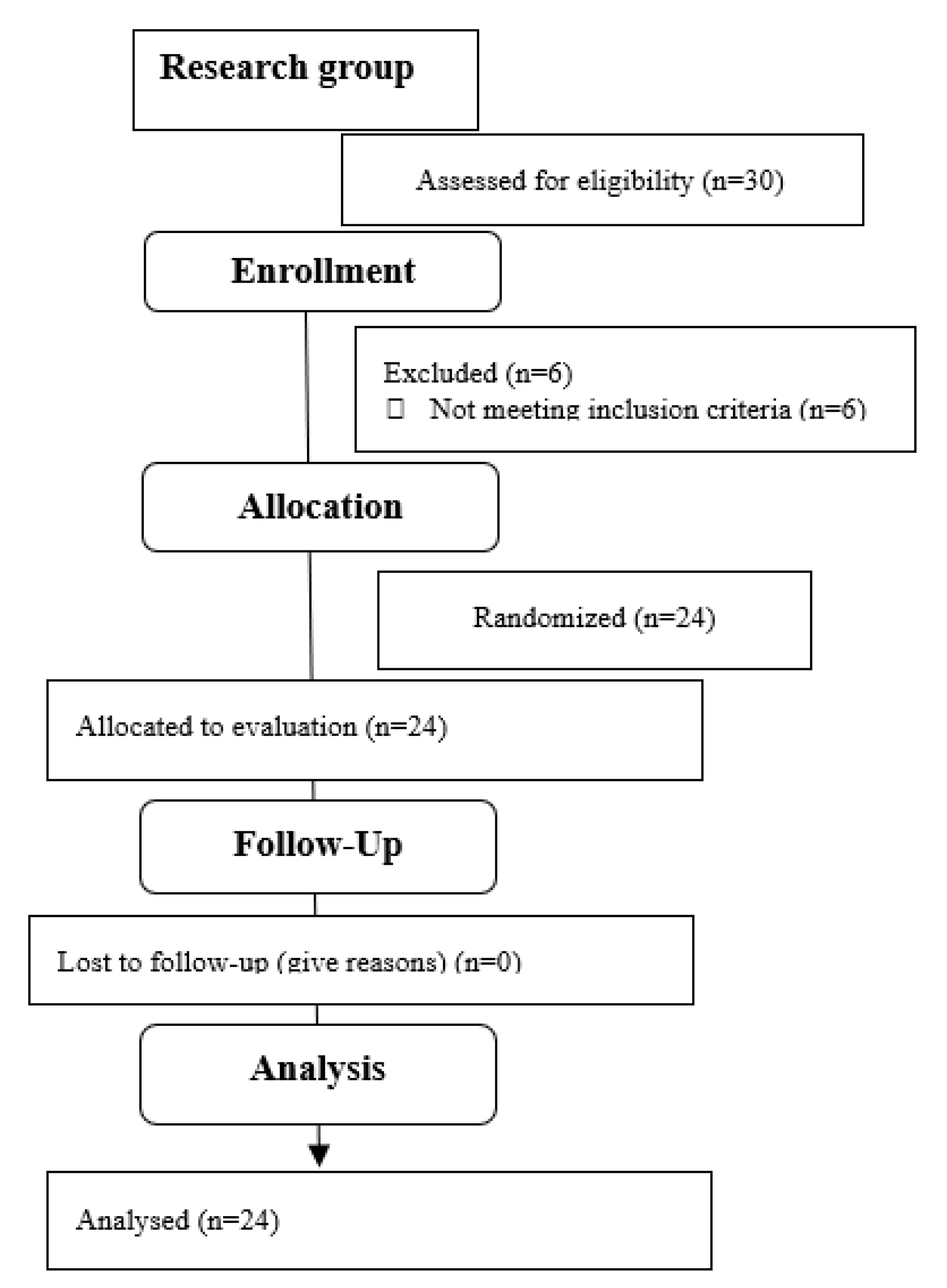
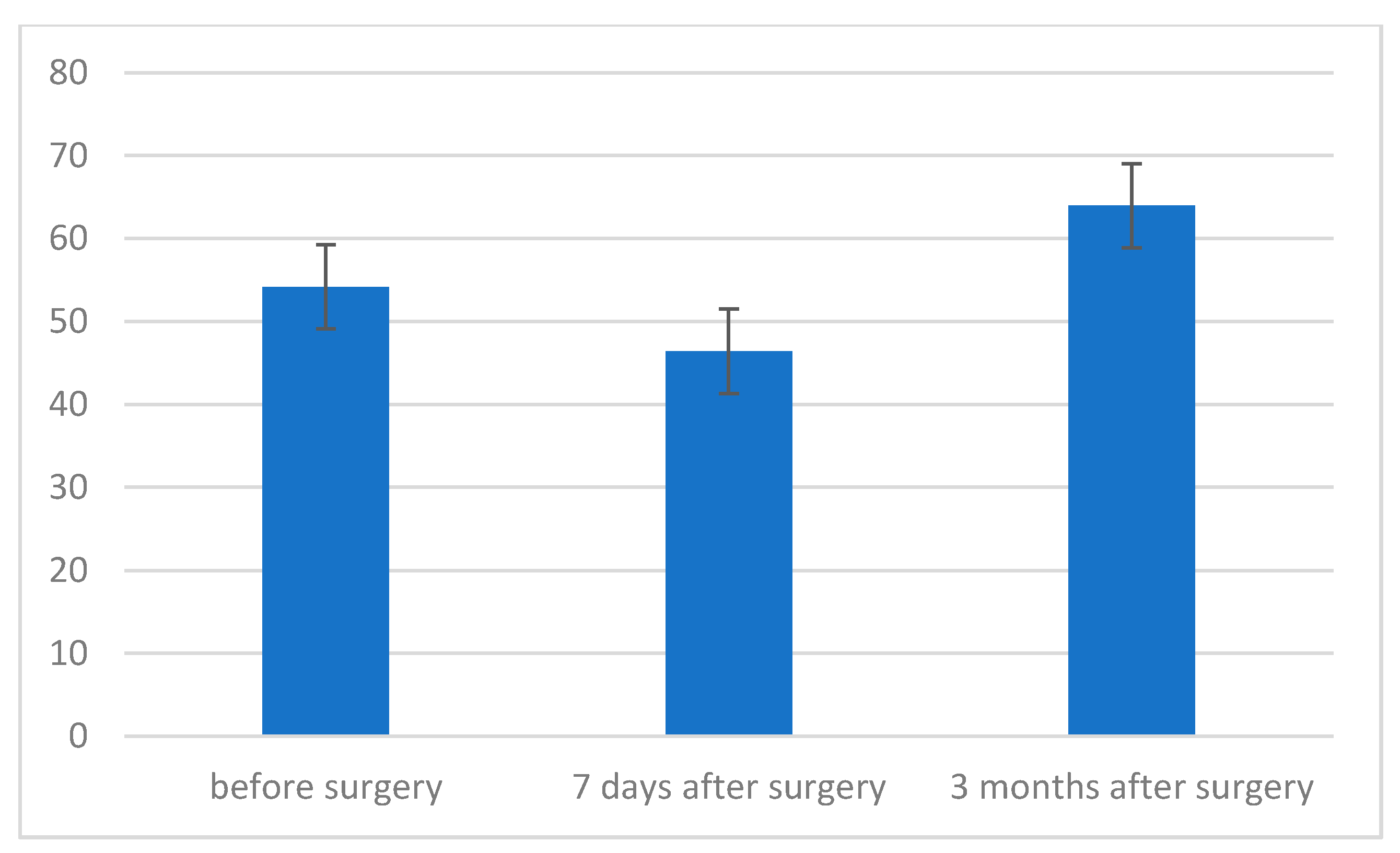
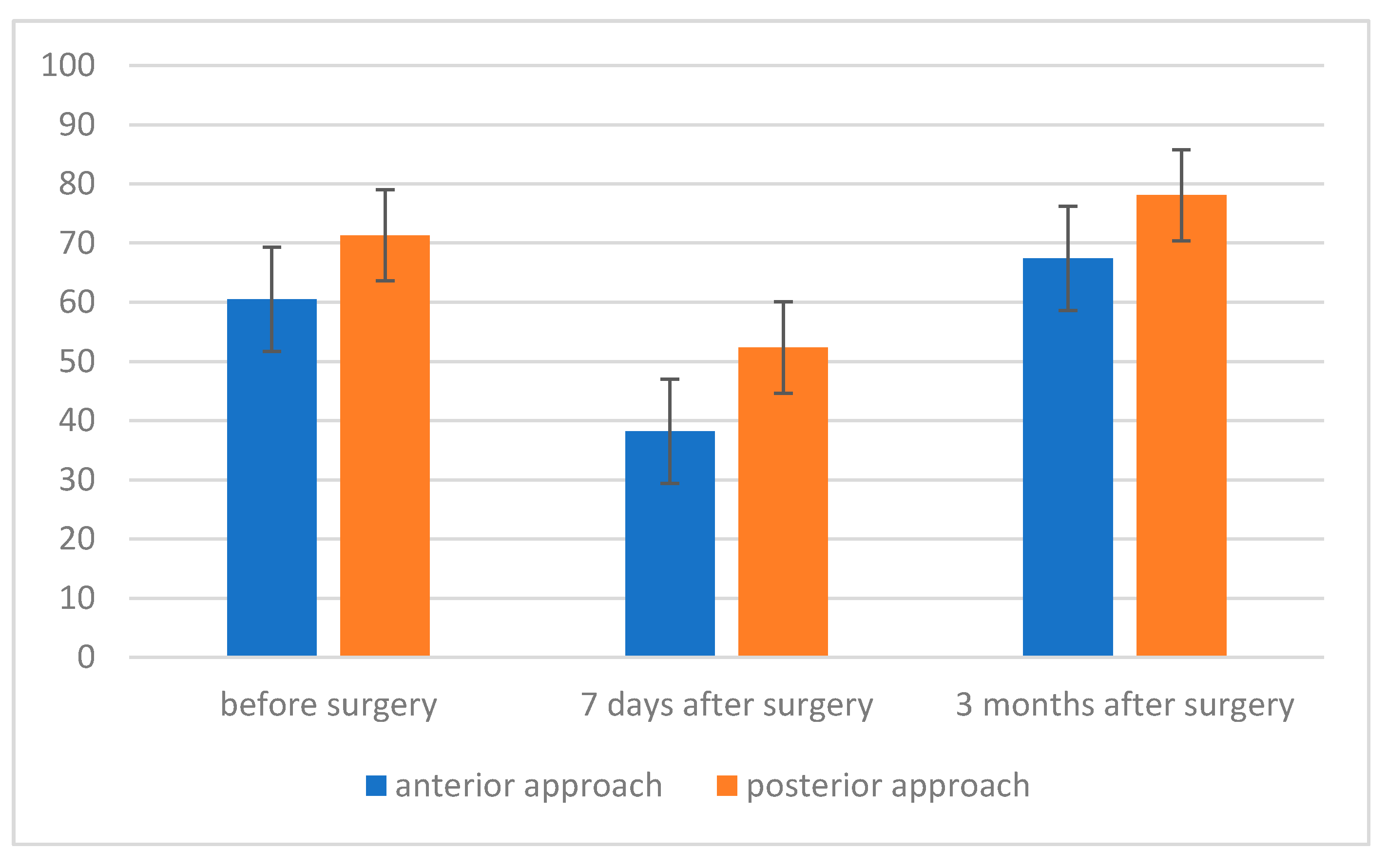
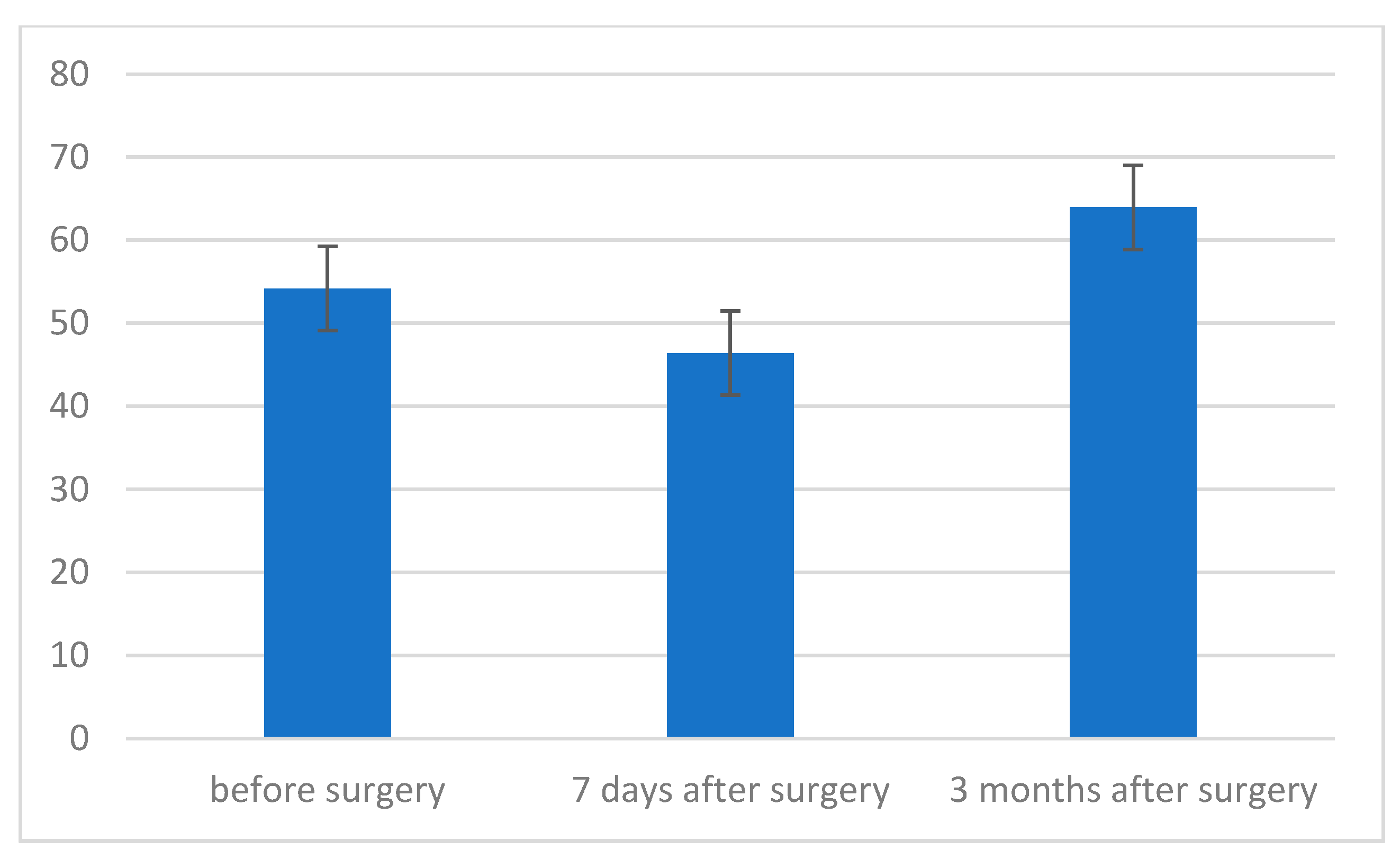
3. Results
4. Discussion
Study Limitations
5. Conclusions
- The degree of curvature of the spine before the procedure does not significantly affect initial values of the strength of respiratory muscles measured by the value of maximum pressure during exhalation (MEP) and the initial value of maximum pressure during inspiration (MIP);
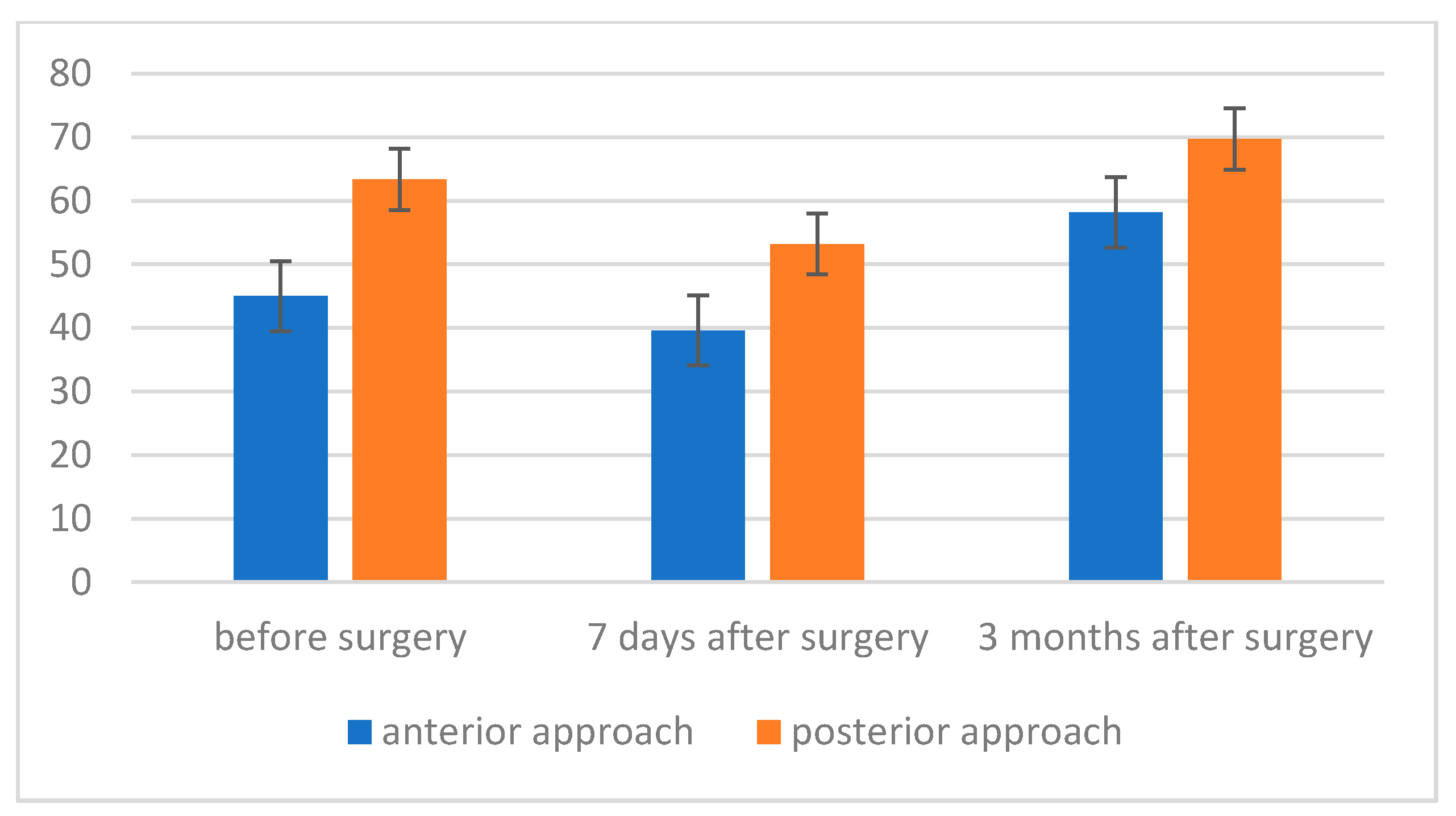
- The level of maximum inspiratory pressure (MIP) is not dependent on the type of surgery. However, a higher MIP value was noted in the group after posterior approach surgery. On the other hand, the averaged maximum pressure during exhalation (MEP) was significantly higher in the group of patients who underwent the procedure using the posterior approach;
- The strength of respiratory muscles decreases significantly immediately after the procedure, but, over time (3 months), its value increases, often exceeding the baseline value.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, J.C.; Castelein, R.M.; Chu, W.C.; Danielsson, A.J.; Dobbs, M.B.; Grivas, T.B.; Gurnett, C.A.; Luk, K.D.; Moreau, A.; Newton, P.O.; et al. Adolescent idiopathic scoliosis. Nat. Rev. Dis. Primers 2015, 1, 15030. [Google Scholar] [CrossRef]
- Negrini, S.; Donzelli, S.; Aulisa, A.G.; Czaprowski, D.; Schreiber, S.; de Mauroy, J.C.; Diers, H.; Grivas, T.B.; Knott, P.; Kotwicki, T.; et al. 2016 SOSORT guidelines: Orthopaedic and rehabilitation treatment of idiopathic scoliosis during growth. Scoliosis Spinal Disord. 2018, 13, 3. [Google Scholar] [CrossRef]
- Lehnert-Schroth, C. Three-Dimensional Treatment for Scoliosis: A Physiotherapeutic Method for Deformities of the Spine, 1st ed.; Martindale Press: Palo Alto, CA, USA, 2007; pp. 11–74. [Google Scholar]
- Kurzeja, P.; Prusak, J.; Tomalak, W. Assessment of respiratory muscle strength during rehabilitation treatment in girls with lateral idiopathic curvature of the spine. Acta Pneumonol. Allergol. Pediatr. 2011, 14, 12–18. [Google Scholar]
- Rinsky, L.A.; Gamble, J.G. Adolescent idiopathic scoliosis. West J. Med. 1998, 148, 182–191. [Google Scholar]
- Tomalak, W. Assessment of respiratory muscles functions. Acta Pneumonol. Allergol. Pediatr. 2001, 4, 3–5. [Google Scholar]
- Hamnegård, C.H.; Wragg, S.; Kyroussis, D.; Aquilina, R.; Moxham, J.; Green, M. Portable measurement of maximum mouth pressures. Eur. Respir. J. 1994, 7, 398–401. [Google Scholar] [CrossRef]
- Tomalak, W.; Pogorzelski, A.; Prusak, J. Normal values for maximal static inspiratory and expiratory pressures in healthy children. Pediatr. Pulmonol. 2002, 34, 42–46. [Google Scholar] [CrossRef]
- Maruyama, T.; Takeshita, K. Surgical treatment of scoliosis: A review of techniques currently applied. Scoliosis 2008, 3, 6. [Google Scholar] [CrossRef]
- Dogar, F.; Argun, M.; Erdem, S.; Gurbuz, K.; Argun, A.S.; Kafadar, I.H. Clinical and radiological results of surgically treated patients with adolescent idiopathic scoliosis and the effects of pulmonary rehabilitation on respiration functions. Medicine (Baltimore) 2021, 100, e24675. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.medicom.com.pl/products/miernik-sily-miesni-oddechowych-microrpm/ (accessed on 10 November 2021).
- Cotes, J.E. Lung Function. Assessment and Application in Medicine; PZWL: Warszawa, Poland, 1969. [Google Scholar]
- Upadhyay, S.S.; Mullaji, A.B.; Luk, K.D.; Leong, J.C. Relation of spinal and thoracic cage deformities and their flexibilities with altered pulmonary functions in adolescent idiopathic scoliosis. Spine 1995, 20, 2415–2420. [Google Scholar] [CrossRef]
- Kose, N.; Campbell, R.M. Congenital scoliosis. Med. Sci. Monit. 2004, 10, 104–110. [Google Scholar]
- Upathyay, S.S.; Mullai, A.B.; Luk, K.D.; Leong, J.C. Evaluation of deformities and pulmonary function in adolescent idiopathic right thoracic scoliosis. Eur. Spine J. 1995, 4, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Liaw, M.Y.; Chen, W.J.; Cheng, P.; Wong, A.M.; Chiou, W.K. Pulmonary function and spinal characteristics: Their relationships in persons with idiopathic and postpoliomyelitic scoliosis. Arch. Phys. Med. Rehabil. 2001, 82, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Barrios, C.; Perez-Encinas, C.; Maruenda, J.I.; Laguia, M. Significant ventilatory functional restriction in adolescents with mild or moderate scoliosis during maximal exercise tolerance test. Spine 2006, 31, 1512. [Google Scholar] [CrossRef]
- Kempen, D.H.R.; Heemskerk, J.L.; Kaçmaz, G.; Altena, M.C.; Reesink, H.J.; Vanhommerig, J.W.; Willigenburg, N.W. Pulmonary function in children and adolescents with untreated idiopathic scoliosis: A systematic review with meta-regression analysis. Spine J. 2021. [Google Scholar] [CrossRef]
- DiRocco, P.J.; Vaccaro, P. Cardiopulmonary functioning in adolescent patients with mild idiopathic scoliosis. Arch. Phys. Med. Rehabil. 1988, 69, 198–201. [Google Scholar]
- Koumbourlis, A.C. Scoliosis and the respiratory system. Pediatr. Respi. Rev. 2006, 7, 152–160. [Google Scholar] [CrossRef]
- Boyer, J.; Nikhil, A.; Taddonio, R. Evidence of airway obstruction in children with idiopathic scoliosis. Chest 1996, 109, 1532–1535. [Google Scholar] [CrossRef]
- Kearon, C.; Vivianii, G.R.; Kirkley, A.; Killian, K.J. Factors Determining pulmonary function in adolescent idiopathic scoliosis. Am. Rev. Respir. Dis. 1993, 148, 288–294. [Google Scholar] [CrossRef]
- Smyth, R.J.; Chapman, K.R.; Wright, T.A.; Crawford, J.S.; Rebuck, A.S. Pulmonary function in adolescents with mild idiopathic scoliosis. Thorax 1984, 29, 901–904. [Google Scholar] [CrossRef]
- Durmała, J.; Tomalak, W. Assessment of respiratory muscle function in adolescents with lateral curvature of the spine. Acta Pneumonol. Allergol. Pediatr. 2002, 5, 14–17. [Google Scholar]
- Banjar, H.H. Pediatric scoliosis and the lung. Saudi Med. J. 2003, 24, 957–963. [Google Scholar] [PubMed]
- Day, G.A.; Upadhyay, S.S.; Ho, E.K.; Leong, J.C.; Ip, M. Pulmonary functions in congenital scoliosis. Spine 1994, 19, 1027–1031. [Google Scholar] [CrossRef]
- Goldberg, C.J.; Gillic, I.; Connaughton, O.; Moore, D.P.; Fogarty, E.E.; Canny, G.J.; Dowling, F.E. Respiratory function and cosmesis at maturity in infantile-onset scoliosis. Spine 2003, 15, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lenke, L.G.; Bridwell, K.H.; Kim, K.L.; Steger-May, K. Pulmonary function in adolescent idiopathic scoliosis relative to the surgical procedure. J. Bone Jt. Surg. 2005, 87, 1534–1541. [Google Scholar] [CrossRef]
- Lenke, L.G.; Bridwell, K.H.; Blanke, K.; Baldus, C. Analysis of pulmonary function and chest cage dimension changes after thoracoplasty in idiopathic scoliosis. Spine 1995, 20, 1343–1350. [Google Scholar] [CrossRef]
- Lenke, L.G.; White, D.K.; Kemp, J.S.; Bridwell, K.H.; Blanke, K.; Engsberg, J.R. Evaluation of ventilatory efficiency during exercise in patients with idiopathic scoliosis undergoing spinal fusion. Spine 2002, 27, 2041–2045. [Google Scholar] [CrossRef]
- Newton, P.O.; Faro, F.D.; Gollogly, S.; Betz, R.R.; Lenke, L.G.; Lowe, T.G. Results of preoperative pulmonary function testing of adolescents with idiopathic scoliosis. A study of six hundred and thirty-one patients. J. Bone Jt. Surg. 2005, 87, 1937–1946. [Google Scholar] [CrossRef]
- Pehrsson, K.; Danielsson, A.; Nachemson, A. Pulmonary function in adolescent idiopathic scoliosis: A 25 year follow up after surgery or start of brace treatment. Thorax 2001, 56, 388–393. [Google Scholar] [CrossRef]
- Sakic, K.; Pecina, M.; Pavicic, M. Cardiorespiratory function in surgically treated thoracic scoliosis with respect to degree and apex of scoliosis curve. Resporation 1992, 59, 327–331. [Google Scholar] [CrossRef]
- Sakic, K.; Pecina, M.; Pavicic, M. Pulmonary function in adolescents with idiopathic thoracic scoliosis before and after surgical correction. Lijec Vjesn 1993, 115, 268–273. [Google Scholar] [PubMed]
- Wong, C.A.; Cole, A.A.; Watson, L.; Webb, J.K.; Johnston, I.D.; Kinnear, V.J. Pulmonary function before and after anterior spinal surgery in adult idiopathic scoliosis. Thorax 1996, 51, 534–536. [Google Scholar] [CrossRef] [PubMed]
- Vedantam, R.; Crawford, A.H. The role of preoperative pulmonary function tests in patients with adolescent idiopathic scoliosis undergoing posterior spinal fusion. Spine 1997, 22, 2731–2734. [Google Scholar] [CrossRef]
- Vedantam, R.; Lenke, L.G.; Bridwell, K.H.; Hass, J.; Linville, D. A prospective evaluation of pulmonary function in patients with adolescent idiopathic scoliosis relative to the surgical approach used for spinal arthrodesis. Spine 2000, 25, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.G.; Wang, W.; Qiu, G.X.; Wang, Y.P.; Weng, X.S.; Xu, H.G. The role of preoperative pulmonary function tests in the surgical treatment of scoliosis. Spine 2005, 30, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Fraire, J.A.; Margetis, M.M.; Skaggs, D.L.; Tolo, V.T.; Keens, T.G. The effect of scoliosis surgery on lung function in the immediate postoperative period. Spine 2005, 1, 2182–2185. [Google Scholar] [CrossRef]
- Saito, W.; Mizuno, K.; Inoue, G.; Imura, T.; Nakazawa, T.; Miyagi, M.; Shirasawa, E.; Uchida, K.; Takaso, M. Perioperative Evaluation of Respiratory Muscle Strength after Scoliosis Correction in Patients with Duchenne Muscular Dystrophy. Asian Spine J. 2017, 11, 787–792. [Google Scholar] [CrossRef][Green Version]
- Flores, F.; Cavaleiro, J.; Lopes, A.A.; Ribeiro, F.; Oliveira, A. Preoperative pulmonary function and respiratory muscle strength in Portuguese adolescents with idiopathic scoliosis. Rev. Port. Pneumol. 2016, 22, 52–53. [Google Scholar] [CrossRef]

| Variable | Scoliosis x ± SD | 95% CI |
|---|---|---|
| Age [yrs] | 13.6 ± 0.6 | 13.36–13.84 |
| Height [cm] | 160.6 ± 7.8 | 157.48–163.72 |
| Body weight [kg] | 50.5 ± 4.3 | 48.78–52.22 |
| Cobb [°] | 59.9 ± 12.6 | 54.85–64.94 |
| X | Me | SD | Sk | Kurt | Min | Max | S–W | p | |
|---|---|---|---|---|---|---|---|---|---|
| Cobb (measurement I) | 59.92 | 56 | 12.61 | 0.67 | 0.11 | 38.64 | 90.5 | 0.95 | 0.339 |
| Cobb (measurement II) | 22.67 | 20.7 | 6.40 | 0.77 | −0.31 | 15 | 37.33 | 0.92 | 0.084 |
| MEP (measurement I) | 66.79 | 69 | 21.41 | 0.22 | −1.03 | 32 | 108 | 0.95 | 0.271 |
| MIP (measurement I) | 55.71 | 55 | 16.05 | 0.63 | 0.61 | 29 | 98 | 0.97 | 0.694 |
| MEP (measurement II) | 46.46 | 43 | 19.75 | 1.33 | 2.21 | 16 | 105 | 0.89 | 0.022 |
| MIP (measurement II) | 47.54 | 46 | 18.16 | 0.00 | −0.49 | 14 | 82 | 0.98 | 0.898 |
| MEP (measurement III) | 73.63 | 68 | 25.62 | 0.71 | 0.33 | 37 | 133 | 0.94 | 0.241 |
| MIP (measurement III) | 64.92 | 62.5 | 17.91 | 0.46 | −0.37 | 33 | 100 | 0.96 | 0.524 |
| Approach | X | SE | |
|---|---|---|---|
| MIP measurement I | anterior approach | 60.50 | 6.70 |
| posterior approach | 71.29 | 5.66 | |
| Total | 65.89 | 4.38 | |
| MIP measurement II | anterior approach | 38.20 | 5.96 |
| posterior approach | 52.36 | 5.03 | |
| Total | 45.28 | 3.90 | |
| MIP measurement III | anterior approach | 67.40 | 8.10 |
| posterior approach | 78.07 | 6.85 | |
| Total | 72.74 | 5.30 | |
| Total | anterior approach | 55.37 | 5.96 |
| posterior approach | 67.24 | 5.04 |
| Approach | X | SE | |
|---|---|---|---|
| MEP measurement I | anterior approach | 45.00 | 4.24 |
| posterior approach | 63.36 | 3.58 | |
| Total | 54.18 | 2.78 | |
| MEP measurement II | anterior approach | 39.60 | 5.44 |
| posterior approach | 53.21 | 4.60 | |
| Total | 46.41 | 3.56 | |
| MEP measurement III | anterior approach | 58.20 | 5.48 |
| posterior approach | 69.71 | 4.63 | |
| Total | 63.96 | 3.59 | |
| Total | anterior approach | 47.60 | 4.32 |
| posterior approach | 62.10 | 3.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasiewicz, B.; Rożek, K.; Kurzeja, P.; Daszkiewicz, E.; Ogrodzka-Ciechanowicz, K. The Influence of Surgical Correction of Idiopathic Scoliosis on the Function of Respiratory Muscles. J. Clin. Med. 2022, 11, 1305. https://doi.org/10.3390/jcm11051305
Jasiewicz B, Rożek K, Kurzeja P, Daszkiewicz E, Ogrodzka-Ciechanowicz K. The Influence of Surgical Correction of Idiopathic Scoliosis on the Function of Respiratory Muscles. Journal of Clinical Medicine. 2022; 11(5):1305. https://doi.org/10.3390/jcm11051305
Chicago/Turabian StyleJasiewicz, Barbara, Karina Rożek, Piotr Kurzeja, Edyta Daszkiewicz, and Katarzyna Ogrodzka-Ciechanowicz. 2022. "The Influence of Surgical Correction of Idiopathic Scoliosis on the Function of Respiratory Muscles" Journal of Clinical Medicine 11, no. 5: 1305. https://doi.org/10.3390/jcm11051305
APA StyleJasiewicz, B., Rożek, K., Kurzeja, P., Daszkiewicz, E., & Ogrodzka-Ciechanowicz, K. (2022). The Influence of Surgical Correction of Idiopathic Scoliosis on the Function of Respiratory Muscles. Journal of Clinical Medicine, 11(5), 1305. https://doi.org/10.3390/jcm11051305






