Developing a Mechanistic Approach to Sudden Death Prevention in Mitral Valve Prolapse
Abstract
1. Introduction
2. Patient Characteristics
3. Mechanisms of Arrhythmogenesis
3.1. Ventricular Ectopy
3.2. Myocardial Abnormalities
3.3. Autonomic Nervous System
4. Electrocardiography (ECG) and Holter Monitor
5. Echocardiography
6. Cardiac Magnetic Resonance (CMR) Imaging
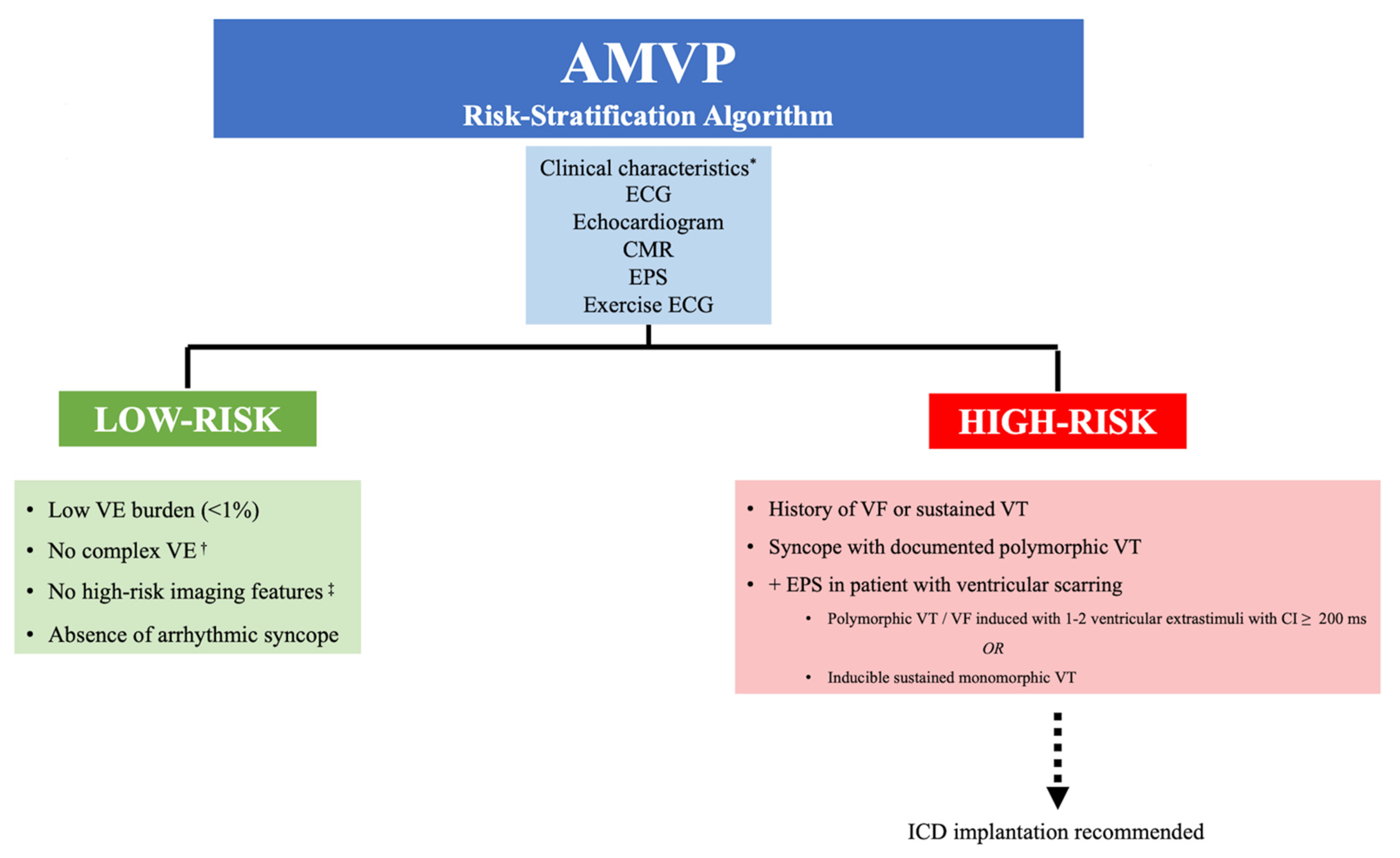
7. Risk Stratification
8. Management
9. Noninvasive/Medical Therapy
10. Implantable Cardioverter-Defibrillators (ICDs)
11. Ventricular Ablation
12. Mitral Valve Surgery
13. Areas of Future Research
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antoine, C.; Michelena, H.I.; Enriquez-Sarano, M. Mitral valve prolapse: Where is the missing link? J. Thorac. Dis. 2016, 8, 2394–2396. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Obel, I.W. Mitral valve billow and prolapse: A brief review at 45 years. Cardiovasc. J. Afr. 2009, 20, 24–26. [Google Scholar] [PubMed]
- Freed, L.A.; Levy, D.; Levine, R.A.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and clinical outcome of mitral-valve prolapse. N. Engl. J. Med. 1999, 341, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Dukkipati, S.R.; Turagam, M.; Liao, S.L.; Adams, D.H.; Reddy, V.Y. Arrhythmic Mitral Valve Prolapse: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2018, 72, 2904–2914. [Google Scholar] [CrossRef] [PubMed]
- Han, H.C.; Ha, F.J.; Teh, A.W.; Calafiore, P.; Jones, E.F.; Johns, J.; Koshy, A.N.; O’Donnell, D.; Hare, D.L.; Farouque, O.; et al. Mitral Valve Prolapse and Sudden Cardiac Death: A Systematic Review. J. Am. Heart Assoc. 2018, 7, e010584. [Google Scholar] [CrossRef]
- Barlow, J.B.; Bosman, C.K.; Pocock, W.A.; Marchand, P. Late systolic murmurs and non-ejection (“mid-late”) systolic clicks. An analysis of 90 patients. Br. Heart J. 1968, 30, 203–218. [Google Scholar] [CrossRef]
- Sriram, C.S.; Syed, F.F.; Ferguson, M.E.; Johnson, J.N.; Enriquez-Sarano, M.; Cetta, F.; Cannon, B.C.; Asirvatham, S.J.; Ackerman, M.J. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J. Am. Coll. Cardiol. 2013, 62, 222–230. [Google Scholar] [CrossRef]
- Kligfield, P.; Levy, D.; Devereux, R.B.; Savage, D.D. Arrhythmias and sudden death in mitral prolapse. Am. Heart J. 1987, 113, 1298. [Google Scholar] [CrossRef]
- Nishimura, R.A.; McGoon, M.D.; Shub, C.; Miller Jr, F.A.; Ilstrup, D.M.; Tajik, A.J. Echocardiographically documented mitral-valve prolapse. Long-term follow-up of 237 patients. N. Engl. J. Med. 1985, 313, 1305–1309. [Google Scholar] [CrossRef]
- Basso, C.; Perazzolo Marra, M.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death. Circulation 2015, 132, 556–566. [Google Scholar] [CrossRef]
- Narayanan, K.; Uy-Evanado, A.; Teodorescu, C.; Reinier, K.; Nichols, G.A.; Gunson, K.; Jui, J.; Chugh, S.S. Mitral valve prolapse and sudden cardiac arrest in the community. Heart Rhythm 2016, 13, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Basso, C.; Iliceto, S.; Thiene, G.; Perazzolo Marra, M. Mitral Valve Prolapse, Ventricular Arrhythmias, and Sudden Death. Circulation 2019, 140, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Delling, F.N.; Aung, S.; Vittinghoff, E.; Dave, S.; Lim, L.J.; Olgin, J.E.; Connolly, A.; Moffatt, E.; Tseng, Z.H. Antemortem and Post-Mortem Characteristics of Lethal Mitral Valve Prolapse Among All Countywide Sudden Deaths. JACC Clin. Electrophysiol. 2021, 7, 1025–1034. [Google Scholar] [CrossRef]
- Dollar, A.L.; Roberts, W.C. Morphologic comparison of patients with mitral valve prolapse who died suddenly with patients who died from severe valvular dysfunction or other conditions. J. Am. Coll. Cardiol. 1991, 17, 921–931. [Google Scholar] [CrossRef]
- Chesler, E.; King, R.A.; Edwards, J.E. The myxomatous mitral valve and sudden death. Circulation 1983, 67, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Perazzolo Marra, M.; Basso, C.; De Lazzari, M.; Rizzo, S.; Cipriani, A.; Giorgi, B.; Lacognata, C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Morphofunctional Abnormalities of Mitral Annulus and Arrhythmic Mitral Valve Prolapse. Circ. Cardiovasc. Imaging 2016, 9, e005030. [Google Scholar] [CrossRef]
- Muthukumar, L.; Rahman, F.; Jan, M.F.; Shaikh, A.; Kalvin, L.; Dhala, A.; Jahangir, A.; Tajik, A.J. The Pickelhaube Sign: Novel Echocardiographic Risk Marker for Malignant Mitral Valve Prolapse Syndrome. JACC Cardiovasc. Imaging 2017, 10, 1078–1080. [Google Scholar] [CrossRef]
- Avierinos, J.F.; Inamo, J.; Grigioni, F.; Gersh, B.; Shub, C.; Enriquez-Sarano, M. Sex differences in morphology and outcomes of mitral valve prolapse. Ann. Intern. Med. 2008, 149, 787–795. [Google Scholar] [CrossRef]
- Zuppiroli, A.; Mori, F.; Favilli, S.; Barchielli, A.; Corti, G.; Montereggi, A.; Dolara, A. Arrhythmias in mitral valve prolapse: Relation to anterior mitral leaflet thickening, clinical variables, and color Doppler echocardiographic parameters. Am. Heart J. 1994, 128, 919–927. [Google Scholar] [CrossRef]
- Essayagh, B.; Sabbag, A.; Antoine, C.; Benfari, G.; Yang, L.T.; Maalouf, J.; Asirvatham, S.; Michelena, H.; Enriquez-Sarano, M. Presentation and Outcome of Arrhythmic Mitral Valve Prolapse. J. Am. Coll. Cardiol. 2020, 76, 637–649. [Google Scholar] [CrossRef]
- Han, H.; Teh, A.W.; Hare, D.L.; Farouque, O.; Lim, H.S. The Clinical Demographics of Arrhythmic Mitral Valve Prolapse. J. Am. Coll. Cardiol. 2020, 22, 2689–2690. [Google Scholar] [CrossRef]
- Delling, F.N.; Rong, J.; Larson, M.G.; Lehman, B.; Osypiuk, E.; Stantchev, P.; Slaugenhaupt, S.A.; Benjamin, E.J.; Levine, R.A.; Vasan, R.S. Familial clustering of mitral valve prolapse in the community. Circulation 2015, 131, 263–268. [Google Scholar] [CrossRef]
- Giudicessi, J.R.; Maleszewski, J.J.; Tester, D.J.; Ackerman, M.J. Prevalence and potential genetic determinants of young sudden unexplained death victims with suspected arrhythmogenic mitral valve prolapse syndrome. Heart Rhythm O2 2021, 2, 431–438. [Google Scholar] [CrossRef]
- Bains, S.; Tester, D.J.; Asirvatham, S.J.; Noseworthy, P.A.; Ackerman, M.J.; Giudicessi, J.R. A Novel Truncating Variant in FLNC-Encoded Filamin C May Serve as a Proarrhythmic Genetic Substrate for Arrhythmogenic Bileaflet Mitral Valve Prolapse Syndrome. Mayo Clin. Proc. 2019, 94, 906–913. [Google Scholar] [CrossRef]
- Boudoulas, H.; Schaal, S.F.; Stang, J.M.; Fontana, M.E.; Kolibash, A.J.; Wooley, C.F. Mitral valve prolapse: Cardiac arrest with long-term survival. Int. J. Cardiol. 1990, 26, 37. [Google Scholar] [CrossRef]
- Syed, F.F.; Ackerman, M.J.; McLeod, C.J.; Kapa, S.; Mulpuru, S.K.; Sriram, C.S.; Cannon, B.C.; Asirvatham, S.J.; Noseworthy, P.A. Sites of Successful Ventricular Fibrillation Ablation in Bileaflet Mitral Valve Prolapse Syndrome. Circ. Arrhythm Electrophysiol. 2016, 9, e004005. [Google Scholar] [CrossRef]
- Wilde, A.A.; Düren, D.R.; Hauer, R.N.; deBakker, J.M.; Bakker, P.F.; Becker, A.E.; Janse, M.J. Mitral Valve Prolapse and Ventricular Arrhythmias: Observations in a Patient with a 20-Year History. J. Cardiovasc. Electrophysiol. 1997, 8, 307–316. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chang, S.L.; Lin, Y.J.; Chen, Y.Y.; Lo, L.W.; Hu, Y.F.; Tuan, T.C.; Chao, T.F.; Chung, F.P.; Liao, J.N.; et al. An observation study on the effect of premature ventricular complex burden on long-term outcome. Medicine 2017, 96, e5476. [Google Scholar] [CrossRef] [PubMed]
- Massing, M.W.; Simpson, R.J.; Rautaharju, P.M.; Schreiner, P.J.; Crow, R.; Heiss, G. Usefulness of ventricular premature complexes to predict coronary heart disease events and mortality (from the Atherosclerosis Risk In Communities cohort). Am. J. Cardiol. 2006, 98, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Yang, M.; Zhong, L.; Lee, Y.H.; Vaidya, V.R.; Asirvatham, S.J.; Ackerman, M.J.; Pislaru, S.V.; Suri, R.M.; Slusser, J.P.; et al. Ventricular premature contraction associated with mitral valve prolapse. Int. J. Cardiol. 2016, 221, 1144–1149. [Google Scholar] [CrossRef]
- Bumgarner, J.M.; Patel, D.; Kumar, A.; Clevenger, J.R.; Trulock, K.M.; Popović, Z.; Griffin, B.P.; Wazni, O.M.; Menon, V.; Desai, M.Y.; et al. Management and outcomes in mitral valve prolapse with ventricular arrhythmias undergoing ablation and/or implantation of ICDs. Pacing Clin. Electrophysiol. 2019, 42, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Enriquez, A.; Shirai, Y.; Huang, J.; Liang, J.; Briceño, D.; Hayashi, T.; Muser, D.; Fulton, B.; Han, Y.; Perez, A.; et al. Papillary muscle ventricular arrhythmias in patients with arrhythmic mitral valve prolapse: Electrophysiologic substrate and catheter ablation outcomes. J. Cardiovasc. Electrophysiol. 2019, 30, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Cheniti, G.; Vlachos, K.; Meo, M.; Puyo, S.; Thompson, N.; Denis, A.; Duchateau, J.; Takigawa, M.; Martin, C.; Frontera, A.; et al. Mapping and Ablation of Idiopathic Ventricular Fibrillation. Front. Cardiovasc. Med. 2018, 5, 123. [Google Scholar] [CrossRef]
- Das, M.K.; Stein, K.M.; Canilang, K.; Markowitz, S.M.; Mittal, S.; Slotwiner, D.J.; Iwai, S.; Lerman, B.B. Significance of sustained monomorphic ventricular tachycardia induced with short coupling intervals in patients with ischemic cardiomyopathy. Am. J. Cardiol. 2002, 89, 987–990. [Google Scholar] [CrossRef]
- Santoro, F.; Di Biase, L.; Hranitzky, P.; Sanchez, J.E.; Santangeli, P.; Perini, A.P.; Burkhardt, J.D.; Natale, A. Ventricular fibrillation triggered by PVCs from papillary muscles: Clinical features and ablation. J. Cardiovasc. Electrophysiol. 2014, 25, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Janse, M.J.; Kleber, A.G.; Capucci, A.; Coronel, R.; Wilms-Schopman, F. Electrophysiological basis for arrhythmias caused by acute ischemia. Role of the subendocardium. J. Mol. Cell. Cardiol. 1986, 18, 339–355. [Google Scholar] [CrossRef]
- Hai, J.J.; Chahal, A.A.; Friedman, P.A.; Vaidya, V.R.; Syed, F.F.; DeSimone, C.V.; Nanda, S.; Brady, P.A.; Madhavan, M.; Cha, Y.M.; et al. Electrophysiologic characteristics of ventricular arrhythmias arising from the aortic mitral continuity-potential role of the conduction system. J. Cardiovasc. Electrophysiol. 2015, 26, 158–163. [Google Scholar] [CrossRef]
- Persson, F.; Andersson, B.; Duker, G.; Jacobson, I.; Carlsson, L. Functional effects of the late sodium current inhibition by AZD7009 and lidocaine in rabbit isolated atrial and ventricular tissue and Purkinje fibre. Eur. J. Pharmacol. 2007, 558, 133–143. [Google Scholar] [CrossRef]
- Robinson, R.B.; Boyden, P.A.; Hoffman, B.F.; Hewett, K.W. Electrical restitution process in dispersed canine cardiac Purkinje and ventricular cells. Am. J. Physiol. 1987, 253, H1018–H1025. [Google Scholar] [CrossRef]
- Dun, W.; Boyden, P.A. The Purkinje cell; 2008 style. J. Mol. Cell. Cardiol. 2008, 45, 617–624. [Google Scholar] [CrossRef]
- Stuyvers, B.D.; Dun, W.; Matkovich, S.; Sorrentino, V.; Boyden, P.A.; ter Keurs, H.E. Ca2+ sparks and waves in canine purkinje cells: A triple layered system of Ca2+ activation. Circ. Res. 2005, 97, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.W.; Lab, M.J. Arrhythmia in heart failure: Role of mechanically induced changes in electrophysiology. Lancet 1989, 1, 1309–1312. [Google Scholar] [CrossRef]
- Han, W.; Chartier, D.; Li, D.; Nattel, S. Ionic remodeling of cardiac Purkinje cells by congestive heart failure. Circulation 2001, 104, 2095–2100. [Google Scholar] [CrossRef] [PubMed]
- Nattel, S.; Maguy, A.; Le Bouter, S.; Yeh, Y.H. Arrhythmogenic ion-channel remodeling in the heart: Heart failure, myocardial infarction, and atrial fibrillation. Physiol. Rev. 2007, 87, 425–456. [Google Scholar] [CrossRef] [PubMed]
- Michowitz, Y.; Belhassen, B. New Insights on Verapamil-Sensitive Idiopathic Left Fascicular Tachycardia. J. Electrocardiol. 2018, 51, 874–878. [Google Scholar] [CrossRef]
- Cha, M.J.; Seo, J.W.; Kim, H.; Kim, M.; Choi, J.; Kang, D.H.; Oh, S. Visualization of left ventricular Purkinje fiber distribution using widefield optical coherence microscopy. Int. J. Clin. Exp. Pathol. 2020, 13, 3013–3020. [Google Scholar]
- Fulton, B.L.; Liang, J.J.; Enriquez, A.; Garcia, F.C.; Supple, G.E.; Riley, M.P.; Schaller, R.D.; Dixit, S.; Callans, D.J.; Marchlinski, F.E.; et al. Imaging characteristics of papillary muscle site of origin of ventricular arrhythmias in patients with mitral valve prolapse. J. Cardiovasc. Electrophysiol. 2018, 29, 146–153. [Google Scholar] [CrossRef]
- Hsia, H.H.; Callans, D.J.; Marchlinski, F.E. Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation 2003, 108, 704–710. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Nademanee, W.; Hocini, M.; Duchateau, J.; André, C.; Lavergne, T.; Takigawa, M.; Sacher, F.; Derval, N.; Pambrun, T.; et al. The Spectrum of Idiopathic Ventricular Fibrillation and J-Wave Syndromes: Novel Mapping Insights. Card. Electrophysiol. Clin. 2019, 11, 699–709. [Google Scholar] [CrossRef]
- Lab, M.J. Mechanoelectric feedback (transduction) in heart: Concepts and implications. Cardiovasc. Res. 1996, 32, 3–14. [Google Scholar] [CrossRef]
- Scatteia, A.; Pascale, C.E.; Gallo, P.; Pezzullo, S.; America, R.; Cappelletti, A.M.; Dalla Vecchia, L.A.; Guarini, P.; Dellegrottaglie, S. Abnormal Papillary Muscle Signal on Cine MRI as a Typical Feature of Mitral Valve Prolapse. Sci. Rep. 2020, 10, 9166. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.H.; Roujol, S.; Foppa, M.; Kissinger, K.V.; Goddu, B.; Hauser, T.H.; Zimetbaum, P.J.; Ngo, L.H.; Manning, W.J.; Nafat, R.; et al. Diffuse myocardial fibrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart 2017, 103, 204–209. [Google Scholar] [CrossRef]
- Han, H.C.; Parsons, S.A.; Curl, C.L.; Teh, A.W.; Raaijmakers, A.J.A.; Koshy, A.N.; Leong, T.; Burrell, L.M.; O’Donnell, D.; Vohra, J.K.; et al. Systematic quantification of histologic ventricular fibrosis in isolated mitral valve prolapse and sudden cardiac death. Heart Rhythm 2021, 18, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Sharma, E.; Arunachalam, K.; Di, M.; Chu, A.; Maan, A. PVCs, PVC-Induced Cardiomyopathy, and the Role of Catheter Ablation. Crit. Pathw. Cardiol. 2017, 16, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Luyten, P.; Heuts, S.; Cheriex, E.; Olsthoorn, J.R.; Crijns, H.J.G.M.; Winkens, B.; Roos-Hesselink, J.W.; Sardari Nia, P.; Schalla, S. Mitral prolapsing volume is associated with increased cardiac dimensions in patients with mitral annular disjunction. Neth. Heart J. 2022, 30, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Sniezek-Maciejewska, M.; Dubiel, J.P.; Piwowarska, W.; Mroczek-Czernecka, D.; Mazurek, S.; Jaśkiewicz, J.; Kitliński, M. Ventricular arrhythmias and the autonomic tone in patients with mitral valve prolapse. Clin. Cardiol. 1992, 15, 720. [Google Scholar] [CrossRef]
- Vergara, P.; Scarfo, I.; Esposito, A.; Colantoni, C.; Palmisano, A.; Altizio, S.; Falasconi, G.; Pannone, L.; Lapenna, E.; Gulletta, S.; et al. Characterization of the electrophysiological substrate in patients with Barlow’s disease. J. Cardiovasc. Electrophysiol. 2021, 32, 3179–3186. [Google Scholar] [CrossRef]
- Giudicessi, J.R.; Rohatgi, R.K.; Bos, J.M.; Ackerman, M.J. Prevalence and clinical phenotype of concomitant long QT syndrome and arrhythmogenic bileaflet mitral valve prolapse. Int. J. Cardiol. 2019, 274, 175–178. [Google Scholar] [CrossRef]
- Kulan, K.; Komsuoğlu, B.; Tuncer, C.; Kulan, C. Significance of QT dispersion on ventricular arrhythmias in mitral valve prolapse. Int. J. Cardiol. 1996, 54, 251–257. [Google Scholar] [CrossRef]
- Yang, P.C.; Kurokawa, J.; Furukawa, T.; Clancy, C.E. Acute effects of sex steroid hormones on susceptibility to cardiac arrhythmias: A simulation study. PLoS Comput. Biol. 2010, 6, e1000658. [Google Scholar] [CrossRef]
- Rubart, M.; von der Lohe, E. Sex steroids and cardiac arrhythmia: More questions than answers. J. Cardiovasc. Electrophysiol. 1998, 9, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Salem, J.E.; Alexandre, J.; Bachelot, A.; Funck-Brentano, C. Influence of steroid hormones on ventricular repolarization. Pharmacol. Ther. 2016, 167, 38–47. [Google Scholar] [CrossRef]
- Steinberg, C.; Davies, B.; Mellor, G.; Tadros, R.; Laksman, Z.W.; Roberts, J.D.; Green, M.; Alqarawi, W.; Angaran, P.; Healey, J.; et al. Short-coupled ventricular fibrillation represents a distinct phenotype among latent causes of unexplained cardiac arrest: A report from the CASPER registry. Eur. Heart J. 2021, 42, 2827–2838. [Google Scholar] [CrossRef]
- Nordhues, B.D.; Siontis, K.C.; Scott, C.G.; Nkomo, V.T.; Ackerman, M.J.; Asirvatham, S.J.; Noseworthy, P.A. Bileaflet Mitral Valve Prolapse and Risk of Ventricular Dysrhythmias and Death. J. Cardiovasc. Electrophysiol. 2016, 27, 463–468. [Google Scholar] [CrossRef]
- Freed, L.A.; Benjamin, E.J.; Levy, D.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Levine, R.A. Mitral valve prolapse in the general population: The benign nature of echocardiographic features in the Framingham Heart Study. J. Am. Coll. Cardiol. 2002, 40, 1298. [Google Scholar] [CrossRef]
- Grigioni, F.; Enriquez-Sarano, M.; Ling, L.H.; Bailey, K.R.; Seward, J.B.; Tajik, A.J.; Frye, R.L. Sudden death in mitral regurgitation due to flail leaflet. J. Am. Coll. Cardiol. 1999, 34, 2078. [Google Scholar] [CrossRef]
- Turker, Y.; Ozaydin, M.; Acar, G.; Ozgul, M.; Hoscan, Y.; Varol, E.; Dogan, A.; Erdogan, D.; Yucel, H. Predictors of ventricular arrhythmias in patients with mitral valve prolapse. Int. J. Cardiovasc. Imaging 2010, 26, 139–145. [Google Scholar] [CrossRef]
- Dejgaard, L.A.; Skjolsvik, E.T.; Lie, O.H.; Ribe, M.; Stokke, M.K.; Hegbom, F.; Scheirlynck, E.S.; Gjertsen, E.; Andresen, K.; Helle-Valle, T.M.; et al. The Mitral Annulus Disjunction Arrhythmic Syndrome. J. Am. Coll. Cardiol. 2018, 72, 1600–1609. [Google Scholar] [CrossRef]
- Essayagh, B.; Sabbag, A.; Antoine, C.; Benfari, G.; Batista, R.; Yang, L.T.; Maalouf, J.; Thapa, P.; Asirvatham, S.; Michelena, H.I.; et al. The Mitral Annular Disjunction of Mitral Valve Prolapse: Presentation and Outcome. JACC Cardiovasc. Imaging 2021, 14, 2073–2087. [Google Scholar] [CrossRef] [PubMed]
- Toh, H.; Mori, S.; Izawa, Y.; Fujita, H.; Miwa, K.; Suzuki, M.; Takahashi, Y.; Toba, T.; Watanabe, Y.; Kono, A.K.; et al. Prevalence and extent of mitral annular disjunction in structurally normal hearts: Comprehensive 3D analysis using cardiac computed tomography. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Ermakov, S.; Gulhar, R.; Lim, L.; Bibby, D.; Fang, Q.; Nah, G.; Abraham, T.P.; Schiller, N.B.; Delling, F.N. Left ventricular mechanical dispersion predicts arrhythmic risk in mitral valve prolapse. Heart 2019, 105, 1063–1069. [Google Scholar] [CrossRef]
- Han, Y.; Peters, D.C.; Salton, J.C.; Bzymek, D.; Nezafat, R.; Goddu, B.; Kissinger, K.V.; Zimetbaum, P.J.; Manning, W.J.; Yeon, S.B. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc. Imaging 2008, 1, 294. [Google Scholar] [CrossRef]
- Constant Dit Beaufils, A.L.; Huttin, O.; Jobbe-Duval, A.; Senage, T.; Filippetti, L.; Piriou, N.; Cueff, C.; Venner, C.; Mandry, D.; Sellal, J.M.; et al. Replacement Myocardial Fibrosis in Patients With Mitral Valve Prolapse: Relation to Mitral Regurgitation, Ventricular Remodeling, and Arrhythmia. Circulation 2021, 143, 1763–1774. [Google Scholar] [CrossRef]
- Kitkungvan, D.; Nabi, F.; Kim, R.J.; Bonow, R.O.; Khan, M.A.; Xu, J.; Little, S.H.; Quinones, M.A.; Lawrie, G.M.; Zoghbi, W.A.; et al. Myocardial Fibrosis in Patients with Primary Mitral Regurgitation With and Without Prolapse. J. Am. Coll. Cardiol. 2018, 72, 823–834. [Google Scholar] [CrossRef]
- Pradella, S.; Grazzini, G.; Brandani, M.; Calistri, L.; Nardi, C.; Mori, F.; Miele, V.; Colagrande, S. Cardiac magnetic resonance in patients with mitral valve prolapse: Focus on late gadolinium enhancement and T1 mapping. Eur. Radiol. 2019, 29, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Moura-Ferreira, S.; Vandenberk, B.; Masci, P.G.; Dresselaers, T.; Garweg, C.; Symons, R.; Willems, R.; Bogaert, J. Left ventricular remodeling in mitral valve prolapse patients: Implications of apical papillary muscle insertion. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, M.E.; Hamer, A.; Gang, E.S.; Oseran, D.S.; Mandel, W.J.; Peter, T. The yield of programmed ventricular stimulation in mitral valve prolapse patients with ventricular arrhythmias. Am. Heart J. 1985, 110, 970–976. [Google Scholar] [CrossRef]
- Morady, F.; Shen, E.; Bhandari, A.; Schwartz, A.; Scheinman, M.M. Programmed ventricular stimulation in mitral valve prolapse: Analysis of 36 patients. Am. J. Cardiol. 1984, 53, 135–138. [Google Scholar] [CrossRef]
- Bardy, G.H.; Lee, K.L.; Mark, D.B.; Poole, J.E.; Packer, D.L.; Boineau, R.; Domanski, M.; Troutman, C.; Anderson, J.; Johnson, G.; et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005, 352, 225–237. [Google Scholar] [CrossRef]
- Al-Khatib, S.M.; Stevenson, W.G.; Ackerman, M.J.; Bryant, W.J.; Callans, D.J.; Curtis, A.B.; Deal, B.J.; Dickfeld, T.; Field, M.E.; Fonarow, G.C.; et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. J. Am. Coll. Cardiol. 2018, 72, 91–220. [Google Scholar] [CrossRef]
- Yokokawa, M.; Siontis, K.C.; Kim, H.M.; Stojanovska, J.; Latchamsetty, R.; Crawford, T.; Jongnarangsin, K.; Ghanbari, H.; Cunnane, R.; Chugh, A.; et al. Value of cardiac magnetic resonance imaging and programmed ventricular stimulation in patients with frequent premature ventricular complexes undergoing radiofrequency ablation. Heart Rhythm 2017, 14, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Marano, P.J.; Lim, L.J.; Sanchez, J.M.; Alvi, R.; Nah, G.; Badhwar, N.; Gerstenfeld, E.P.; Tseng, Z.H.; Marcus, G.M.; Delling, F.N. Long-term outcomes of ablation for ventricular arrhythmias in mitral valve prolapse. J. Interv. Card. Electrophysiol. 2021, 61, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Cronin, E.M.; Bogun, F.M.; Maury, P.; Peichl, P.; Chen, M.; Namboodiri, N.; Aguinaga, L.; Leite, L.R.; Al-Khatib, S.M.; Anter, E.; et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. Europace 2019, 21, 1143–1144. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Hamilton-Craig, C.; Denman, R.; Haqqani, H.M. Catheter ablation of papillary muscle arrhythmias: Implications of mitral valve prolapse and systolic dysfunction. Pacing Clin. Electrophysiol. 2018, 41, 750–758. [Google Scholar] [CrossRef]
- El-Eshmawi, A.; Pandis, D.; Miller, M.A.; Boateng, P.; Dukkipati, S.R.; Reddy, V.Y.; Adams, D.H. Surgical Cryoablation of Papillary Muscle PVCs during Mitral Valve Surgery: Therapeutic Consideration for Malignant MVP. J. Am. Coll. Cardiol. 2020, 76, 3061–3062. [Google Scholar] [CrossRef]
- Hosseini, S.; Rezaei, Y.; Samiei, N.; Emkanjoo, Z.; Dehghani, M.R.; Haghjoo, M.; Badano, L.P. Effects of mitral valve repair on ventricular arrhythmia in patients with mitral valve prolapse syndrome: A report of two cases. Int. J. Cardiol. 2016, 222, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.R.; DeSimone, C.V.; Damle, N.; Naksuk, N.; Syed, F.F.; Ackerman, M.J.; Ponamgi, S.P.; Nkomo, V.T.; Suri, R.M.; Noseworthy, P.A.; et al. Reduction in malignant ventricular arrhythmia and appropriate shocks following surgical correction of bileaflet mitral valve prolapse. J. Interv. Card. Electrophysiol. 2016, 46, 137–143. [Google Scholar] [CrossRef]
- Naksuk, N.; Syed, F.F.; Krittanawong, C.; Anderson, M.J.; Ebrille, E.; DeSimone, C.V.; Vaidya, V.R.; Ponamgi, S.P.; Suri, R.M.; Ackerman, M.J.; et al. The effect of mitral valve surgery on ventricular arrhythmia in patients with bileaflet mitral valve prolapse. Indian Pacing Electrophysiol. J. 2016, 16, 187–191. [Google Scholar] [CrossRef]
- Hou, Y.; Zhou, Q.; Po, S.S. Neuromodulation for cardiac arrhythmia. Heart Rhythm 2016, 13, 584–592. [Google Scholar] [CrossRef]
- Hawson, J.; Harmer, J.A.; Cowan, M.; Virk, S.; Campbell, T.; Bennett, R.G.; Anderson, R.D.; Kalman, J.; Lee, G.; Kumar, S. Renal Denervation for the Management of Refractory Ventricular Arrhythmias: A Systematic Review. J. Am. Coll. Cardiol. 2021, 7, 100–108. [Google Scholar]
- Okeagu, E.; Abid, A.; Jensen, B.C.; Caranasos, T.G.; Syed, F.F. Refractory ventricular arrhythmia in a patient with Lamin A/C (LMNA) cardiomyopathy successfully treated with thoracic bilateral stellate ganglionectomy. Heart Rhythm. 2022, 8, 110–113. [Google Scholar] [CrossRef]
- Scheirlynck, E.; Dejgaard, L.A.; Skjolsvik, E.; Lie, O.H.; Motoc, A.; Hopp, E.; Tanaka, K.; Ueland, T.; Ribe, M.; Collet, C.; et al. Increased levels of sST2 in patients with mitral annulus disjunction and ventricular arrhythmias. Open Heart 2019, 6, e001016. [Google Scholar] [CrossRef]
- Songia, P.; Chiesa, M.; Alfieri, V.; Massaiu, I.; Moschetta, D.; Myasoedova, V.; Valerio, V.; Fusini, L.; Gripari, P.; Zanobini, M.; et al. Putative circulating microRNAs are able to identify patients with mitral valve prolapse and severe mitral regurgitation. Int. J. Mol. Sci. 2021, 22, 2102. [Google Scholar] [CrossRef]
- Lee, J.H.; Uhm, J.S.; Suh, Y.J.; Kim, M.; Kim, I.S.; Jin, M.N.; Cho, M.S.; Yu, H.T.; Kim, T.H.; Hong, Y.J.; et al. Usefulness of cardiac magnetic resonance images for prediction of sudden cardiac arrest in patients with mitral valve prolapse: A multicenter retrospective cohort study. BMC Cardiovasc. Disord. 2021, 21, 546. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Adams, D.H.; Pandis, D.; Robson, P.M.; Pawale, A.; Pyzik, R.; Liao, S.L.; El-Eshmawi, A.; Boateng, P.; Garg, J.; et al. Hybrid Positron Emission Tomography/Magnetic Resonance Imaging in Arrhythmic Mitral Valve Prolapse. JAMA Cardiol. 2020, 5, 1000–1005. [Google Scholar] [CrossRef]
- Tison, G.H.; Abreau, S.; Lim, L.; Barrios, J.; Hu, G.; Nguyen, T.; Dixit, S.; Nah, G.; Lee, Y.J.; Delling, F.N. Abstract 13321: Identifying Mitral Valve Prolapse at Risk for Ventricular Arrhythmias and Myocardial Fibrosis From 12-lead ECGs Using Deep Learning. Circulation 2021, 144, A13321. [Google Scholar]
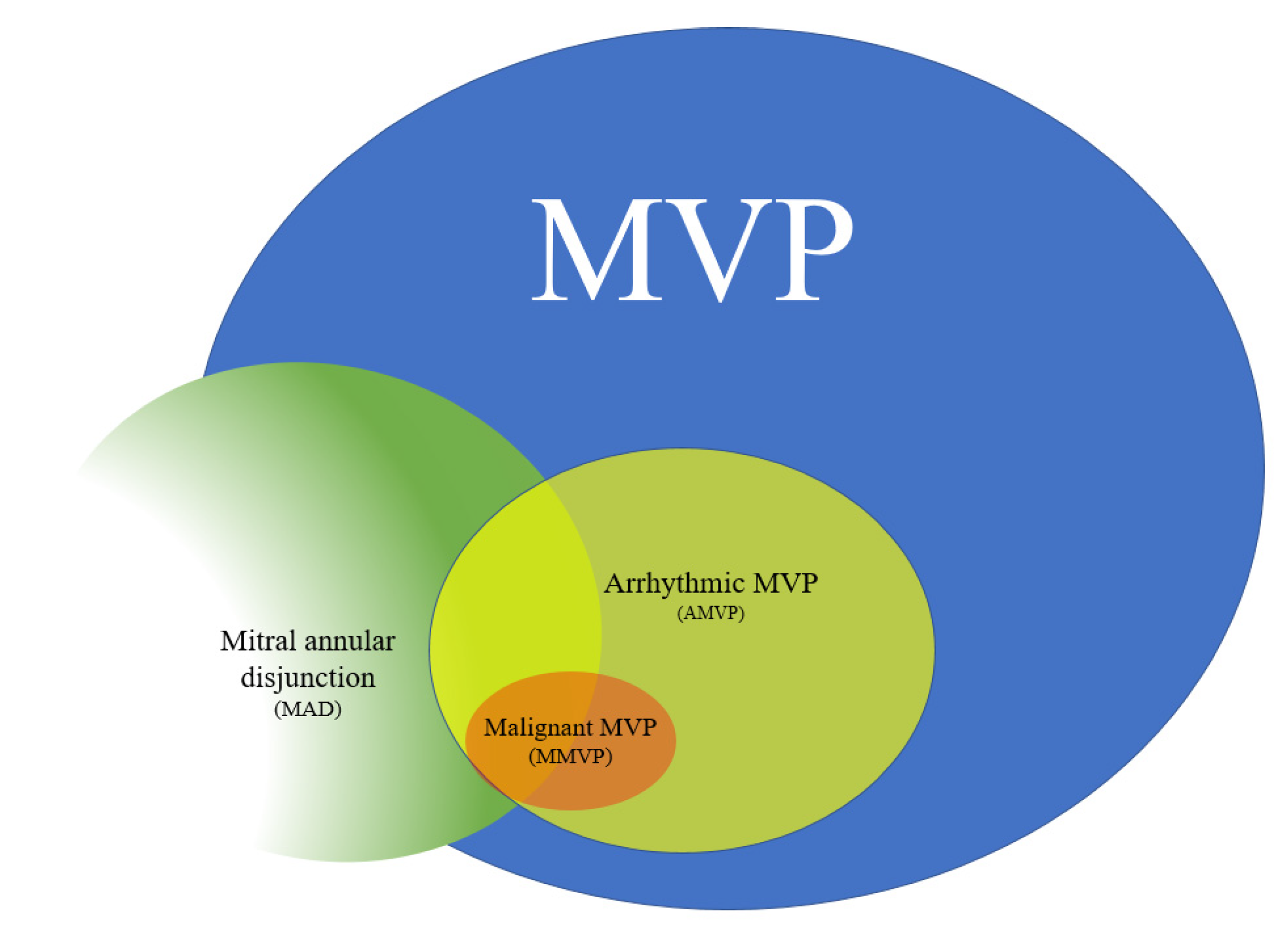
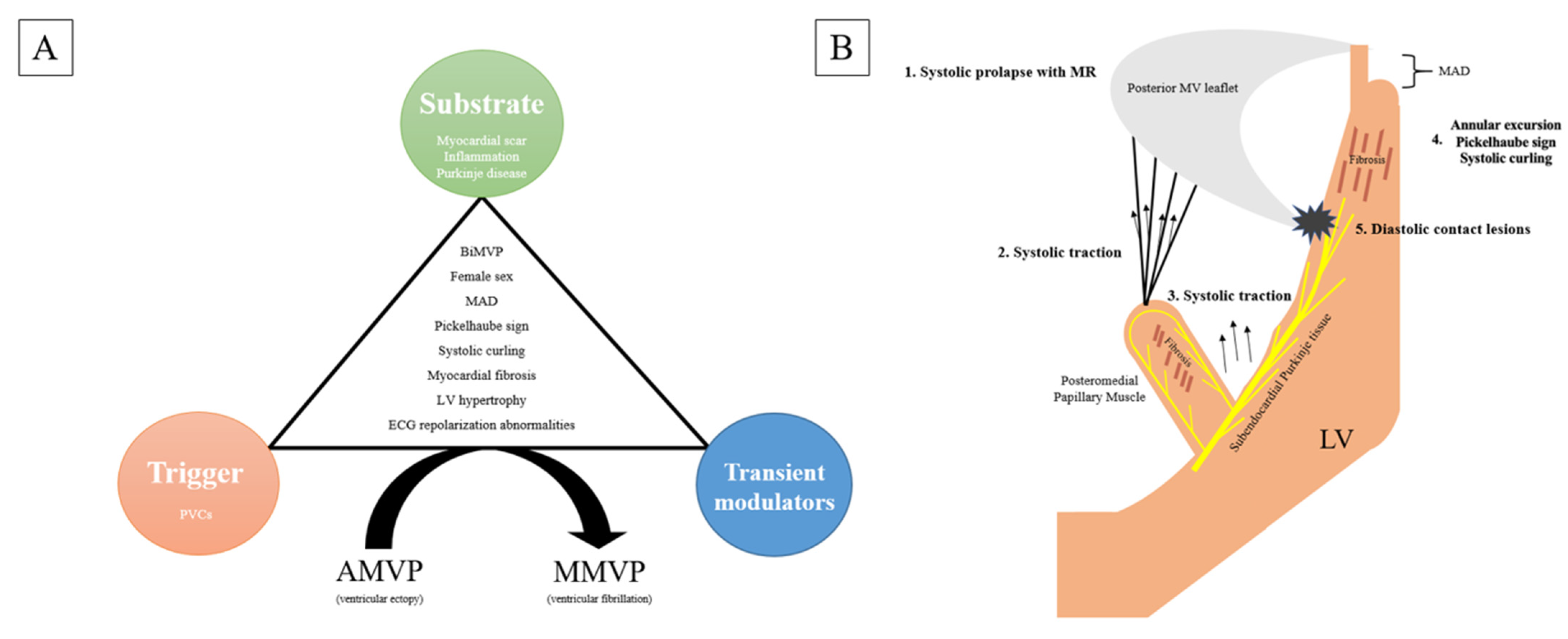
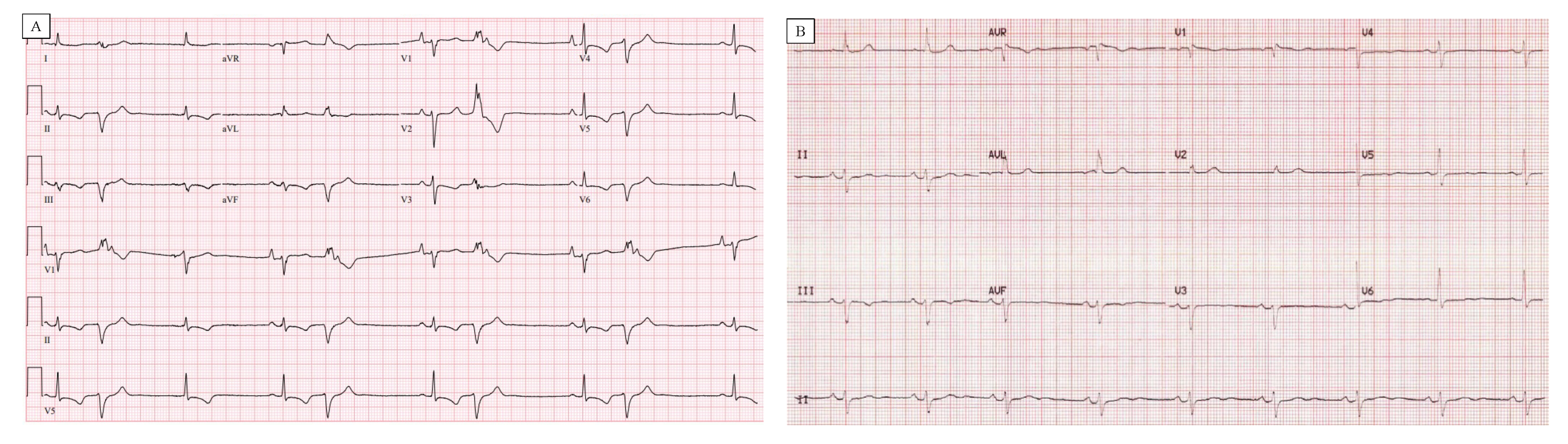
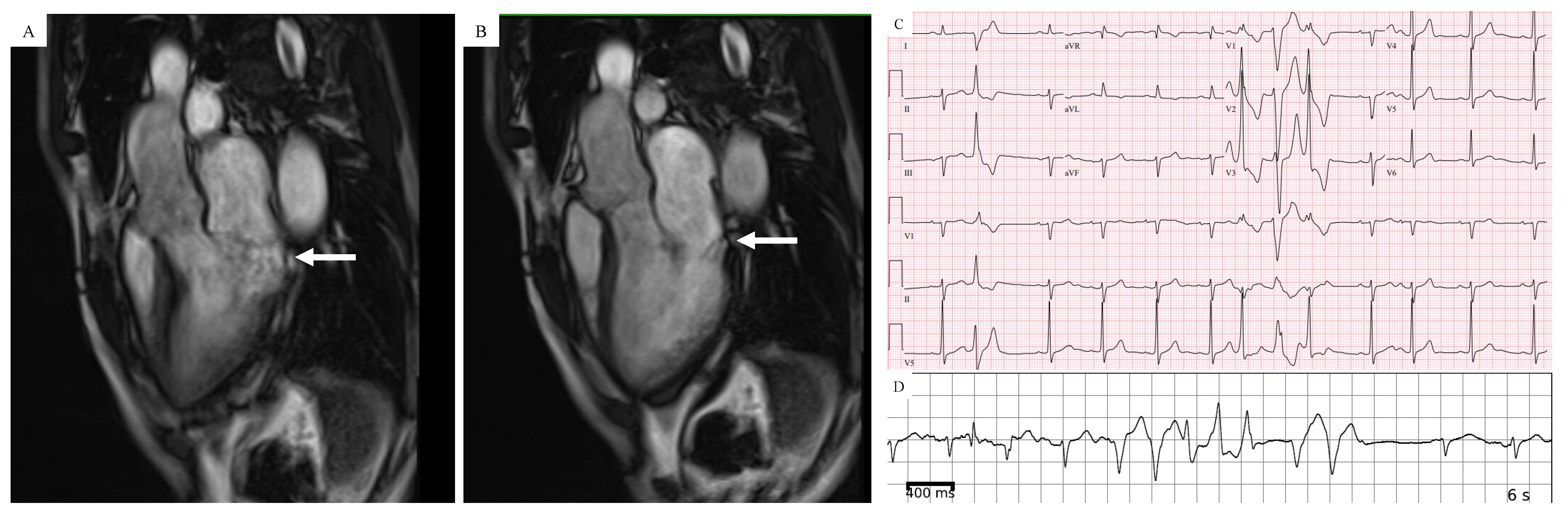
| Study (Ref) | Number of Patients | Increased Cardiac Mass (%) | LV Dilatation (%) | LV Hypertrophy (%) | LV Fibrosis/Scar (%) | PM Fibrosis (%) |
|---|---|---|---|---|---|---|
| Dollar et al. [14] | 56 | 100 | 5/48 (10) | 5/48 (10) | - | |
| Han HC et al. [5] | 70 | 69 | - | 49/70 (70) | 52/70 (74) | 2/70 (2) |
| Chesler E et al. [15] | 14 | 50 | - | 11/14 (78%) | - |
| Study Name | No. of Participants | Follow Up Time (Years) | Repeated Ablation (n) | Recurrent PVCs (n) | Recurrent VT/VF (n) | Successful Ablation (n) |
|---|---|---|---|---|---|---|
| Marano et al. [82] | 15 | 9 | - | 3 | 5 | 7 |
| Syed et al. [26] | 8 | ~2 | 3 | 3 | - | 5 |
| Lee et al. [84] | 9 | 6 | 3 | 2 | - | 7 |
| Bumgarner et al. [29] | 30 | 2.5 | - | - | 11 | 20 |
| Total | 62 | 6/17 (35%) | 8/32 (25%) | 16/45 (35%) | 39/62 (62.9%) |
| Study Name | No. of Patients (n) | ICD in Place | PVC Reduction (n, %) | VF/VT Reduction (Events Per Person-Year) | ICD Shock Reduction |
|---|---|---|---|---|---|
| Naksuk et al. [88] | 32 | No | >10% (n = 17, [53%]) | - | - |
| Vaidya et al. [87] | 8 | Yes | - | ~70% (0.6 to 0.14)/~80% (0.4 to 0.05) | ~80% (0.95 to 0.19) |
| El-Eshmawi et al. [85] | 3 | Yes | 97.7% (n = 3, [100%]) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelley, B.P.; Chaudry, A.M.; Syed, F.F. Developing a Mechanistic Approach to Sudden Death Prevention in Mitral Valve Prolapse. J. Clin. Med. 2022, 11, 1285. https://doi.org/10.3390/jcm11051285
Kelley BP, Chaudry AM, Syed FF. Developing a Mechanistic Approach to Sudden Death Prevention in Mitral Valve Prolapse. Journal of Clinical Medicine. 2022; 11(5):1285. https://doi.org/10.3390/jcm11051285
Chicago/Turabian StyleKelley, Brian P., Abdul Mateen Chaudry, and Faisal F. Syed. 2022. "Developing a Mechanistic Approach to Sudden Death Prevention in Mitral Valve Prolapse" Journal of Clinical Medicine 11, no. 5: 1285. https://doi.org/10.3390/jcm11051285
APA StyleKelley, B. P., Chaudry, A. M., & Syed, F. F. (2022). Developing a Mechanistic Approach to Sudden Death Prevention in Mitral Valve Prolapse. Journal of Clinical Medicine, 11(5), 1285. https://doi.org/10.3390/jcm11051285







