QRS Narrowing Following CRT Implantation: Predictors, Dynamics, and Association with Improved Long-Term Outcome
Abstract
:1. Introduction
2. Methods
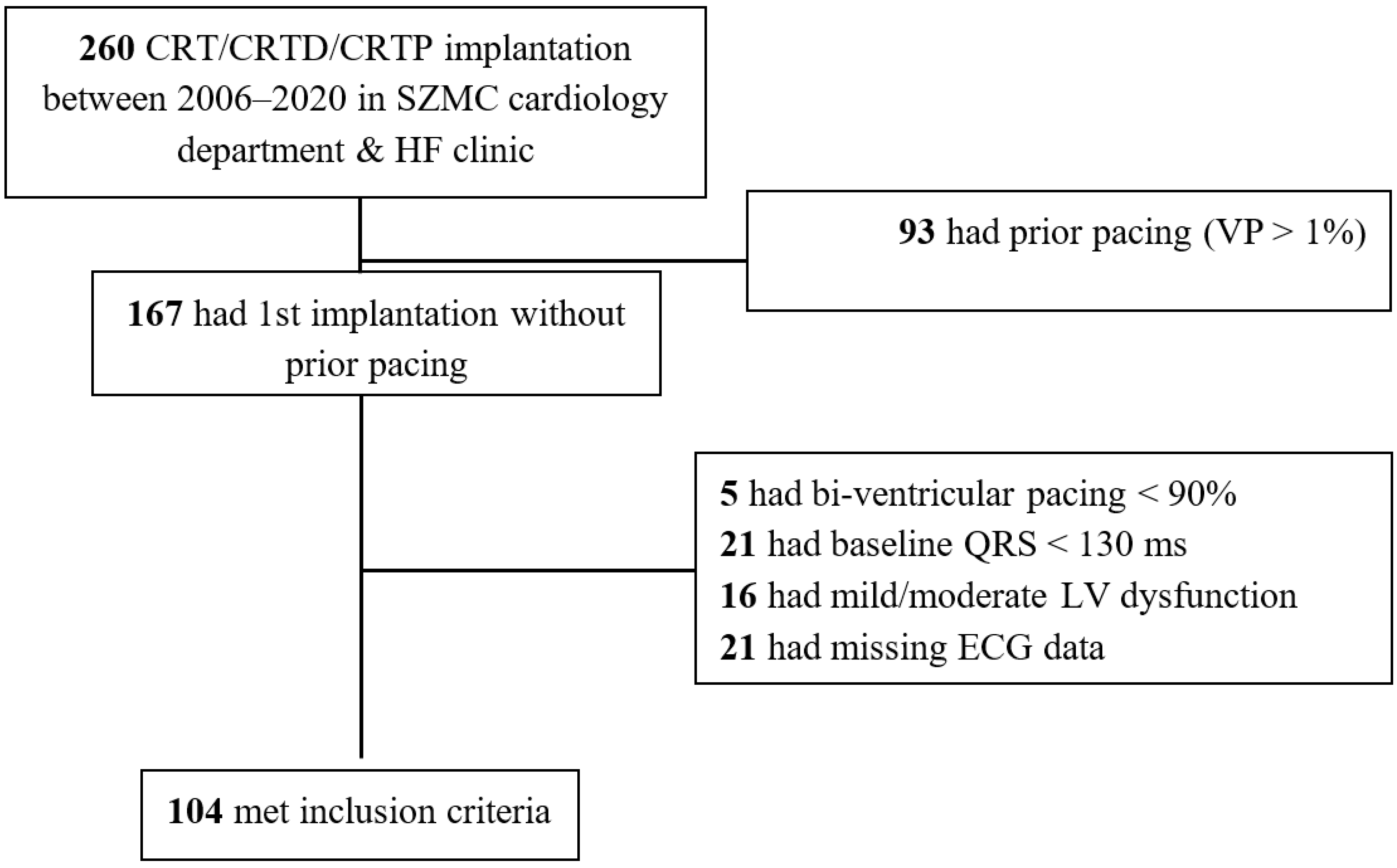
2.1. Inclusion and Exclusion Criteria
2.2. Follow-Up and Outcomes
2.3. QRS Morphology and Duration Measurement
2.4. Statistical Analysis
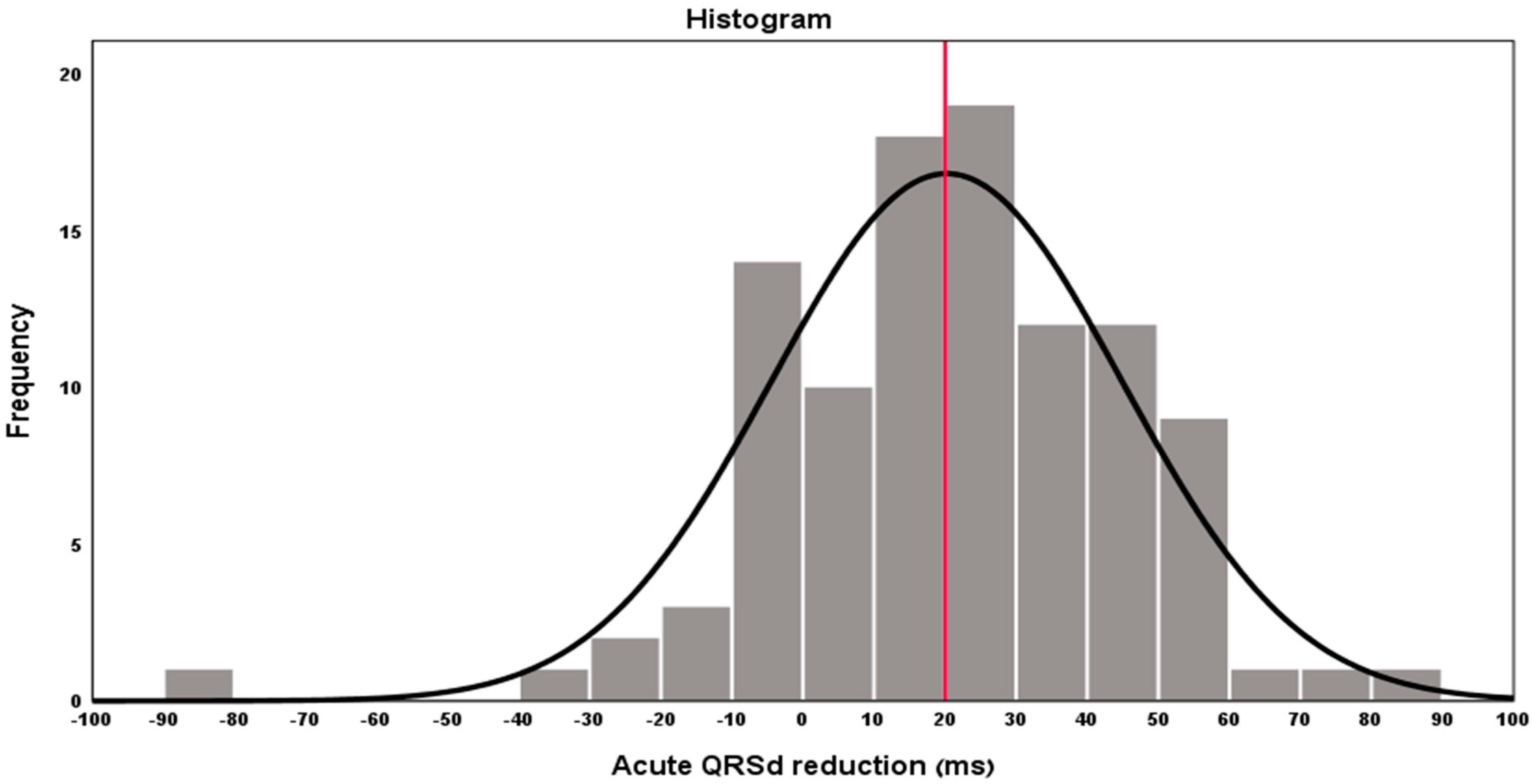
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cazeau, S.; Leclercq, C.; Lavergne, T.; Walker, S.; Varma, C.; Linde, C.; Garrigue, S.; Kappenberger, L.; Haywood, G.A.; Santini, M.; et al. Effects of Multisite Biventricular Pacing in Patients with Heart Failure and Intraventricular Conduction Delay. N. Engl. J. Med. 2001, 344, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Fisher, W.G.; Smith, A.L.; Delurgio, D.B.; Leon, A.R.; Loh, E.; Kocovic, D.Z.; Packer, M.; Clavell, A.L.; Hayes, D.L.; et al. Cardiac Resynchronization in Chronic Heart Failure. N. Engl. J. Med. 2002, 346, 1845–1853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleland, J.G.F.; Daubert, J.-C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L. The Effect of Cardiac Resynchronization on Morbidity and Mortality in Heart Failure. N. Engl. J. Med. 2005, 352, 1539–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, A.S.L.; Wells, G.A.; Talajic, M.; Arnold, M.O.; Sheldon, R.; Connolly, S.; Hohnloser, S.H.; Nichol, G.; Birnie, D.H.; Sapp, J.L.; et al. Cardiac-Resynchronization Therapy for Mild-to-Moderate Heart Failure. N. Engl. J. Med. 2010, 363, 2385–2395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; Barrabés, J.A.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2021, 42, 3427–3520. [Google Scholar]
- Zareba, W.; Klein, H.; Cygankiewicz, I.; Hall, W.J.; McNitt, S.; Brown, M.; Cannom, D.; Daubert, J.P.; Eldar, M.; Gold, M.R.; et al. Effectiveness of Cardiac Resynchronization Therapy by QRS Morphology in the Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy (MADIT-CRT). Circulation 2011, 123, 1061–1072. [Google Scholar] [CrossRef] [Green Version]
- Dupont, M.; Rickard, J.; Baranowski, B.; Varma, N.; Dresing, T.; Gabi, A.; Finucan, M.; Mullens, W.; Wilkoff, B.L.; Tang, W.H.W. Differential Response to Cardiac Resynchronization Therapy and Clinical Outcomes According to QRS Morphology and QRS Duration. J. Am. Coll. Cardiol. 2012, 60, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Klein, H.; Brown, M.W.; Daubert, J.P.; Estes, N.A.M., III; Foster, E.; Greenberg, H.; Higgins, S.L.; et al. Cardiac-Resynchronization Therapy for the Prevention of Heart-Failure Events. N. Engl. J. Med. 2009, 361, 1329–1338. [Google Scholar] [CrossRef] [Green Version]
- Sipahi, I. Impact of QRS Duration on Clinical Event Reduction with Cardiac Resynchronization Therapy. Arch. Intern. Med. 2011, 171, 1454. [Google Scholar] [CrossRef] [Green Version]
- Arshad, A.; Moss, A.J.; Foster, E.; Padeletti, L.; Barsheshet, A.; Goldenberg, I.; Goldenberg, I.; Greenberg, H.; Hall, W.J.; McNitt, S.; et al. Cardiac Resynchronization Therapy Is More Effective in Women Than in Men. J. Am. Coll. Cardiol. 2011, 57, 813–820. [Google Scholar] [CrossRef] [Green Version]
- Barsheshet, A.; Goldenberg, I.; Moss, A.J.; Eldar, M.; Huang, D.T.; McNitt, S.; Klein, H.W.; Hall, W.J.; Brown, M.W.; Goldberger, J.J.; et al. Response to preventive cardiac resynchronization therapy in patients with ischaemic and nonischaemic cardiomyopathy in MADIT-CRT. Eur. Heart J. 2010, 32, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Auricchio, A.; Prinzen, F.W. Non-Responders to Cardiac Resynchronization Therapy—The Magnitude of the Problem and the Issues. Circ. J. 2011, 75, 521–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, E.S.; Leon, A.R.; Tavazzi, L.; Sun, J.-P.; Nihoyannopoulos, P.; Merlino, J.; Abraham, W.T.; Ghio, S.; Leclercq, C.; Bax, J.J.; et al. Results of the Predictors of Response to CRT (PROSPECT) Trial. Circulation 2008, 117, 2608–2616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorcsan, J.; Oyenuga, O.; Habib, P.J.; Tanaka, H.; Adelstein, E.C.; Hara, H.; McNamara, D.M.; Saba, S. Relationship of Echocardiographic Dyssynchrony to Long-Term Survival After Cardiac Resynchronization Therapy. Circulation 2010, 122, 1910–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, M.O.; Hellkamp, A.S.; van Bommel, R.J.; Schalij, M.J.; Jan Willem Borleffs, C.; Bax, J.J. QRS fusion complex analysis using wave interference to predict reverse remodeling during cardiac resynchronization therapy. Heart Rhythm 2014, 11, 806–813. [Google Scholar] [CrossRef]
- Rickard, J.; Popovic, Z.; Verhaert, D.; Sraow, D.; Baranowski, B.; Martin, D.; Lindsay, B.D.; Varma, N.; Tchou, P.; Grimm, R.A.; et al. The QRS Narrowing Index Predicts Reverse Left Ventricular Remodeling Following Cardiac Resynchronization Therapy. Pacing Clin. Electrophysiol. 2011, 34, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Surawicz, B.; Childers, R.; Deal, B.J.; Gettes, L.S.; Bailey, J.J.; Gorgels, A.; Hancock, E.W.; Josephson, M.; Kligfield, P.; Kors, J.A.; et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part III: Intraventricular conduction disturbances: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll. Cardiol. 2009, 53, 976–981. [Google Scholar] [PubMed] [Green Version]
- Lecoq, G.; Leclercq, C.; Leray, E.; Crocq, C.; Alonso, C.; de Place, C.; Mabo, P.; Daubert, C. Clinical and electrocardiographic predictors of a positive response to cardiac resynchronization therapy in advanced heart failure. Eur. Heart J. 2005, 26, 1094–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menet, A.; Bardet-Bouchery, H.; Guyomar, Y.; Graux, P.; Delelis, F.; Castel, A.-L.; Heuls, S.; Cuvelier, E.; Gevaert, C.; Ennezat, P.-V.; et al. Prognostic importance of postoperative QRS widening in patients with heart failure receiving cardiac resynchronization therapy. Heart Rhythm 2016, 13, 1636–1643. [Google Scholar] [CrossRef]
- Gervais, R.; Leclercq, C.; Shankar, A.; Jacobs, S.; Eiskjaer, H.; Johannessen, A.; Freemantle, N.; Cleland, J.G.F.; Tavazzi, L.; Daubert, C.; et al. Surface electrocardiogram to predict outcome in candidates for cardiac resynchronization therapy: A sub-analysis of the CARE-HF trial. Eur. J. Heart Fail. 2009, 11, 699–705. [Google Scholar] [CrossRef]
- Friedman, D.J.; Bao, H.; Spatz, E.S.; Curtis, J.P.; Daubert, J.P.; Al-Khatib, S.M. Association Between a Prolonged PR Interval and Outcomes of Cardiac Resynchronization Therapy. Circulation 2016, 134, 1617–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, H.-J.; Park, K.-M.; Lee, S.S.; Park, Y.J.; On, Y.K.; Kim, J.S.; Park, S.-J. Electrical Reverse Remodeling of the Native Cardiac Conduction System after Cardiac Resynchronization Therapy. J. Clin. Med. 2020, 9, 2152. [Google Scholar] [CrossRef] [PubMed]
- Hsing, J.M.; Selzman, K.A.; Leclercq, C.; Pires, L.A.; McLaughlin, M.G.; McRae, S.E.; Peterson, B.J.; Zimetbaum, P.J. Paced Left Ventricular QRS Width and ECG Parameters Predict Outcomes After Cardiac Resynchronization Therapy. Circ. Arrhythmia Electrophysiol. 2011, 4, 851–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaca, O.; Omaygenc, M.O.; Cakal, B.; Cakal, S.D.; Gunes, H.M.; Barutcu, I.; Barutcu, I.; Boztosun, B.; Kilicaslan, F. Effect of QRS Narrowing After Cardiac Resynchronization Therapy on Functional Mitral Regurgitation in Patients with Systolic Heart Failure. Am. J. Cardiol. 2016, 117, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Rickard, J.; Jackson, G.; Spragg, D.D.; Cronin, E.M.; Baranowski, B.; Tang, W.H.W.; Wilkoff, B.L.; Varma, N. QRS prolongation induced by cardiac resynchronization therapy correlates with deterioration in left ventricular function. Heart Rhythm 2012, 9, 1674–1678. [Google Scholar] [CrossRef] [PubMed]
- Bonakdar, H.R.; Jorat, M.V.; Fazelifar, A.F.; Alizadeh, A.; Givtaj, N.; Sameie, N.; Sadeghpour, A.; Haghjoo, M. Prediction of response to cardiac resynchronization therapy using simple electrocardiographic and echocardiographic tools. Europace 2009, 11, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębski, M.; Baranchuk, A.; Fijorek, K.; Kisiel, R.; Kukla, P.; Sondej, T.; Czarnecka, D. Cardiac resynchronization therapy-induced acute shortening of QRS duration predicts long-term mortality only in patients with left bundle branch block. EP Eur. 2019, 21, 281–289. [Google Scholar] [CrossRef]
- Végh, E.M.; Kandala, J.; Januszkiewicz, L.; Ren, J.; Miller, A.; Orencole, M.; Blendea, D.; Merkely, B.; Gellér, L.; Singh, J.P.; et al. A new simplified electrocardiographic score predicts clinical outcome in patients treated with CRT. Europace 2018, 20, 492–500. [Google Scholar] [CrossRef] [Green Version]
- Rav-Acha, M.; Nujidat, A.; Farkash, R.; Medina, A.; Ilan, M.; Klutstein, M.; Butnaru, A.; Weitsman, T.; Glikson, M.; Hasin, T. Delayed Prolongation of the QRS Interval in Patients with Left Ventricular Dysfunction. Int. J. Cardiol. 2019, 296, 71–75. [Google Scholar] [CrossRef]

| All (n = 104) | Acute QRS Narrowing ≥ 20 ms (n = 55) | Acute QRS Narrowing <20 ms (n = 49) | Univariate 1 | Multivariate 2 | ||
|---|---|---|---|---|---|---|
| p-Value | Adjusted OR (95% CI) | p-Value | ||||
| Type of device | 1.000 3 | |||||
| CRTD | 98 (94.2%) | 52 (94.5%) | 46 (93.8%) | |||
| CRTP | 2 (1.9%) | 1 (1.8%) | 1 (2.0%) | |||
| CRT | 4 (3.9%) | 2 (3.6%) | 2 (4.1%) | |||
| Age (Mean ± SD, years) | 66.8 ± 11.7 | 65.8 ± 11.1 | 68.0 ± 12.3 | 0.346 | 0.989 (0.948; 1.031) | 0.594 |
| Gender (Female) | 26 (25%) | 20 (36.3%) | 6 (12.2%) | 0.005 | 4.454 (1.521; 13.046) | 0.006 |
| CHF etiology (Non-ischemic) | 45 (43.3%) | 28 (50.9%) | 17 (34.7%) | 0.096 | ||
| HTN | 71 (68.3%) | 35 (63.6%) | 36 (73.5%) | 0.282 | ||
| DM | 46 (44.2%) | 22 (40%) | 24 (45.0%) | 0.357 | ||
| HYPERLIPIDEMIA | 62 (59.6%) | 31 (56.3%) | 31 (63.3%) | 0.474 | ||
| SMOKING | 29 (27.9%) | 14 (25.4%) | 15 (30.6%) | 0.558 | ||
| Ischemic history | ||||||
| Prior PCI | 57 (54.8%) | 25 (45.5%) | 32 (65.3%) | 0.042 | 0.93 (0.339; 2.87) | 0.993 |
| Prior MI | 48 (46.1%) | 22 (40.0%) | 26 (53.1%) | 0.182 | ||
| Prior CABG | 23 (22.1%) | 7 (12.8%) | 16 (32.7%) | 0.015 | 0.414 (0.125; 1.368) | 0.148 |
| Prior Valve surgery | 14 (13.5%) | 6 (10.9%) | 8 (16.3%) | 0.419 | ||
| Prior ICD | 4 (3.9%) | 1 (1.8%) | 3 (6.1%) | 0.341 3 | ||
| Prior STROKE | 8 (7.7%) | 2 (3.6%) | 6 (12.2%) | 0.276 3 | ||
| RENAL FAILURE | 27 (26%) | 12 (21.8%) | 15 (30.6%) | 0.307 | ||
| Admission medications | ||||||
| B-Blockers | 96 (92.3%) | 50 (90.9%) | 46 (93.9%) | 0.719 3 | ||
| Diuretics | 81 (77.9%) | 39 (70.9%) | 42 (85.7%) | 0.069 | ||
| ARB | 24 (23.1%) | 14 (25.4%) | 10 (20.4%) | 0.542 | ||
| ACEI | 59 (56.7%) | 30 (54.5%) | 29 (59.2%) | 0.634 | ||
| ARNI | 10 (9.6%) | 5 (9.1%) | 5 (10.2%) | 1.000 3 | ||
| MRA | 75 (72.1%) | 40 (72.7%) | 35 (71.4%) | 0.883 | ||
| CCB | 5 (4.8%) | 2 (3.6%) | 3 (6.1%) | 0.665 3 | ||
| DIGOXIN | 15 (14.4%) | 5 (9.1%) | 10 (20.4%) | 0.101 | ||
| Anti-coagulation | 33 (31.7%) | 14 (25.4%) | 19 (38.8%) | 0.145 | ||
| Echocardiographic parameters | ||||||
| Pre-LV function | ||||||
| Moderate-Severe | 0.488 | |||||
| Severe | 43 (41.4%) | 21 (38.2%) | 22 (44.9%) | |||
| Fractional shortening | 61 (58.6%) | 34 (61.8%) | 27 (55.1%) | |||
| (Mean ± SD, %) | 14.9 ± 4.8 | 15.0 ± 5.3 | 14.8 ± 4.2 | 0.876 | ||
| Electrocardiographic parameters | ||||||
| Sinus Rhythm | 91/101 (90.1%) | 48/52 (92.3%) | 43/49 (87.8%) | |||
| Heart Rate (Mean ± SD, bpm) | 72.8 ± 13 | 73.4 ± 12.7 | 72.1 ± 13.5 | |||
| PR interval (Mean ± SD, ms) | 198.4 ± 45.4 | 192.1 ± 47.2 | 205.1 ± 42.9 | 0.518 3 | ||
| QTc 4 interval (Mean ± SD, ms) | 471.6 ± 50.4 | 471.2 ± 62.6 | 472.1 ± 34.6 | 0.636 | ||
| Pre-QRSd average | 151.6 ± 14.3 | 156.8 ± 14.5 | 145.8 ± 11.8 | 0.184 | ||
| (Mean ± SD, ms) | 0.931 | |||||
| LBBB morphology | 97 (93.3%) | 51 (92.7%) | 46 (93.9%) | <0.001 | 1.068 (1.031; 1.106) | <0.001 |
| All-Cause Mortality | All-Cause Mortality or HF Hospitalization | |||||
|---|---|---|---|---|---|---|
| Univariante 1 | Multivariate 2,a | Univariante 1 | Multivariante 2,a | |||
| p-Value | HR (95% CI) | p-Value | p-Value | HR (95% CI) | p-Value | |
| Age | 0.004 | 1.032 (0.982; 1.086) | 0.215 | 0.104 | 0.988 (0.950; 1.028) | 0.561 |
| Gender (Male) | 0.214 | 0.678 (0.206; 2.230) | 0.522 | 0.158 | 0.386 (0.144; 1.037) | 0.059 |
| CHF etiology (Ischemic) | 0.019 | 0.240 (0.019; 3.007) | 0.269 | 0.006 | 1.239 (0.232; 6.625) | 0.802 |
| Hypertension | 0.703 | 0.160 | ||||
| Diabetes mellitus | 0.119 | 0.021 | 1.059 (0.498; 2.252) | 0.882 | ||
| HYPERLIPIDEMIA | 0.779 | 0.046 | 1.508 (0.702; 3.239) | 0.292 | ||
| SMOKING | 0.483 | 0.752 | ||||
| Ischemic history | ||||||
| Prior PCI | 0.030 | 3.366 (0.535; 21.165) | 0.196 | 0.012 | 1.019 (0.152; 6.833) | 0.985 |
| Prior MI | 0.002 | 2.813 (1.217; 6.504) | 0.016 | 0.002 | 2.770 (1.391; 5.515) | 0.004 |
| Prior CABG | 0.002 | 1.044 (0.356; 3.059) | 0.938 | 0.004 | 1.104 (0.466; 2.614) | 0.822 |
| Prior Valve surgery | 0.000 | 3.387 (1.352; 8.483) | 0.009 | 0.001 | 2.413 (0.814; 7.151) | 0.112 |
| Prior ICD | 0.491 | 0.841 | ||||
| Prior STROKE | 0.048 | 0.702 | ||||
| RENAL FAILURE | 0.077 | 0.257 | ||||
| Echocardiographic parameters | ||||||
| Pre-LV function (Severe) | 0.786 | 0.253 | ||||
| Electrocardiographic parameters | ||||||
| Sinus Rhythm | <0.001 | 0.251 (0.042; 1.484) | 0.127 | 0.007 | 1.589 (0.131; 19.342) | 0.716 |
| Heart Rate (bpm) | 0.005 | 0.959 (0.921; 0.999) b | 0.045 b | 0.093 | ||
| PR interval (ms) | 0.070 | <0.001 | 1.015 (1.008; 1.021) | <0.001 | ||
| QTc 4 interval (ms) | 0.399 | 0.231 | ||||
| Pre-QRSd average | 0.768 | 0.876 | ||||
| Acute QRS narrowing | 0.037 | 1.922 (0.796; 4.640) | 0.146 | 0.005 | 3.243 (1.593; 6.603) | 0.001 |
| <20 ms | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapidot, D.; Rav-Acha, M.; Bdolah-Abram, T.; Farkash, R.; Glikson, M.; Hasin, T. QRS Narrowing Following CRT Implantation: Predictors, Dynamics, and Association with Improved Long-Term Outcome. J. Clin. Med. 2022, 11, 1279. https://doi.org/10.3390/jcm11051279
Lapidot D, Rav-Acha M, Bdolah-Abram T, Farkash R, Glikson M, Hasin T. QRS Narrowing Following CRT Implantation: Predictors, Dynamics, and Association with Improved Long-Term Outcome. Journal of Clinical Medicine. 2022; 11(5):1279. https://doi.org/10.3390/jcm11051279
Chicago/Turabian StyleLapidot, Daniel, Moshe Rav-Acha, Tali Bdolah-Abram, Rivka Farkash, Michael Glikson, and Tal Hasin. 2022. "QRS Narrowing Following CRT Implantation: Predictors, Dynamics, and Association with Improved Long-Term Outcome" Journal of Clinical Medicine 11, no. 5: 1279. https://doi.org/10.3390/jcm11051279
APA StyleLapidot, D., Rav-Acha, M., Bdolah-Abram, T., Farkash, R., Glikson, M., & Hasin, T. (2022). QRS Narrowing Following CRT Implantation: Predictors, Dynamics, and Association with Improved Long-Term Outcome. Journal of Clinical Medicine, 11(5), 1279. https://doi.org/10.3390/jcm11051279








