Outcomes of Vitrectomy with Fovea-Sparing and Inverted ILM Flap Technique for Myopic Foveoschisis
Abstract
:1. Introduction
2. Materials and Methods
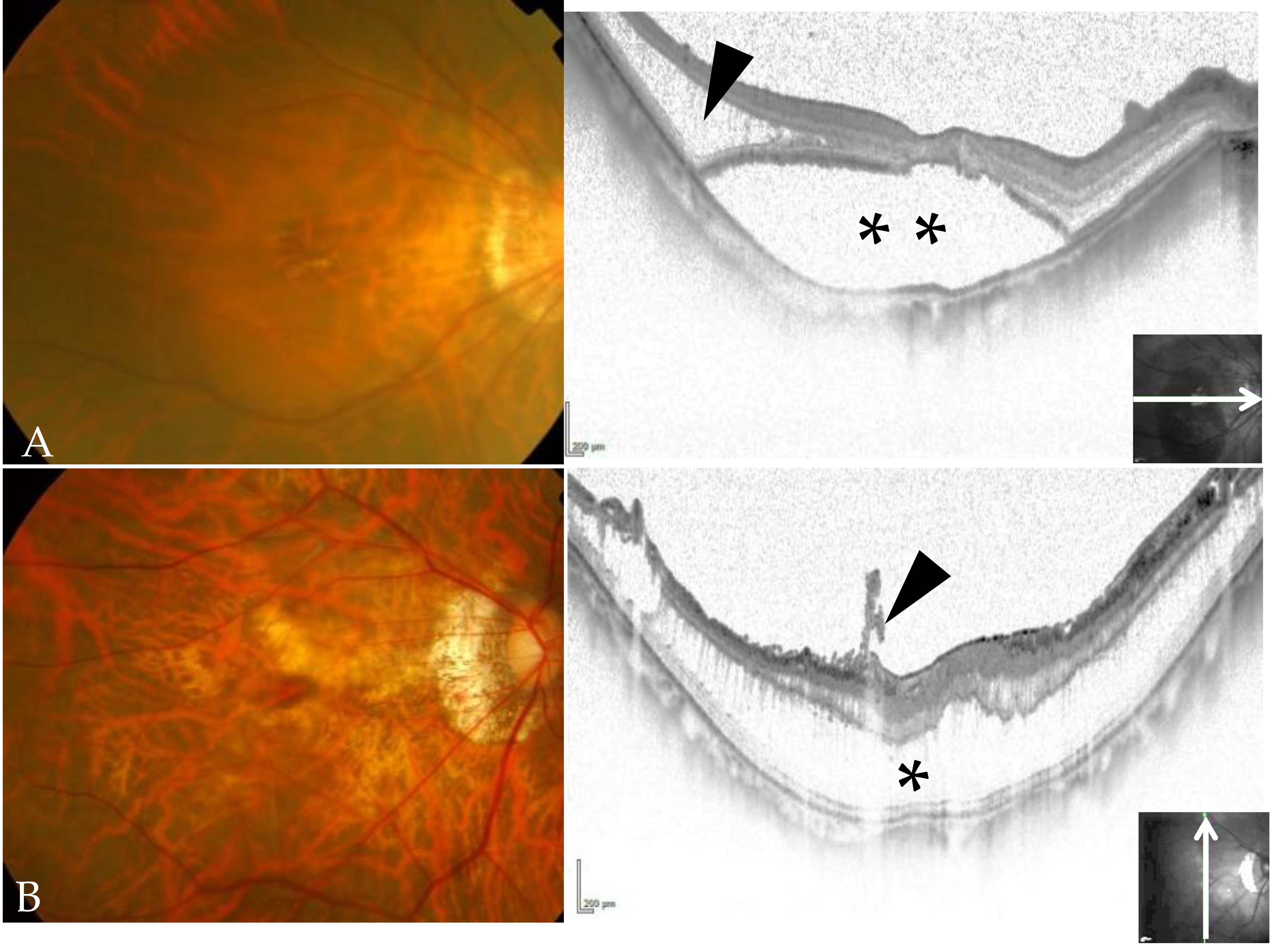
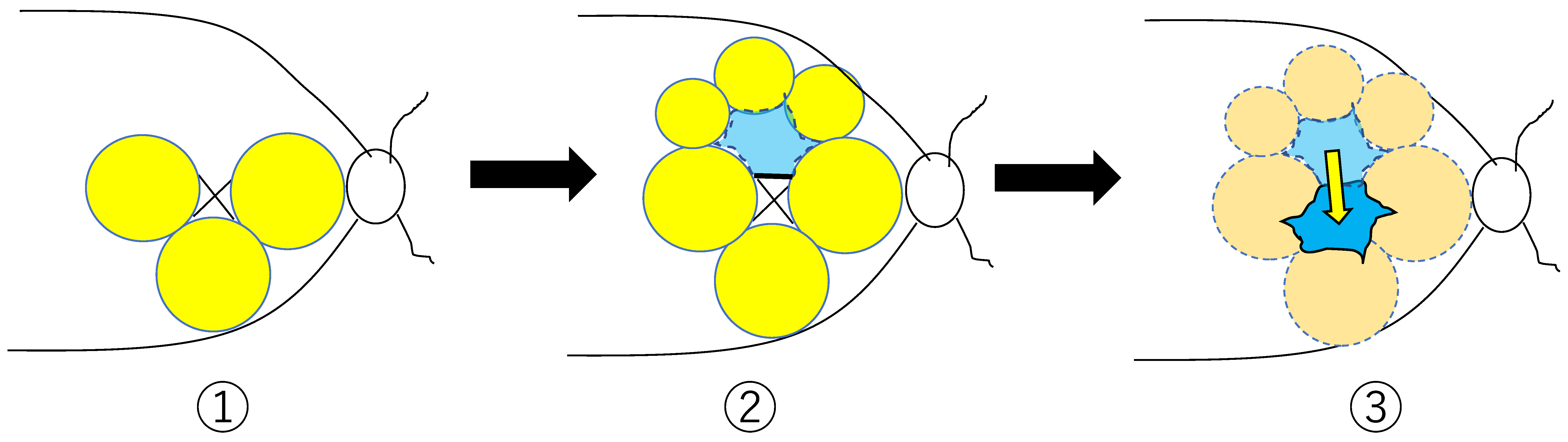
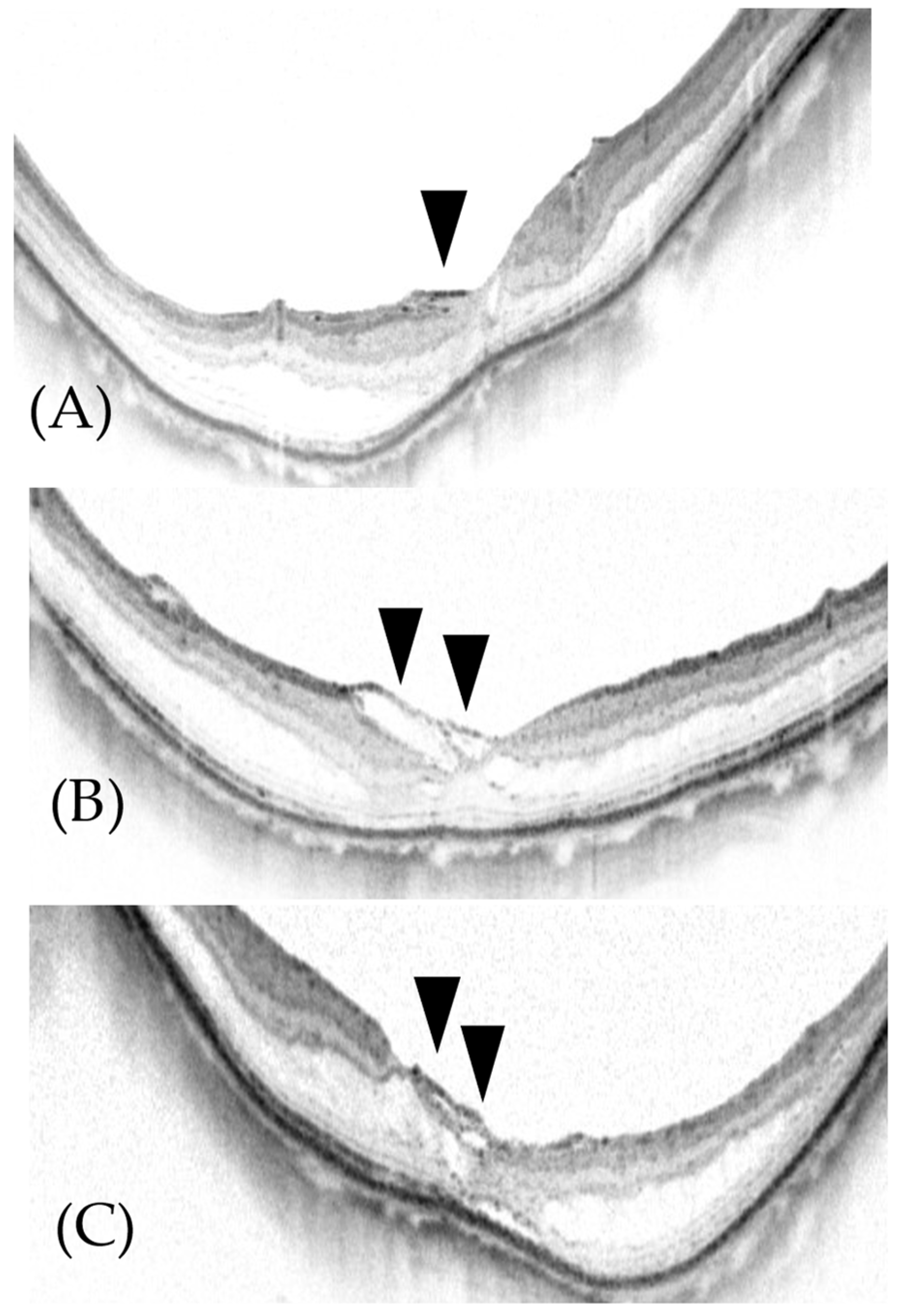
2.1. Surgical Methods (ILM Flap Procedure)
2.2. Statistics
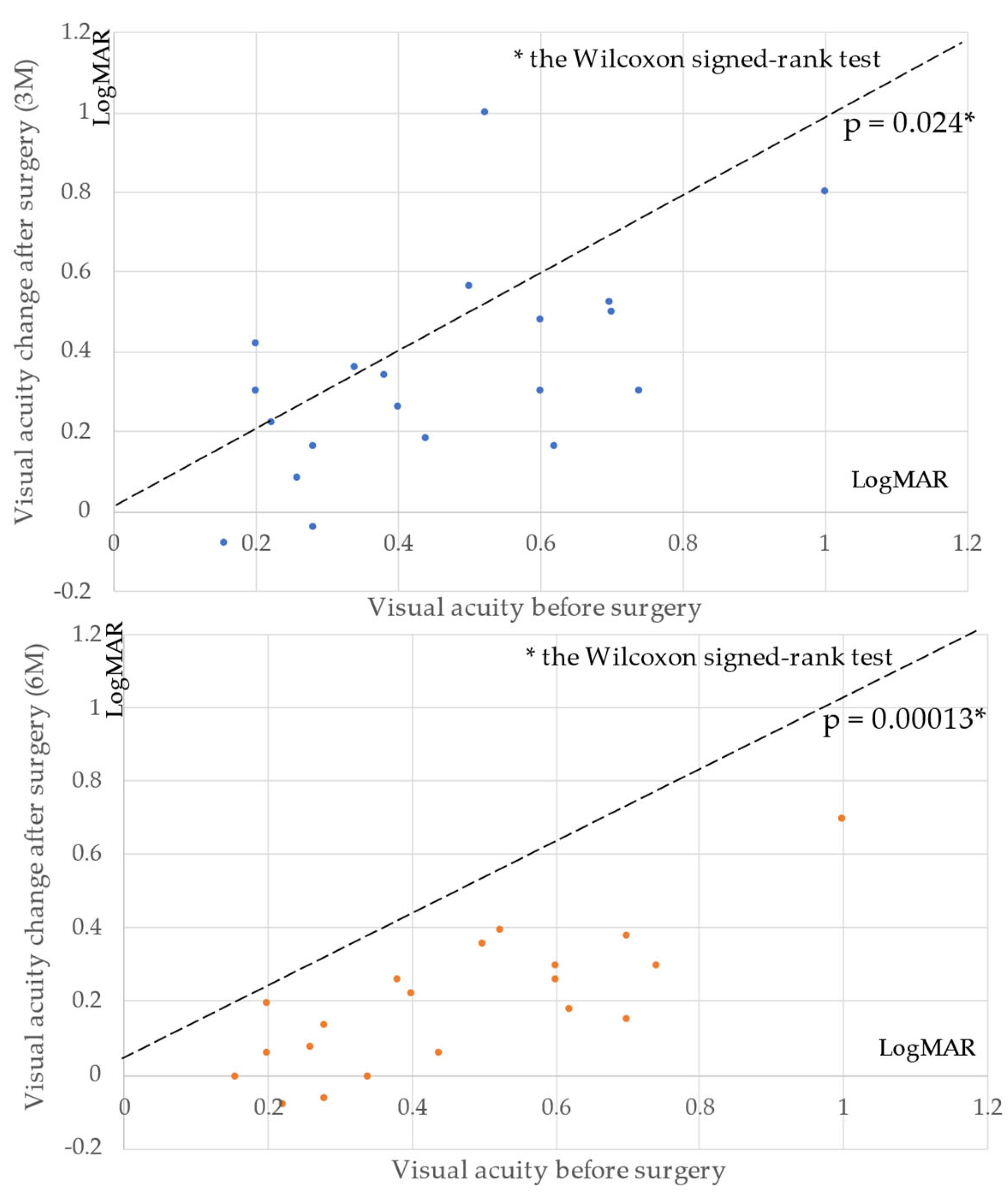
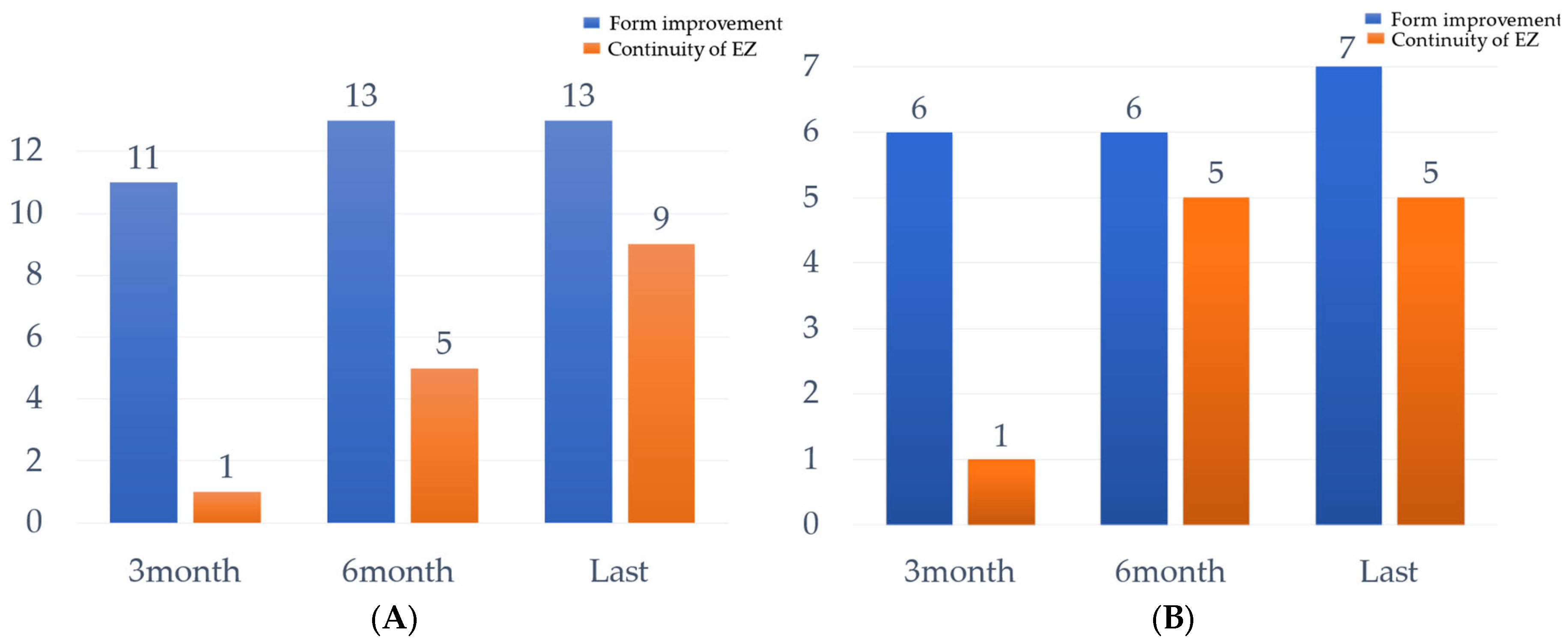
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verkicharla, P.K.; Ohno-Matsui, K.; Saw, S.M. Current and predicted demographics of high myopia and an update of its associated pathological changes. Ophthalmic Physiol. Opt. 2015, 35, 465–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, C.I. Retinal detachment at the posterior pole. Br. J. Ophthalmol. 1958, 42, 749–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takano, M.; Kishi, S. Foveal retinoschisis and retinal detachment in severely myopic eyes with posterior staphyloma. Am. J. Ophthalmol. 1999, 128, 472–476. [Google Scholar] [CrossRef]
- Wu, P.C.; Chen, Y.J.; Chen, Y.H.; Chen, C.H.; Shin, S.J.; Tsai, C.L.; Kuo, H.K. Factors associated with foveoschisis and foveal detachment without macular hole in high myopia. Eye 2009, 23, 356–361. [Google Scholar] [CrossRef]
- Baba, T.; Ohno-Matsui, K.; Futagami, S.; Yoshida, T.; Yasuzumi, K.; Kojima, A.; Tokoro, T.; Mochizuki, M. Prevalence and characteristics of foveal retinal detachment without macular hole in high myopia. Am. J. Ophthalmol. 2003, 135, 338–342. [Google Scholar] [CrossRef]
- Panozzo, G.; Mercanti, A. Optical coherence tomography findings in myopic traction maculopathy. Arch. Ophthalmol. 2004, 122, 1455–1460. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Chen, X.; Li, M.; Li, S.; You, R.; Wang, Y. Association between morphological characteristics of the optic disc and other anatomical features of the fundus in highly myopic eyes. Eur J Ophthalmol. 2021, 31, 2329–2338. [Google Scholar] [CrossRef]
- Futagami, S.; Inoue, M.; Hirakata, A. Removal of internal limiting membrane for recurrent myopic traction maculopathy. Clin. Exp. Ophthalmol. 2008, 36, 782–785. [Google Scholar] [CrossRef]
- Kumagai, K.; Furukawa, M.; Ogino, N.; Larson, E. Factors correlated with postoperative visual acuity after vitrectomy and internal limiting membrane peeling for myopic foveoschisis. Retina 2010, 30, 874–880. [Google Scholar] [CrossRef]
- Ikuno, Y.; Sayanagi, K.; Soga, K.; Oshima, Y.; Ohji, M.; Tano, Y. Foveal anatomical status and surgical results in vitrectomy for myopic foveoschisis. Jpn. J. Ophthalmol. 2008, 52, 269–276. [Google Scholar] [CrossRef]
- Mii, M.; Matsuoka, M.; Matsuyama, K.; Otsu, Y.; Nishimura, T. Favorable anatomic and visual outcomes with 25-gauge vitrectomy for myopic foveoschisis. Clin. Ophthalmol. 2014, 8, 1837–1844. [Google Scholar] [CrossRef] [Green Version]
- Hirakata, A.; Hida, T. Vitrectomy for myopic posterior retinoschisis or foveal detachment. Jpn. J. Ophthalmol. 2006, 50, 53–61. [Google Scholar] [CrossRef]
- Gao, X.; Ikuno, Y.; Fujimoto, S.; Nishida, K. Risk factors for development of full-thickness macular holes after pars plana vitrectomy for myopic foveoschisis. Am. J. Ophthalmol. 2013, 155, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Uchida, A.; Shinoda, H.; Koto, T.; Mochimaru, H.; Nagai, N.; Tsubota, K.; Ozawa, Y. Vitrectomy for myopic foveoschisis with internal limiting membrane peeling and no gas tamponade. Retina 2014, 34, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Kwon, Y.H.; Kim, S.H.; You, Y.S.; Kwon, O.W. Vitrectomy and internal limiting membrane peeling without gas tamponade for myopic foveoschisis. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 1573–1577. [Google Scholar] [CrossRef] [PubMed]
- Shimada, N.; Sugamoto, Y.; Ogawa, M.; Takase, H.; Ohno-Matsui, K. Fovea-sparing internal limiting membrane peeling for myopic traction maculopathy. Am. J. Ophthalmol. 2012, 154, 693–701. [Google Scholar] [CrossRef]
- Shimada, N.; Tanaka, Y.; Tokoro, T.; Ohno-Matsui, K. Natural course of myopic traction maculopathy and factors associated with progression or resolution. Am. J. Ophthalmol. 2013, 156, 948–957. [Google Scholar] [CrossRef]
- Gaucher, D.; Haouchine, B.; Tadayoni, R.; Massin, P.; Erginay, A.; Benhamou, N.; Gaudric, A. Long-term follow-up of high myopic foveoschisis: Natural course and surgical outcome. Am. J. Ophthalmol. 2007, 143, 455–462. [Google Scholar] [CrossRef]
- Sulkes, D.J.; Smiddy, W.E.; Flynn, H.W.; Feuer, W. Outcomes of macular hole surgery in severely myopic eyes: A case-control study. Am. J. Ophthalmol. 2000, 130, 335–339. [Google Scholar] [CrossRef]
- Patel, S.; Loo, R.H.; Thompson, J.T.; Sjaarda, R.N. Macular hole surgery in high myopia. Ophthalmology 2001, 108, 377–380. [Google Scholar] [CrossRef]
- Jo, Y.; Ikuno, Y.; Nishida, K. Retinoschisis: A predictive factor in vitrectomy for macular holes without retinal detachment in highly myopic eyes. Br. J. Ophthalmol. 2012, 96, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Michalewska, Z.; Michalewski, J.; Adelman, R.A.; Nawrocki, J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology 2010, 117, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Michalewska, Z.; Michalewski, J.; Dulczewska-Cichecka, K.; Adelman, R.A.; Nawrocki, J. Temporal inverted internal limiting membrane flap technique versus classic inverted internal limiting membrane flap technique: A Comparative Study. Retina 2015, 35, 1844–1850. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Qian, H.; Fransisca, S.; Gu, X.; Ji, J.; Wang, J.; Liu, Q.; Xie, P. Minimal internal limiting membrane peeling with ILM flap technique for idiopathic macular holes: A preliminary study. BMC Ophthalmol. 2020, 20, 228. [Google Scholar] [CrossRef]
- Kuriyama, S.; Hayashi, H.; Jingami, Y.; Kuramoto, N.; Akita, J.; Matsumoto, M. Efficacy of inverted internal limiting membrane flap technique for the treatment of macular hole in high myopia. Am. J. Ophthalmol. 2013, 156, 125–131. [Google Scholar] [CrossRef]
- Marques, R.E.; Sousa, D.C.; Leal, I.; Faria, M.Y.; Marques-Neves, C. Complete ILM peeling versus inverted flap technique for macular hole surgery: A meta-analysis. Ophthalmic Surg. Lasers Imaging Retin. 2020, 51, 187-A2. [Google Scholar] [CrossRef]
- Ho, T.C.; Yang, C.M.; Huang, J.S.; Yang, C.H.; Chen, M.S. Foveola nonpeeling internal limiting membrane surgery to prevent inner retinal damages in early stage 2 idiopathic macula hole. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1553–1560. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, Q.; Zhao, P. Fovea sparing internal limiting membrane peeling using multiple parafoveal curvilinear peels for myopic foveoschisis: Technique and outcome. BMC Ophthalmol. 2016, 16, 180. [Google Scholar] [CrossRef] [Green Version]
- Tian, T.; Jin, H.; Zhang, Q.; Zhang, X.; Zhang, H.; Zhao, P. Long-term surgical outcomes of multiple parfoveolar curvilinear internal limiting membrane peeling for myopic foveoschisis. Eye 2018, 32, 1783–1789. [Google Scholar] [CrossRef]
- Matsumura, N.; Ikuno, Y.; Tano, Y. Posterior vitreous detachment and macular hole formation in myopic foveoschisis. Am. J. Ophthalmol. 2004, 138, 1071–1073. [Google Scholar] [CrossRef]
- Lin, J.P.; Yang, C.M. Combined fovea-sparing internal limiting membrane peeling with internal limiting membrane flap technique for progressive myopic traction maculopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Yang, C.M.; Huang, J.S.; Yang, C.H.; Yeh, P.T.; Chen, T.C.; Ho, A.; Chen, M.S. Long-term outcome of foveolar internal limiting membrane nonpeeling for myopic traction maculopathy. Retina 2014, 34, 1833–1840. [Google Scholar] [CrossRef] [PubMed]


| Patient No. | Age (y) | Sex | Eye | Preoperational Type by OCT | Axial Length (mm) | Tamponade | Observation Period (mo) | Operative Method |
|---|---|---|---|---|---|---|---|---|
| 1 | 45 | Male | Left | Retinoschisis | 27.75 | Air | 16 | Vitrectomy |
| 2 | 61 | Male | Right | Retinoschisis | 29.81 | C3F8 | 6 | PEA + IOL, vitrectomy |
| 3 | 62 | Male | Left | Retinoschisis | 28.64 | SF6 | 11 | PEA + IOL, vitrectomy |
| 4 | 67 | Male | Right | Retinoschisis | 29.40 | Air | 11 | PEA + IOL, vitrectomy |
| 5 | 71 | Male | Right | Retinoschisis | 30.16 | SF6 | 14 | PEA + IOL, vitrectomy |
| 6 | 70 | Female | Left | Retinoschisis | 31.10 | SF6 | 7 | PEA + IOL, vitrectomy |
| 7 | 71 | Female | Right | Retinoschisis | 28.54 | SF6 | 6 | PEA + IOL, vitrectomy |
| 8 | 70 | Female | Right | Foveal detachment | 28.35 | Air | 13 | Vitrectomy |
| 9 | 66 | Female | Left | Foveal detachment | 30.70 | C3F8 | 26 | PEA + IOL, vitrectomy |
| 10 | 57 | Female | Right | Foveal detachment | 29.98 | C3F8 | 68 | PEA + IOL, vitrectomy |
| 11 | 61 | Female | Left | Foveal detachment | 29.18 | C3F8 | 93 | PEA + IOL, vitrectomy |
| 12 | 68 | Female | Left | Foveal detachment | 30.01 | C3F8 | 19 | PEA + IOL, vitrectomy |
| 13 | 73 | Female | Right | Foveal detachment | 27.52 | SF6 | 12 | Vitrectomy |
| 14 | 26 | Female | Right | Foveal detachment | 30.67 | Air | 14 | Vitrectomy |
| 15 | 64 | Female | Left | Foveal detachment | 30.20 | C3F8 | 13 | PEA + IOL, vitrectomy |
| 16 | 69 | Female | Left | Foveal detachment | 25.59 * | SF6 | 6 | PEA + IOL, vitrectomy |
| 17 | 67 | Male | Right | Foveal detachment | 33.27 | SF6 | 11 | Vitrectomy |
| 18 | 61 | Female | Right | Foveal detachment | 27.60 | SF6 | 46 | PEA + IOL, vitrectomy |
| 19 | 67 | Male | Left | Foveal detachment | 26.50 | SF6 | 14 | PEA + IOL, vitrectomy |
| 20 | 46 | Female | Left | Foveal detachment | 30.66 | SF6 | 12 | PEA + IOL, vitrectomy |
| Patient No. | Foveal Contour (3 mo) | EZ (3 mo) | Foveal Contour (6 mo) | EZ (6 mo) | Foveal Contour (Last Observation) | EZ (Last Observation) |
|---|---|---|---|---|---|---|
| 1 | Improvement | Irregular | Improvement | Continuous | Recovered | Continuous |
| 2 | Improvement | Absent | Improvement | Continuous | Improvement | Continuous |
| 3 | Improvement | Continuous | Improvement | Continuous | Improvement | Continuous |
| 4 | Improvement | Absent | Improvement | Continuous | Recovered | Continuous |
| 5 | Worse | Absent | Worse | Absent | Recovered | Absent |
| 6 | Improvement | Recovered | Recovered | Absent | Irregular | Irregular |
| 7 | Improvement | Recovered | Recovered | Irregular | Continuous | Continuous |
| 8 | Worse | Absent | Improvement | Absent | Recovered | Continuous |
| 9 | Worse | Absent | Improvement | Absent | Recovered | Absent |
| 10 | Improvement | Absent | Recovered | Continuous | Recovered | Continuous |
| 11 | Improvement | Absent | Improvement | Absent | Recovered | Continuous |
| 12 | Recovered | Absent | Recovered | Continuous | Recovered | Continuous |
| 13 | Improvement | Absent | Improvement | Absent | Recovered | Absent |
| 14 | Improvement | Absent | Recovered | Absent | Recovered | Continuous |
| 15 | Recovered | Absent | Recovered | Absent | Recovered | Absent |
| 16 | Recovered | Absent | Recovered | Continuous | Recovered | Continuous |
| 17 | Recovered | Continuous | Recovered | Continuous | Recovered | Continuous |
| 18 | Recovered | Absent | Recovered | Absent | Recovered | Absent |
| 19 | Improvement | Absent | Improvement | Irregular | Recovered | Continuous |
| 20 | Improvement | Absent | Recovered | Continuous | Recovered | Continuous |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakatsuki, Y.; Nakashizuka, H.; Tanaka, K.; Mori, R.; Shimada, H. Outcomes of Vitrectomy with Fovea-Sparing and Inverted ILM Flap Technique for Myopic Foveoschisis. J. Clin. Med. 2022, 11, 1274. https://doi.org/10.3390/jcm11051274
Wakatsuki Y, Nakashizuka H, Tanaka K, Mori R, Shimada H. Outcomes of Vitrectomy with Fovea-Sparing and Inverted ILM Flap Technique for Myopic Foveoschisis. Journal of Clinical Medicine. 2022; 11(5):1274. https://doi.org/10.3390/jcm11051274
Chicago/Turabian StyleWakatsuki, Yu, Hiroyuki Nakashizuka, Koji Tanaka, Ryusaburo Mori, and Hiroyuki Shimada. 2022. "Outcomes of Vitrectomy with Fovea-Sparing and Inverted ILM Flap Technique for Myopic Foveoschisis" Journal of Clinical Medicine 11, no. 5: 1274. https://doi.org/10.3390/jcm11051274
APA StyleWakatsuki, Y., Nakashizuka, H., Tanaka, K., Mori, R., & Shimada, H. (2022). Outcomes of Vitrectomy with Fovea-Sparing and Inverted ILM Flap Technique for Myopic Foveoschisis. Journal of Clinical Medicine, 11(5), 1274. https://doi.org/10.3390/jcm11051274






