Superiority of a Novel Multifunctional Amorphous Hydrogel Containing Olea europaea Leaf Extract (EHO-85) for the Treatment of Skin Ulcers: A Randomized, Active-Controlled Clinical Trial
Abstract
:1. Introduction
2. Patients and Methods
2.1. Design
2.2. Patients (Inclusion and Exclusion Criteria)
2.3. Investigational Product, Comparator, and Treatment Description
2.4. Randomization and Stratification
2.5. Procedures
2.6. Efficacy Endpoints
2.7. Safety Endpoints
2.8. Data Collection and Database
2.9. Statistical Analysis
2.9.1. Sample Size Calculation
2.9.2. Treatment of Missing Values
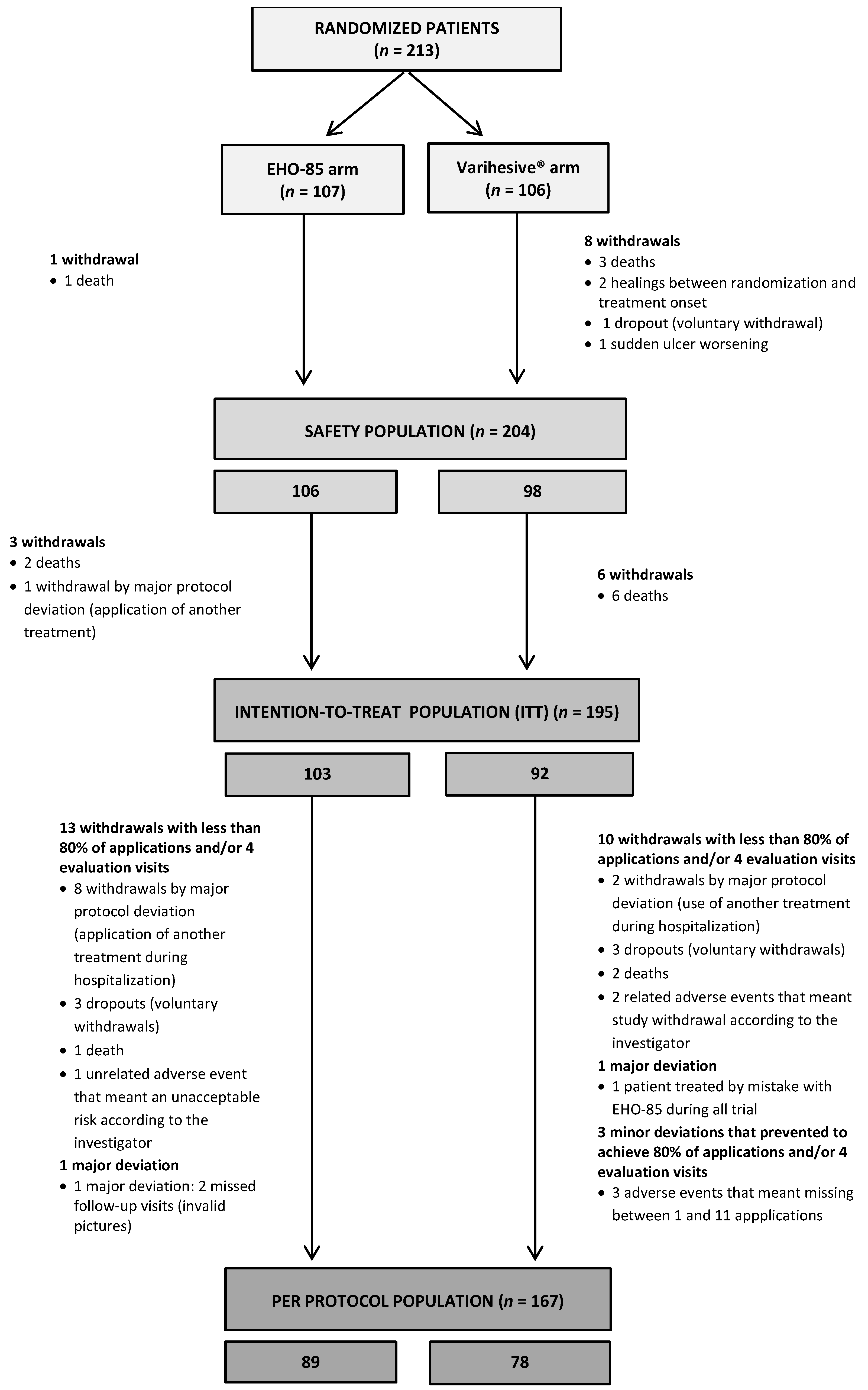
2.9.3. Study Populations
- 1.
- Safety study population (Safety): All randomized patients who received at least one application of the study product.
- 2.
- Intention-to-treat study population (ITT): All patients randomized and treated at least once from whom at least one post-treatment evaluation (digital picture) of the primary efficacy endpoint was obtained, whether or not they met the inclusion/exclusion criteria.
- 3.
- Per protocol study population (PP): Patients who have fulfilled the inclusion/exclusion requirements and the rest of the activities foreseen according to the protocol:
- (1)
- The study/control product had been applied in >80% of the visits foreseen in the protocol (minimum 20 applications) or had achieved complete healing.
- (2)
- Would have undergone one loss in the scheduled evaluation visits at most.
Baseline Values: Descriptive Analysis
Efficacy Outcomes Analysis
3. Results
3.1. Baseline Characteristics
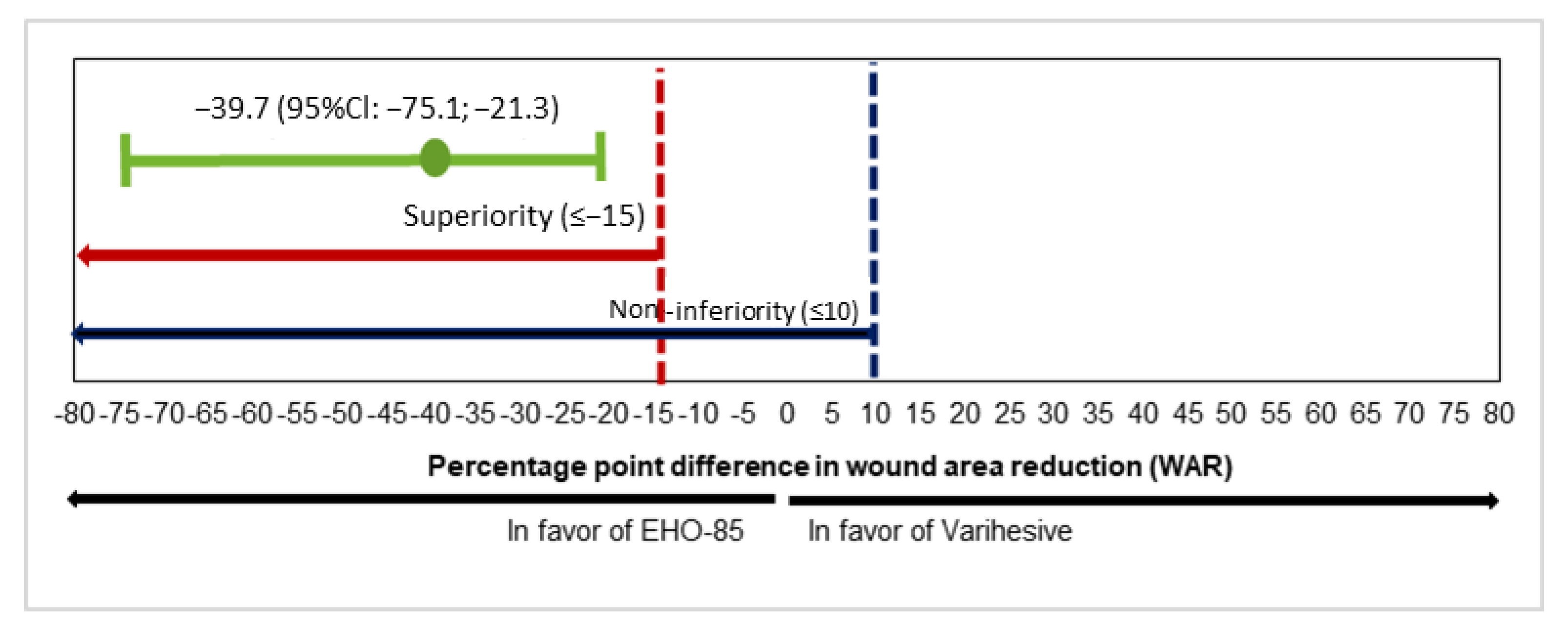
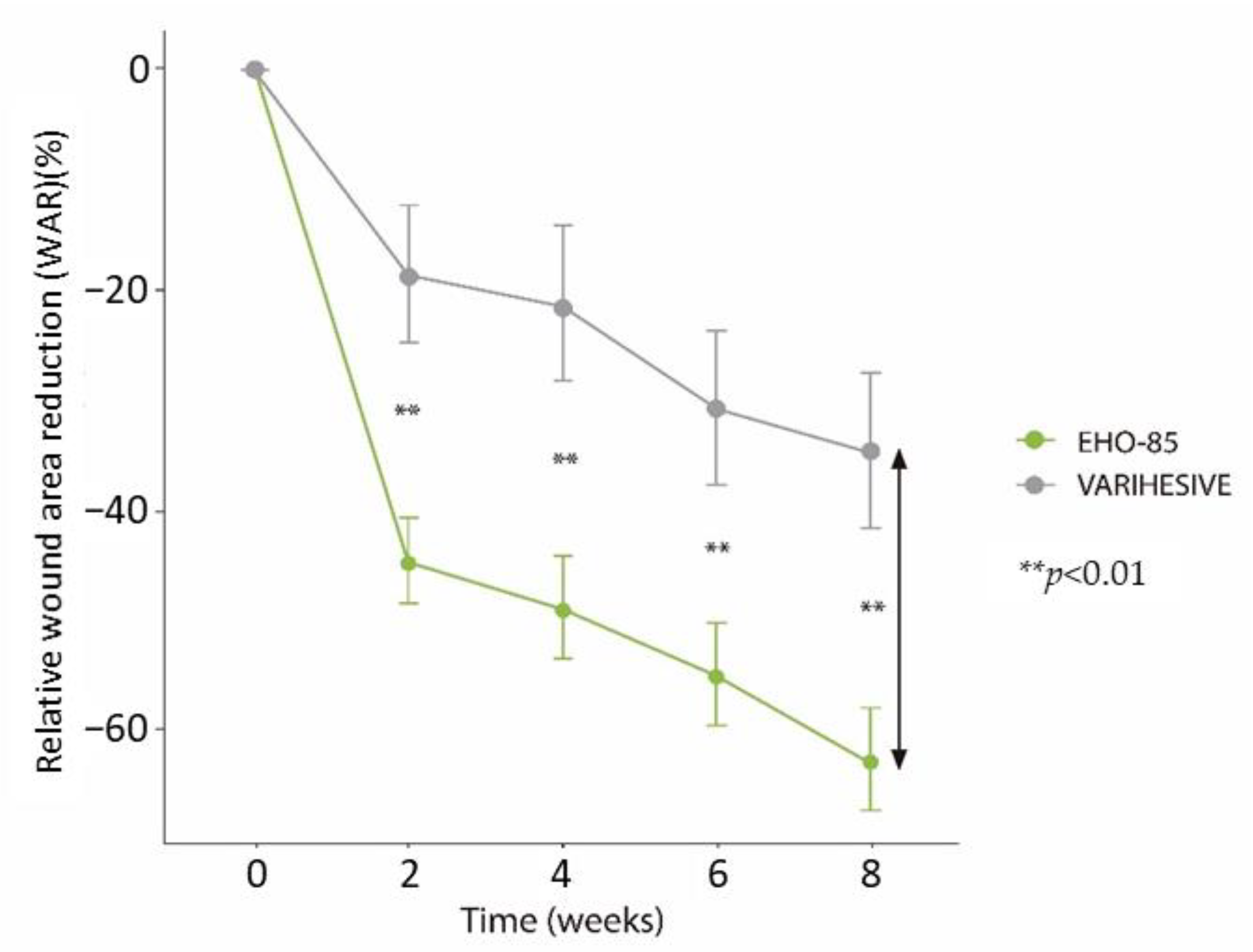
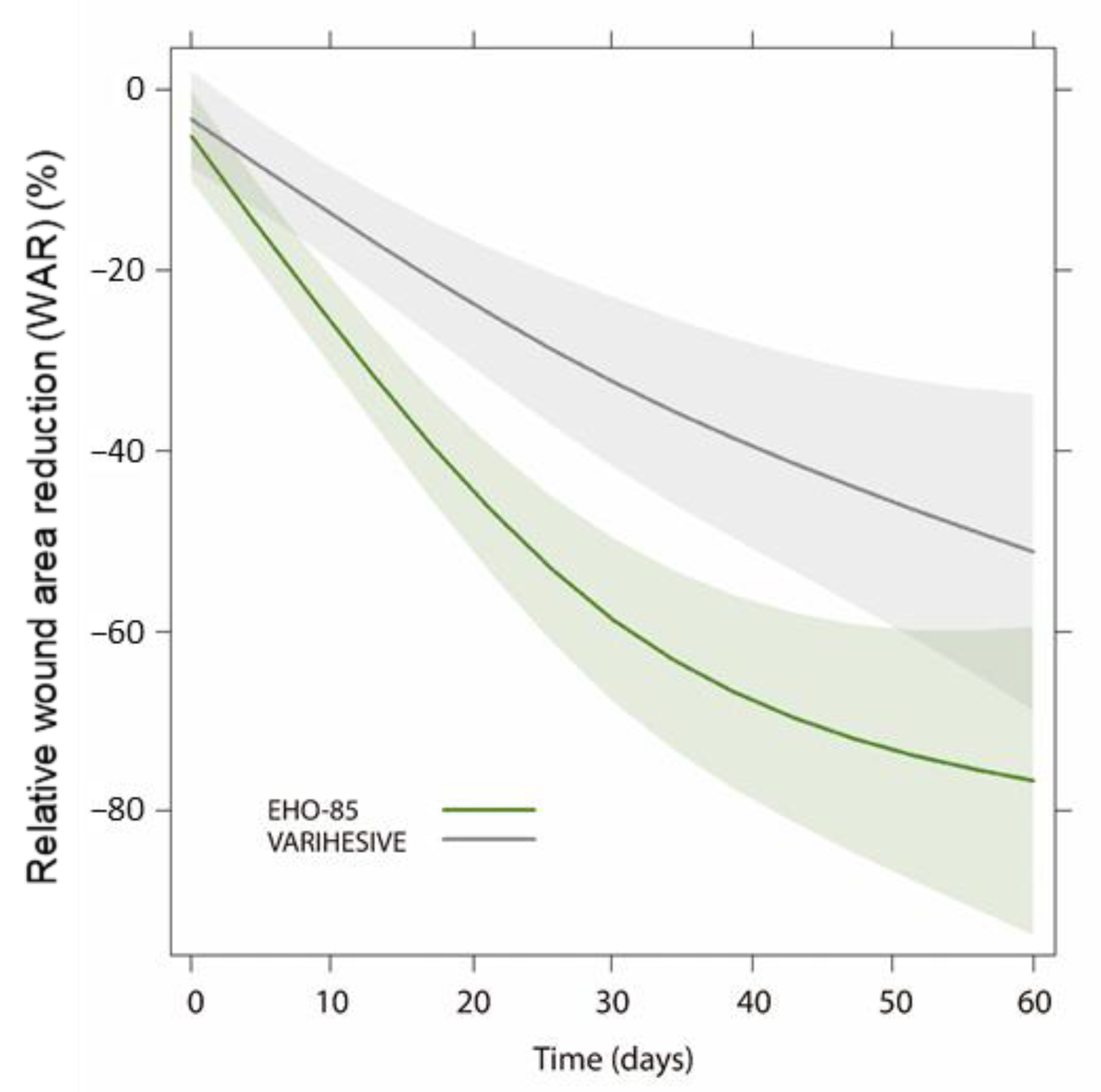
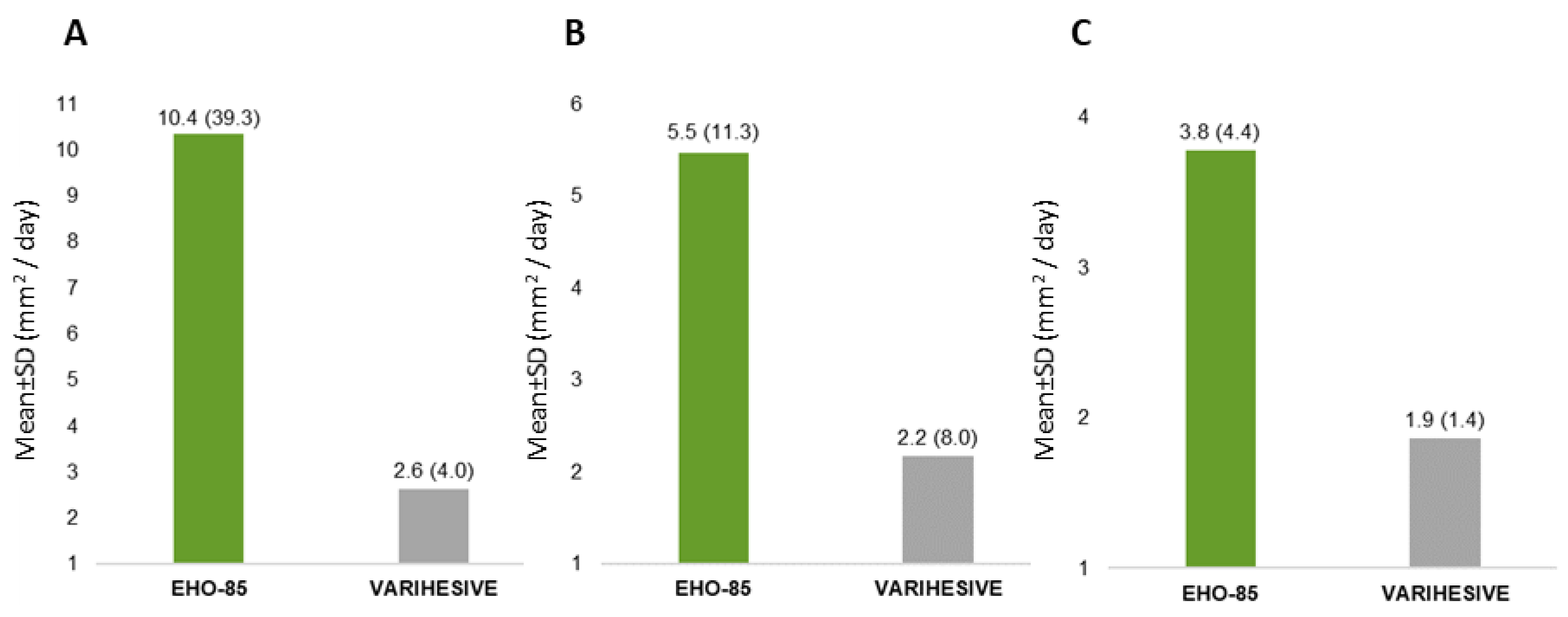
3.2. Wound Area Reduction (WAR)
3.3. Secondary Endpoints
3.4. Subgroup Analysis: Treatment Efficacy According to Ulcer Etiology
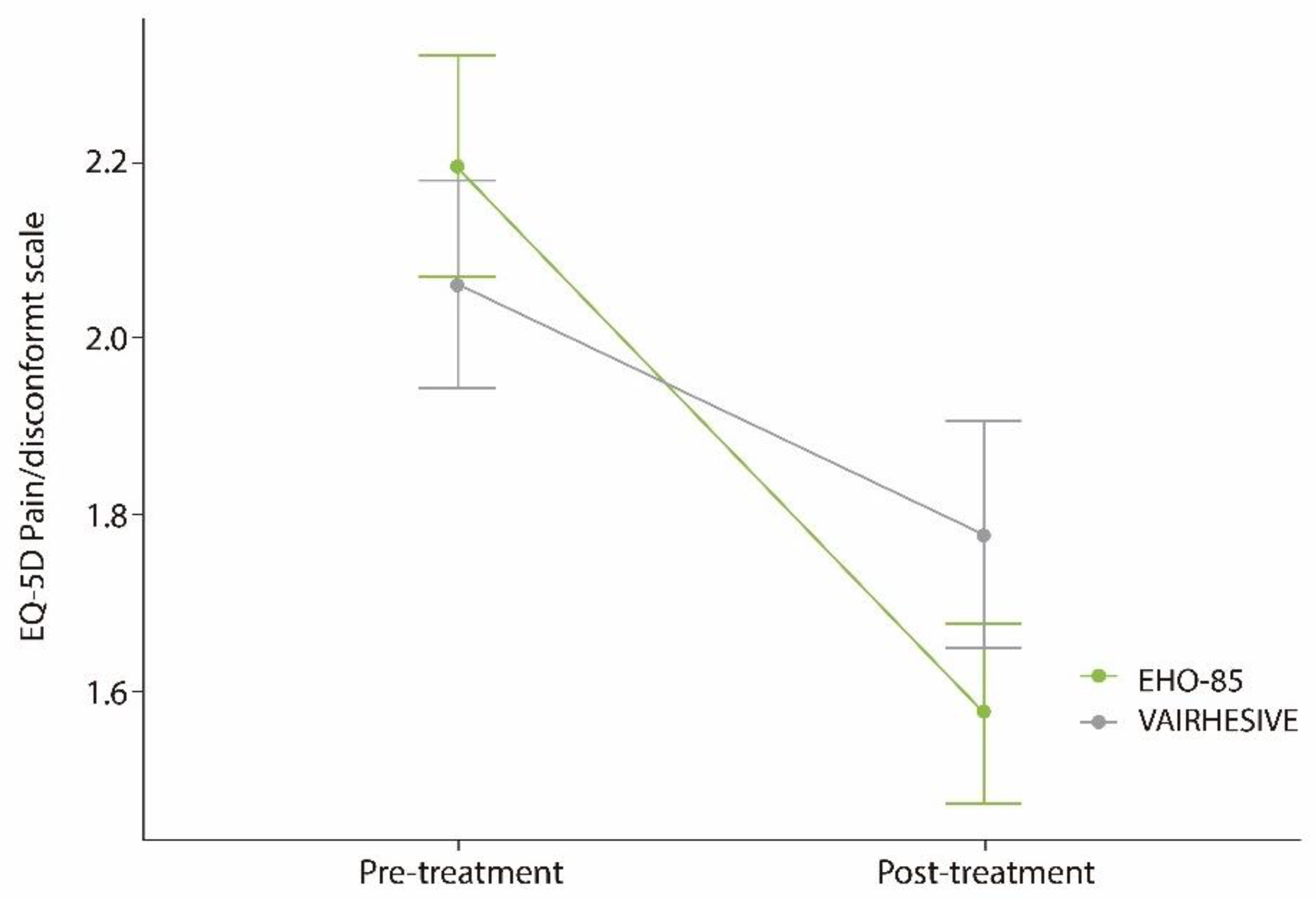
3.5. Ulcer Pain or Discomfort Relief
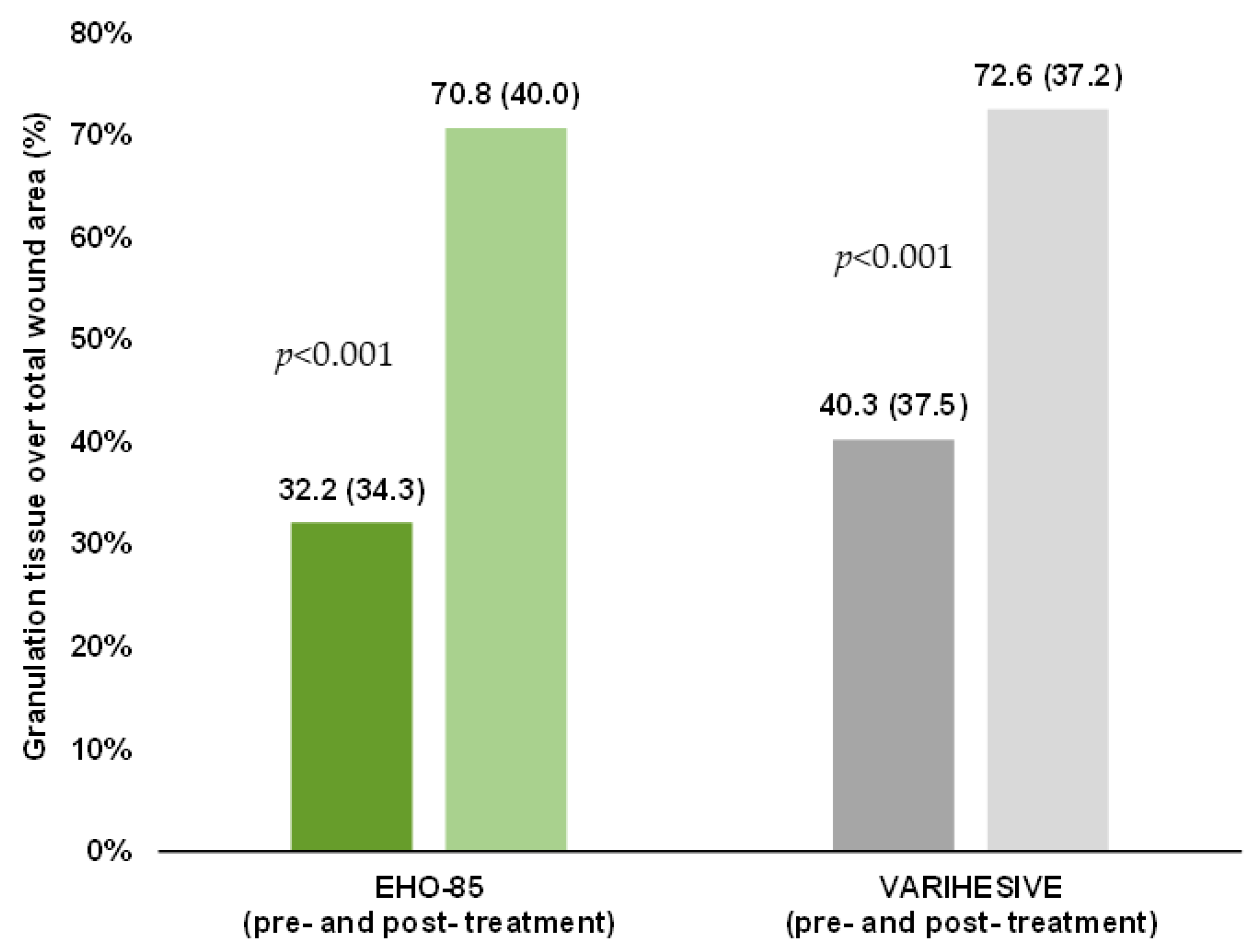
3.6. Granulation Tissue Formation
3.7. Cost Effectiveness: Amount of Product Needed to Fulfill the 8 Weeks of Treatment
3.8. Safety
3.8.1. Total Adverse Events
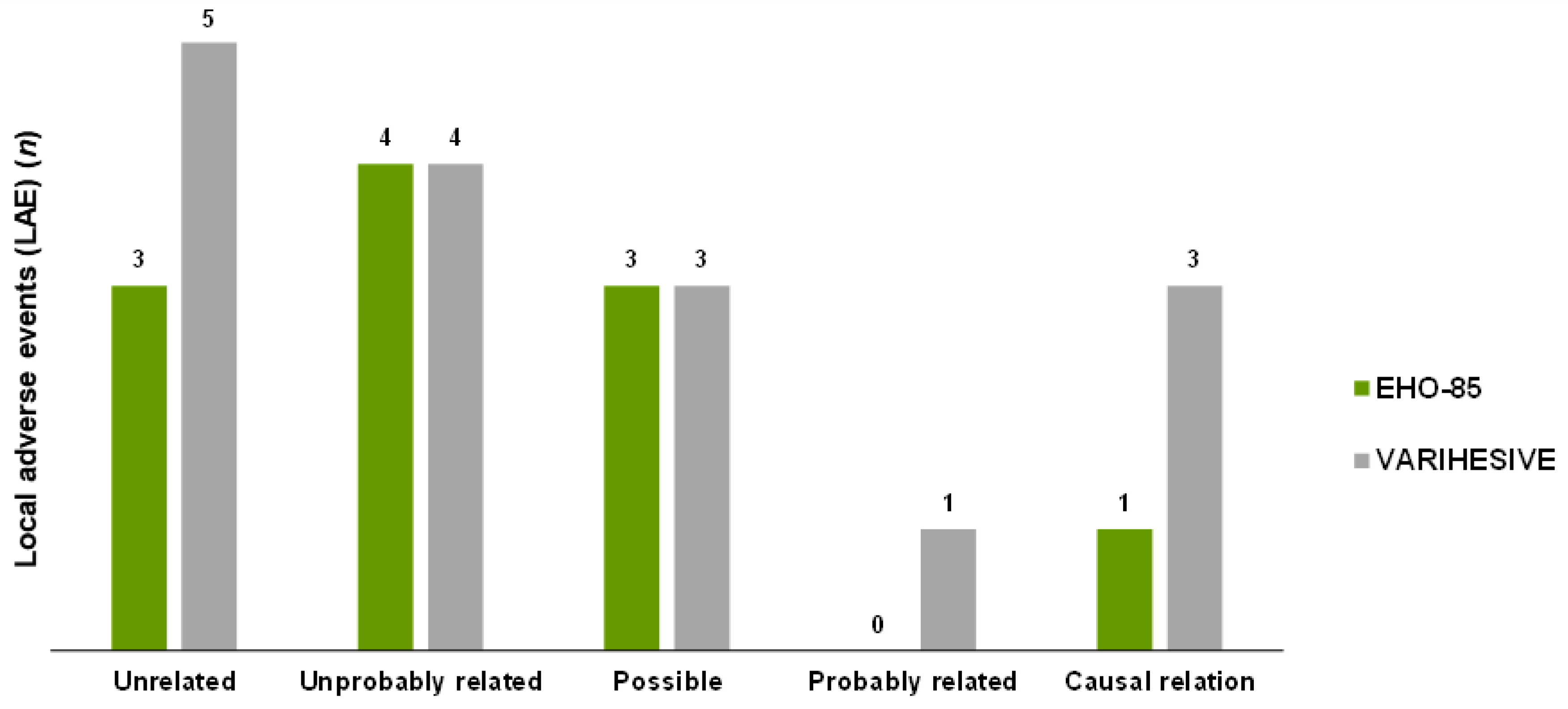
3.8.2. Local Adverse Events
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snyder, R.J.; Jensen, J.; Applewhite, A.J.; Couch, K.; Joseph, W.S.; Lantis Ii, J.C.; Serena, T.E. A Standardized Approach to Evaluating Lower Extremity Chronic Wounds Using a Checklist. Wounds 2019, 31 (Suppl. 5), S29–S44. [Google Scholar] [PubMed]
- Lindholm, C.; Searle, R. Wound management for the 21st century: Combining effectiveness and efficiency. Int. Wound J. 2016, 13 (Suppl. 2), 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salenius, J.E.; Suntila, M.; Ahti, T.; Huhtala, H.; Vaalasti, A.; Salmi, T.T.; Kimpimäki, T. Long-term Mortality among Patients with Chronic Ulcers. Acta Derm. Venereol. 2021, 101, adv00455. [Google Scholar] [CrossRef]
- Bowers, S.; Franco, E. Chronic Wounds: Evaluation and Management. Am. Fam. Physician 2020, 101, 159–166. [Google Scholar]
- Moreno-Giménez, J.C.; Galán-Gutiérrez, M.; Jiménez-Puya, R. Treatment of chronic ulcers. Actas Dermosifiliogr. 2005, 96, 133–146. [Google Scholar] [CrossRef]
- Meaume, S.; Truchetet, F.; Cambazard, F.; Lok, C.; Debure, C.; Dalac, S.; Lazareth, I.; Sigal, M.L.; Sauvadet, A.; Bohbot, S.; et al. A randomized, controlled, double-blind prospective trial with a Lipido-Colloid Technology-Nano-OligoSaccharide Factor wound dressing in the local management of venous leg ulcers. Wound Repair Regen. 2012, 20, 500–511. [Google Scholar] [CrossRef]
- Castro, B.; Bastida, F.D.; Segovia, T.; López Casanova, P.; Soldevilla, J.J.; Verdú-Soriano, J. The use of an antioxidant dressing on hard-to-heal wounds: A multicentre, prospective case series. J. Wound Care 2017, 26, 742–750. [Google Scholar] [CrossRef]
- Barrett, S. Wound-bed preparation: A vital step in the healing process. Br. J. Nurs. 2017, 26 (Suppl. 12), S24–S31. [Google Scholar] [CrossRef] [Green Version]
- Westby, M.J.; Dumville, J.C.; Soares, M.O.; Stubbs, N.; Norman, G. Dressings and topical agents for treating pressure ulcers. Cochrane Database Syst. Rev. 2017, 6, CD011947. [Google Scholar] [CrossRef] [Green Version]
- Walker, R.M.; Gillespie, B.M.; Thalib, L.; Higgins, N.S.; Whitty, J.A. Foam dressings for treating pressure ulcers. Cochrane Database Syst. Rev. 2017, 10, CD011332. [Google Scholar] [CrossRef] [Green Version]
- Norman, G.; Westby, M.J.; Rithalia, A.D.; Stubbs, N.; Soares, M.O.; Dumville, J.C. Dressings and topical agents for treating venous leg ulcers. Cochrane Database Syst. Rev. 2018, 6, CD012583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Norman, G.; Dumville, J.C.; O’Meara, S.; Bell-Syer, S.E. Dressings for treating foot ulcers in people with diabetes: An overview of systematic reviews. Cochrane Database Syst. Rev. 2015, 2015, CD010471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumville, J.C.; Stubbs, N.; Keogh, S.J.; Walker, R.M.; Liu, Z. Hydrogel dressings for treating pressure ulcers. Cochrane Database Syst. Rev. 2015, 2, CD011226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casado-Diaz, A.; Moreno-Rojas, J.M.; Verdú-Soriano, J.; Lázaro-Martínez, J.L.; Rodríguez-Mañas, L.; Tunez, I.; La Torre, M.; Pérez, M.B.; Priego-Capote, F.; Pereira-Caro, G. Evaluation of Antioxidant and Wound-Healing Properties of EHO-85, a Novel Multifunctional Amorphous Hydrogel Containing Olea europaea Leaf Extract. Pharmaceutics 2022, 14, 349. [Google Scholar] [CrossRef]
- European Pressure Ulcer Advisory Panel (EPUAP) and American National Pressure Ulcer Advisory Panel (NPUAP). Prevention and Treatment of Pressure Ulcers: Quick Reference Guide; National Pressure Ulcer Advisory Panel: Washington, DC, USA, 2009. [Google Scholar]
- Wagner, F.W., Jr. The dysvascular foot: A system for diagnosis and treatment. Foot Ankle 1981, 2, 64–122. [Google Scholar] [CrossRef] [PubMed]
- Chaby, G.; Viseux, V.; Ramelet, A.A.; Ganry, O.; Billet, A.; Lok, C. Refractory venous leg ulcers: A study of risk factors. Dermatol. Surg. 2006, 32, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Margolis, D.J.; Allen-Taylor, L.; Hoffstad, O.; Berlin, J.A. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound Repair Regen. 2004, 12, 163–168. [Google Scholar] [CrossRef]
- Margolis, D.J.; Berlin, J.A.; Strom, B.L. Which venous leg ulcers will heal with limb compression bandages? Am. J. Med. 2000, 109, 15–19. [Google Scholar] [CrossRef]
- Stacey, M.C.; Phillips, S.A.; Farrokhyar, F.; Swaine, J.M. Reliability and measurement error of digital planimetry for the measurement of chronic venous leg ulcers. Wound Repair Regen. 2017, 25, 901–905. [Google Scholar] [CrossRef]
- EQ-5D-5L–EQ-5D [Internet]. Available online: https://euroqol.org/eq-5d-instruments/eq-5d-5l-about/ (accessed on 2 May 2021).
- Schmutz, J.L.; Meaume, S.; Fays, S.; Ourabah, Z.; Guillot, B.; Thirion, V.; Collier, M.; Barrett, S.; Smith, J.; Bohbot, S.; et al. Evaluation of the nano-oligosaccharide factor lipido-colloid matrix in the local management of venous leg ulcers: Results of a randomised, controlled trial. Int. Wound J. 2008, 5, 172–182. [Google Scholar] [CrossRef]
- Dereure, O.; Mikosinki, J.; Zegota, Z.; Allaert, F.A. RCT to evaluate a hyaluronic acid containing gauze pad in leg ulcers of venous or mixed aetiology. J. Wound Care 2012, 21, 539–542, 544, 546–547. [Google Scholar] [CrossRef] [PubMed]
- Jünger, M.; Wollina, U.; Kohnen, R.; Rabe, E. Efficacy and tolerability of an ulcer compression stocking for therapy of chronic venous ulcer compared with a below-knee compression bandage: Results from a prospective, randomized, multicentre trial. Curr. Med. Res. Opin. 2004, 20, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Bonett, D.G.; Price, R.M. Statistical inference for a linear function of medians: Confidence intervals, hypothesis testing, and sample size requirements. Psychol. Methods 2002, 7, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef]
- Singer, A.J.; Tassiopoulos, A.; Kirsner, R.S. Evaluation and Management of Lower-Extremity Ulcers. N. Engl. J. Med. 2017, 377, 1559–1567. [Google Scholar] [CrossRef]
- Jones, R.E.; Foster, D.S.; Longaker, M.T. Management of Chronic Wounds-2018. JAMA 2018, 320, 1481–1482. [Google Scholar] [CrossRef]
- Cardinal, M.; Eisenbud, D.E.; Phillips, T.; Harding, K. Early healing rates and wound area measurements are reliable predictors of later complete wound closure. Wound Repair Regen. 2008, 16, 19–22. [Google Scholar] [CrossRef]
- Papazoglou, E.S.; Zubkov, L.; Mao, X.; Neidrauer, M.; Rannou, N.; Weingarten, M.S. Image analysis of chronic wounds for determining the surface area. Wound Repair Regen. 2010, 18, 349–358. [Google Scholar] [CrossRef]
- Meaume, S.; Ourabah, Z.; Romanelli, M.; Manopulo, R.; De Vathaire, F.; Salomon, D.; Saurat, J.H. Efficacy and tolerance of a hydrocolloid dressing containing hyaluronic acid for the treatment of leg ulcers of venous or mixed origin. Curr. Med. Res. Opin. 2008, 24, 2729–2739. [Google Scholar] [CrossRef]
- Margolis, D.J.; Gelfand, J.M.; Hoffstad, O.; Berlin, J.A. Surrogate end points for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2003, 26, 1696–1700. [Google Scholar] [CrossRef] [Green Version]
- Gelfand, J.M.; Hoffstad, O.; Margolis, D.J. Surrogate endpoints for the treatment of venous leg ulcers. J. Investig. Dermatol. 2002, 119, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, M. Improving accuracy of wound measurement in clinical practice. Ostomy Wound Manag. 2003, 49, 28–40. [Google Scholar]
- Flanagan, M. Wound measurement: Can it help us to monitor progression to healing? J. Wound Care 2003, 12, 189–194. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | EHO-85 (n = 103) Mean ± SD or n (%) | VariHesive® (n = 92) Mean ± SD or n (%) | p |
|---|---|---|---|
| Sex, women | 70 (68.0%) | 59 (64.1%) | 0.68 |
| Age, years | 78.0 ± 13.1 | 79.5 ± 14.8 | 0.18 |
| BMI, kg/m2 | 27.4 ± 6.0 | 28.8 ± 8.1 | 0.36 |
| Ankle/brachial index (ABI) (VLU only) | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.98 |
| Diabetes mellitus | 41 (39.8%) | 30 (32.6%) | 0.37 |
| Current smoker | 7 (6.8%) | 5 (5.4%) | 0.31 |
| Alcohol intake | 9 (8.7%) | 7 (7.6%) | 0.98 |
| Place of patient care | 0.74 | ||
| Health center | 22 (21.4%) | 24 (26.1%) | |
| Own home | 52 (50.5%) | 44 (47.8%) | |
| Nursing center | 29 (28.1%) | 24 (26.1%) | |
| Autonomy level | 0.18 | ||
| He/she can walk easily | 22 (21.4%) | 24 (26.1%) | |
| He/she has some difficulty to walking | 45 (43.7%) | 47 (51.1%) | |
| Unable to walk, bedridden | 36 (34.9%) | 21 (22.8%) | |
| Blood test Serum album (3.40–5.0 g/dl) | 3.7 ± 0.5 | 3.5 ± 0.5 | 0.13 |
| Creatinine clearance (80–120 mL/min) | 105.1 ± 46.8 | 107.3 ± 57.1 | 0.93 |
| Characteristic | EHO-85 (n = 103) Mean ± SD or n (%) | VariHesive® (n = 92) Mean ± SD or n (%) | p |
|---|---|---|---|
| Etiology | 0.81 | ||
| Venous | 36 (34.9%) | 36 (39.1%) | |
| Pressure | 62 (60.2%) | 51 (55.4%) | |
| EPUAP II | 42 (67.7%) | 33 (64.7%) | |
| EPUAP III | 20 (32.3%) | 18 (35.3%) | |
| Diabetic foot | 5 (4.9%) | 5 (5.4%) | |
| Wagner I | 2 (40.0%) | 3 (60.0%) | |
| Wagner II | 3 (60.0%) | 2 (40.0%) | |
| Total number of ulcers per patient a | 0.65 | ||
| 1 | 68 (66.0%) | 55 (59.8%) | |
| 2 | 19 (18.5%) | 19 (20.6%) | |
| ≥3 | 16 (15.5%) | 18 (19.6%) | |
| Evolution time, months | 7.1 ± 8.5 | 8.2 ± 9.4 | 0.56 |
| Duration >6 months | 35 (34.0%) | 34 (37.0%) | 0.78 |
| Wound area, cm2 | 5.4 ± 9.0 | 3.4 ± 4.2 | 0.45 |
| Wound area >10 cm2 | 28 (27.2%) | 25 (27.2%) | 1.00 |
| Granulation tissue, % over total ulcer | 71.0 ± 40.4 | 79.9 (35.6) | 0.14 |
| Exudate | 0.82 | ||
| None | 14 (13.6%) | 9 (9.8%) | |
| Low | 47 (45.6%) | 45 (48.9%) | |
| Intermediate | 36 (35.0%) | 34 (37.0%) | |
| High | 6 (5.8%) | 4 (4.3%) | |
| Recurrent ulcer | 35 (34.0%) | 31 (33.7%) | 1.00 |
| Previous hospitalizations due to the treated ulcer | 9 (8.7%) | 3 (3.3%) | 0.20 |
| Ulcer pain intensity b | 0.77 | ||
| No pain nor discomfort | 16 (28.6%) | 14 (28.6%) | |
| Slight pain or discomfort | 17 (30.4%) | 19 (38.8%) | |
| Moderate pain or discomfort | 19 (33.9%) | 15 (30.6%) | |
| Intense pain or discomfort | 4 (7.1%) | 1 (2.0%) | |
| Extreme pain or discomfort | 0 (0.0%) | 0 (0.0%) | |
| Cures during the previous month | 0.75 | ||
| Cure in dry environment | 16 (15.5%) | 16 (17.4%) | |
| Cure in moist environment | 82 (79.6%) | 74 (80.4%) | |
| Both | 2 (1.9%) | 0 (0.0%) | |
| Unknown | 3 (2.9%) | 2 (2.2%) | |
| Debridement in the last month | 45 (43.7%) | 39 (42.4%) | 0.97 |
| Sharp/surgical debridement 24–72 h before visit 1 | 9 (8.7%) | 16 (17.4%) | 0.11 |
| EHO-85 Gel (n = 103) | VariHesive® (n = 92) | p | ||
|---|---|---|---|---|
| Absolute wound area reduction (mm2) | Mean ± SD | 251.92 (553.61) | 108.18 (247.26) | 0.002 |
| Median (range) | 115.80 (−451.0; 4187.7) | 51.15 (−797.0; 1162.6) | ||
| Daily healing (mm2/day) | Mean ± SD | 8.33 (31.19) | 2.40 (5.78) | 0.002 |
| Median (range) | 2.77 (−34.5; 298.9) | 1.18 (−24.5; 20.8) | ||
| N° of patients with complete healing (100%) | N° of patients (%) | 34 (33.01%) | 22 (23.91%) | 0.205 |
| N° average days until complete healing | Mean ± SD | 28.68 (17.13) | 28.64 (12.23) | 0.794 |
| Median (range) | 23.00 (−100.0; 111.0) | 29.00 (7.0; 49.9) | ||
| N° of patients with healing ≥40% | N° of patients (%) | 78 (75.73%) | 45 (48.90%) | <0.001 |
| N° average days until healing ≥40% | Mean ± SD | 14.58 (11.51) | 15.31 (10.49) | 0.508 |
| Median (range) | 10.12 (2.8; 54.1) | 10.43 (2.8; 40.7) | ||
| EHO-85 Gel (n = 62) | VariHesive® (n = 51) | p | ||
| Pressure ulcer | Mean ± SD | −63.92 (39.95) | −37.33 (59.26) | 0.007 |
| Median [range] | −79.05 [−100.0; 79.5] | −36.82 [−100.0; 200.00] | ||
| EHO-85 gel (n = 36) | VariHesive® (n = 36) | p | ||
| Venous ulcer | Mean ± SD | −56.98 (51.22) | −28.45 (80.97) | 0.196 |
| Median [range] | −70.39 [−100.0; 111.0] | −58.71 [−100.0; 200.00] | ||
| EHO-85 gel (n = 5) | VariHesive® (n = 5) | p | ||
| Diabetic foot ulcer | Mean ± SD | −88.49 (25.75) | −51.50 (39.85) | 0.058 |
| Median [range] | −100.00 [−100.0; −42.4] | −36.02 [−100.0; 14.57] |
| Total (n = 27) | EHO-85 Gel (n = 11) | VariHesive® (n = 16) | |
|---|---|---|---|
| Wound infection | 15 | 5 | 10 |
| Satelite lesions | 3 | 2 | 1 |
| Ulcer pain | 2 | 1 | 1 |
| Perilesional erythema | 2 | 1 | 1 |
| Hypergranulation | 2 | 1 | 1 |
| Wound impairment | 1 | 0 | 1 |
| Tissue bleeding | 1 | 1 | 0 |
| Perilesional maceration | 1 | 0 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdú-Soriano, J.; de Cristino-Espinar, M.; Luna-Morales, S.; Dios-Guerra, C.; Caballero-Villarraso, J.; Moreno-Moreno, P.; Casado-Díaz, A.; Berenguer-Pérez, M.; Guler-Caamaño, I.; Laosa-Zafra, O.; et al. Superiority of a Novel Multifunctional Amorphous Hydrogel Containing Olea europaea Leaf Extract (EHO-85) for the Treatment of Skin Ulcers: A Randomized, Active-Controlled Clinical Trial. J. Clin. Med. 2022, 11, 1260. https://doi.org/10.3390/jcm11051260
Verdú-Soriano J, de Cristino-Espinar M, Luna-Morales S, Dios-Guerra C, Caballero-Villarraso J, Moreno-Moreno P, Casado-Díaz A, Berenguer-Pérez M, Guler-Caamaño I, Laosa-Zafra O, et al. Superiority of a Novel Multifunctional Amorphous Hydrogel Containing Olea europaea Leaf Extract (EHO-85) for the Treatment of Skin Ulcers: A Randomized, Active-Controlled Clinical Trial. Journal of Clinical Medicine. 2022; 11(5):1260. https://doi.org/10.3390/jcm11051260
Chicago/Turabian StyleVerdú-Soriano, José, Marisol de Cristino-Espinar, Silvia Luna-Morales, Caridad Dios-Guerra, Javier Caballero-Villarraso, Paloma Moreno-Moreno, Antonio Casado-Díaz, Miriam Berenguer-Pérez, Ipek Guler-Caamaño, Olga Laosa-Zafra, and et al. 2022. "Superiority of a Novel Multifunctional Amorphous Hydrogel Containing Olea europaea Leaf Extract (EHO-85) for the Treatment of Skin Ulcers: A Randomized, Active-Controlled Clinical Trial" Journal of Clinical Medicine 11, no. 5: 1260. https://doi.org/10.3390/jcm11051260
APA StyleVerdú-Soriano, J., de Cristino-Espinar, M., Luna-Morales, S., Dios-Guerra, C., Caballero-Villarraso, J., Moreno-Moreno, P., Casado-Díaz, A., Berenguer-Pérez, M., Guler-Caamaño, I., Laosa-Zafra, O., Rodríguez-Mañas, L., & Lázaro-Martínez, J. L. (2022). Superiority of a Novel Multifunctional Amorphous Hydrogel Containing Olea europaea Leaf Extract (EHO-85) for the Treatment of Skin Ulcers: A Randomized, Active-Controlled Clinical Trial. Journal of Clinical Medicine, 11(5), 1260. https://doi.org/10.3390/jcm11051260









