Tumor Microenvironment and Microvascular Density in Follicular Lymphoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. CD4, CD8, CD68, CD163, Tryptase, CD34, and PDL-1 Immunohistochemistry
2.3. Statistical Analysis
3. Results
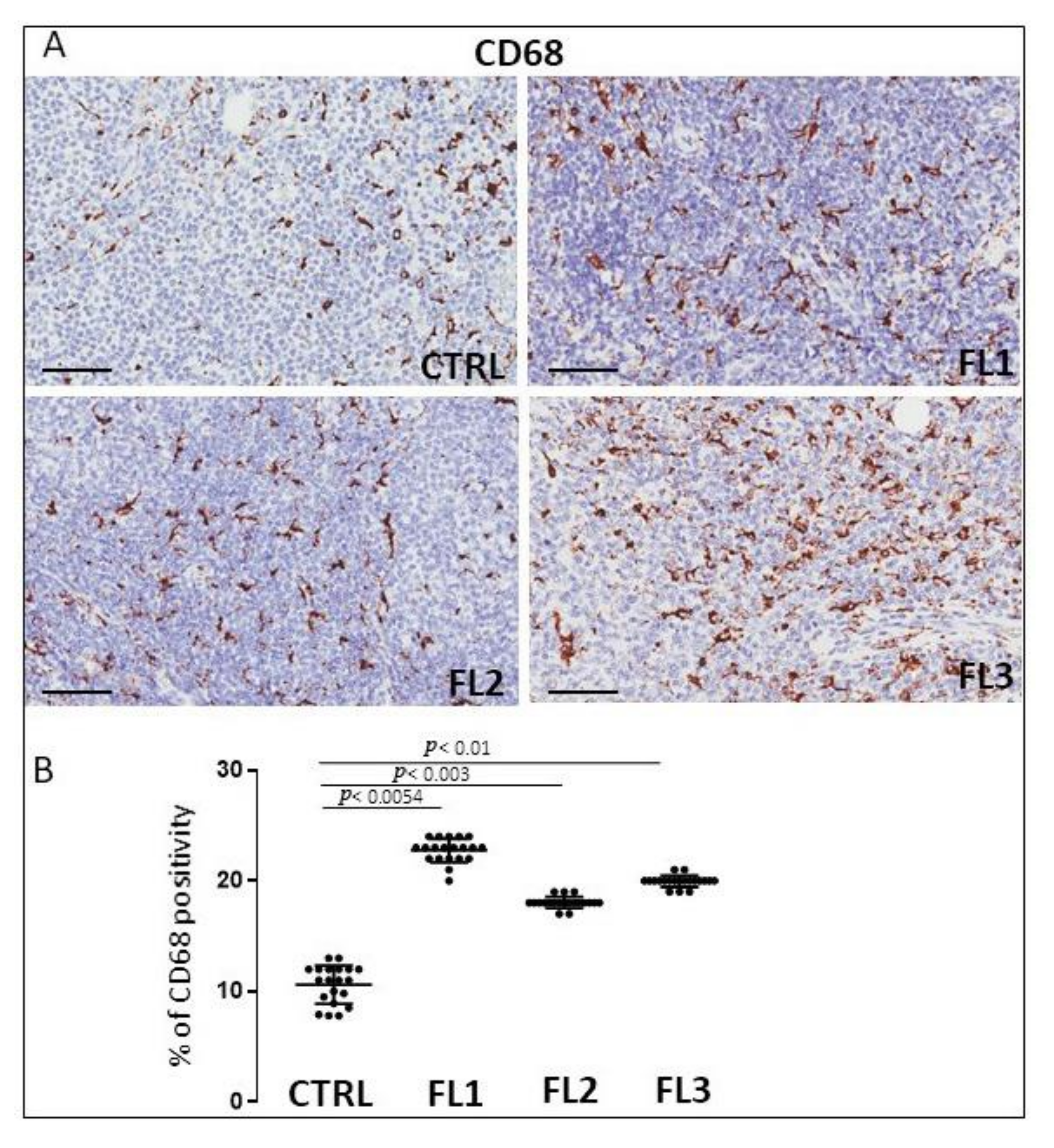
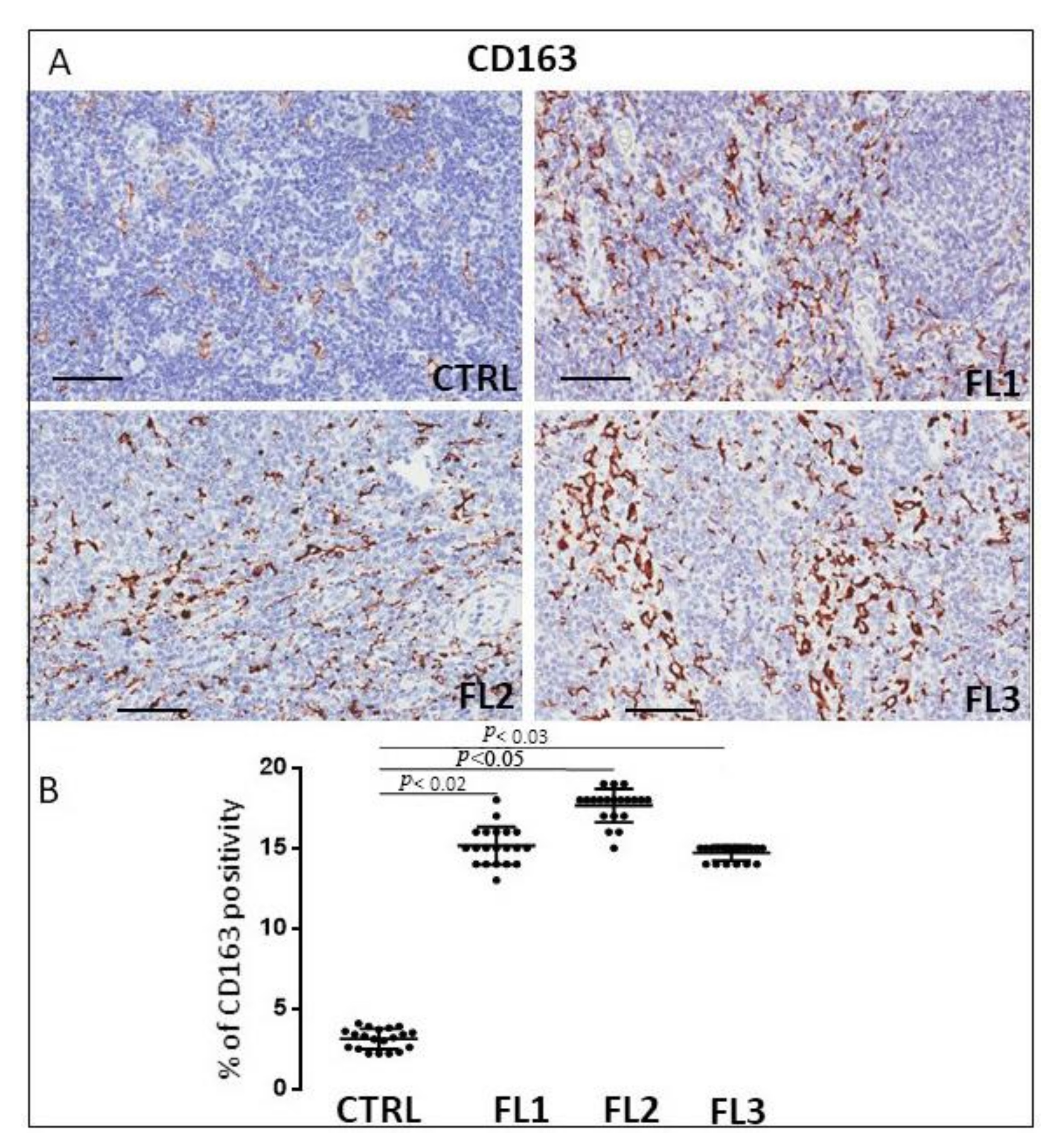
3.1. CD68, CD163, and Tryptase Immunohistochemistry
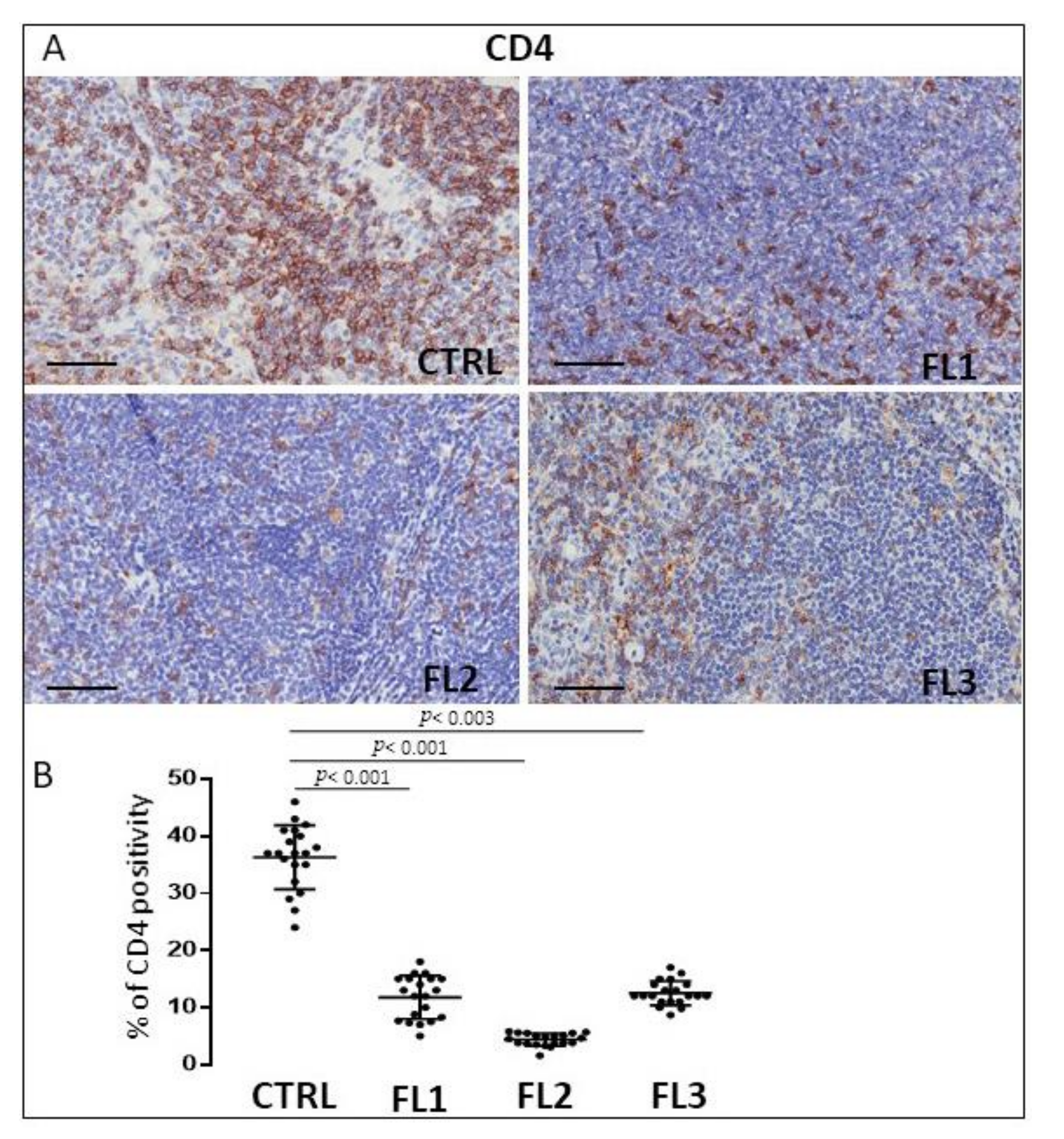
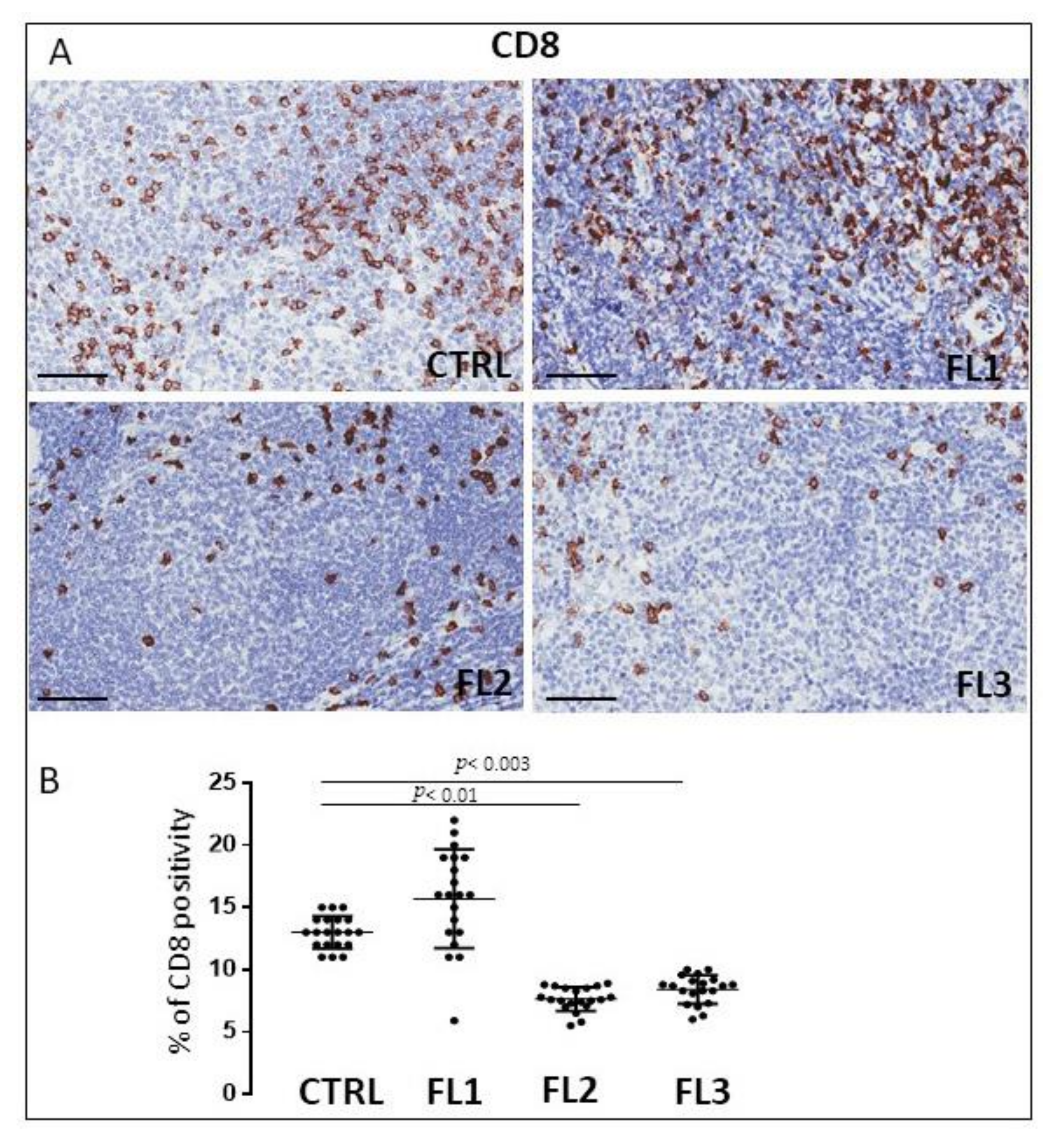
3.2. CD4 and CD8 Immunohistochemistry
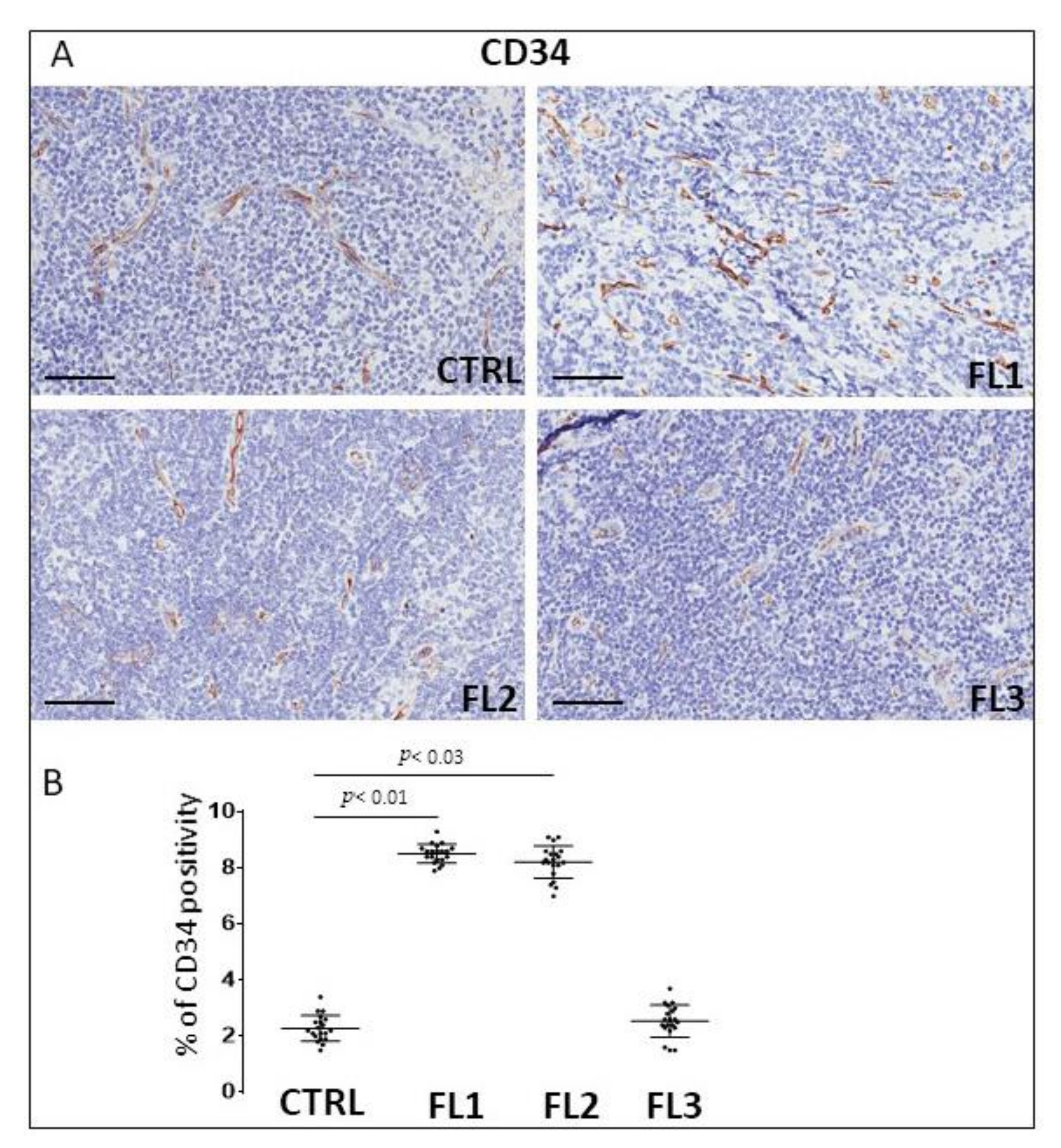
3.3. CD34 and PD-L1 Immunohistochemistry
3.4. Correlation Analysis
4. Discussion
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood 2011, 117, 5019–5032. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Batlevi, C.L.; Sha, F.; Alperovich, A.; Ni, A.; Smith, K.; Ying, Z.; Soumerai, J.D.; Caron, P.C.; Falchi, L.; Hamilton, A.; et al. Follicular lymphoma in the modern era: Survival, treatment outcomes, and identification of high-risk subgroups. Blood Cancer J. 2020, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- Leich, E.; Salaverria, I.; Bea, S.; Zettl, A.; Wright, G.; Moreno, V.; Gascoyne, R.D.; Chan, W.C.; Braziel, R.M.; Rimsza, L.M.; et al. Follicular lymphomas with and without translocation t(14;18) differ in gene expression profiles and genetic alterations. Blood 2009, 114, 826–834. [Google Scholar] [CrossRef]
- Mlynarczyk, C.; Fontan, L.; Melnick, A. Germinal center-derived lymphomas: The darkest side of humoral immunity. Immunol. Rev. 2019, 288, 214–239. [Google Scholar] [CrossRef]
- Lamaison, C.; Tarte, K. B cell/stromal cell crosstalk in health, disease, and treatment: Follicular lymphoma as a paradigm. Immunol. Rev. 2021, 302, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Freedman, A. Follicular lymphoma: 2015 update on diagnosis and management. Am. J. Hematol. 2015, 90, 1171–1178. [Google Scholar] [CrossRef]
- Rusconi, C.; Anastasia, A.; Chiarenza, A.; Marcheselli, L.; Cavallo, F.; Rattotti, S.; Botto, B.; Ferrari, A.; Nassi, L.; Pagani, C.; et al. Outcome of transformed follicular lymphoma worsens according to the timing of transformation and to the number of previous therapies. A retrospective multicenter study on behalf of Fondazione Italiana Linfomi (FIL). Br. J. Haematol. 2019, 185, 713–717. [Google Scholar] [CrossRef]
- Kobayashi, T. The current therapeutic landscape for follicular lymphoma. Rinsho Ketsueki 2021, 62, 1070–1076. [Google Scholar]
- Wahlin, B.E.; Sander, B.; Christensson, B.; Ostenstad, B.; Holte, H.; Brown, P.D.; Sundstrom, C.; Kimby, E. Entourage: The immune microenvironment following follicular lymphoma. Blood Cancer J. 2012, 2, e52. [Google Scholar] [CrossRef]
- Inoue, H.; Rai, S.; Tanaka, H.; Espinoza, J.L.; Watatani, Y.; Kumode, T.; Serizawa, K.; Nakayama, S.; Taniguchi, Y.; Morita, Y.; et al. Tumour-immune microenvironment in duodenal-type follicular lymphoma. Br. J. Haematol. 2020, 191, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Dobano-Lopez, C.; Araujo-Ayala, F.; Serrat, N.; Valero, J.G.; Perez-Galan, P. Follicular Lymphoma Microenvironment: An Intricate Network Ready for Therapeutic Intervention. Cancers 2021, 13, 641. [Google Scholar] [CrossRef] [PubMed]
- Townsend, W.; Pasikowska, M.; Yallop, D.; Phillips, E.H.; Patten, P.E.M.; Salisbury, J.R.; Marcus, R.; Pepper, A.; Devereux, S. The architecture of neoplastic follicles in follicular lymphoma; analysis of the relationship between the tumor and follicular helper T cells. Haematologica 2020, 105, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Solal-Celigny, P.; Roy, P.; Colombat, P.; White, J.; Armitage, J.O.; Arranz-Saez, R.; Au, W.Y.; Bellei, M.; Brice, P.; Caballero, D.; et al. Follicular lymphoma international prognostic index. Blood 2004, 104, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Tsakiroglou, A.M.; Astley, S.; Dave, M.; Fergie, M.; Harkness, E.; Rosenberg, A.; Sperrin, M.; West, C.; Byers, R.; Linton, K. Immune infiltrate diversity confers a good prognosis in follicular lymphoma. Cancer Immunol. Immunother. 2021, 70, 3573–3585. [Google Scholar] [CrossRef] [PubMed]
- Tamma, R.; Ingravallo, G.; Gaudio, F.; Annese, T.; Albano, F.; Ruggieri, S.; Dicataldo, M.; Maiorano, E.; Specchia, G.; Ribatti, D. STAT3, tumor microenvironment, and microvessel density in diffuse large B cell lymphomas. Leuk. Lymphoma 2020, 61, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Tamma, R.; Ingravallo, G.; Annese, T.; De Giorgis, M.; Di Giovanni, F.; Gaudio, F.; Perrone, T.; Musto, P.; Specchia, G.; Ribatti, D. Tumor Cell Microenvironment and Microvessel Density Analysis in MALT Type Lymphoma. Anticancer Res. 2021, 41, 1291–1297. [Google Scholar] [CrossRef]
- Tamma, R.; Ranieri, G.; Ingravallo, G.; Annese, T.; Oranger, A.; Gaudio, F.; Musto, P.; Specchia, G.; Ribatti, D. Inflammatory Cells in Diffuse Large B Cell Lymphoma. J. Clin. Med. 2020, 9, 2418. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Pathria, P.; Louis, T.L.; Varner, J.A. Targeting Tumor-Associated Macrophages in Cancer. Trends Immunol. 2019, 40, 310–327. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Cang, H.; Guo, B. Crosstalk between cancer and immune cells: Role of tumor-associated macrophages in the tumor microenvironment. Cancer Med. 2019, 8, 4709–4721. [Google Scholar] [CrossRef] [PubMed]
- Rabenhorst, A.; Schlaak, M.; Heukamp, L.C.; Forster, A.; Theurich, S.; von Bergwelt-Baildon, M.; Buttner, R.; Kurschat, P.; Mauch, C.; Roers, A.; et al. Mast cells play a protumorigenic role in primary cutaneous lymphoma. Blood 2012, 120, 2042–2054. [Google Scholar] [CrossRef]
- Franco, G.; Guarnotta, C.; Frossi, B.; Piccaluga, P.P.; Boveri, E.; Gulino, A.; Fuligni, F.; Rigoni, A.; Porcasi, R.; Buffa, S.; et al. Bone marrow stroma CD40 expression correlates with inflammatory mast cell infiltration and disease progression in splenic marginal zone lymphoma. Blood 2014, 123, 1836–1849. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maciel, T.T.; Moura, I.C.; Hermine, O. The role of mast cells in cancers. F1000Prime Rep. 2015, 7, 09. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Muller, S.; Do, C.; Al-Saati, T.; Allart, S.; Larocca, L.M.; Hohaus, S.; Duchez, S.; Quillet-Mary, A.; Laurent, G.; et al. Distribution, function, and prognostic value of cytotoxic T lymphocytes in follicular lymphoma: A 3-D tissue-imaging study. Blood 2011, 118, 5371–5379. [Google Scholar] [CrossRef]
- Liu, X.; Venkataraman, G.; Lin, J.; Kiyotani, K.; Smith, S.; Montoya, M.; Nakamura, Y.; Kline, J. Highly clonal regulatory T-cell population in follicular lymphoma—Inverse correlation with the diversity of CD8(+) T cells. Oncoimmunology 2015, 4, e1002728. [Google Scholar] [CrossRef][Green Version]
- Kiaii, S.; Clear, A.J.; Ramsay, A.G.; Davies, D.; Sangaralingam, A.; Lee, A.; Calaminici, M.; Neuberg, D.S.; Gribben, J.G. Follicular lymphoma cells induce changes in T-cell gene expression and function: Potential impact on survival and risk of transformation. J. Clin. Oncol. 2013, 31, 2654–2661. [Google Scholar] [CrossRef]
- Umetsu, D.T.; Esserman, L.; Donlon, T.A.; DeKruyff, R.H.; Levy, R. Induction of proliferation of human follicular (B type) lymphoma cells by cognate interaction with CD4+ T cell clones. J. Immunol. 1990, 144, 2550–2557. [Google Scholar]
- Burack, W.R.; Spence, J.M.; Spence, J.P.; Spence, S.A.; Rock, P.J.; Shenoy, G.N.; Shultz, L.D.; Bankert, R.B.; Bernstein, S.H. Patient-derived xenografts of low-grade B-cell lymphomas demonstrate roles of the tumor microenvironment. Blood Adv. 2017, 1, 1263–1273. [Google Scholar] [CrossRef]
- Mondello, P.; Fama, A.; Larson, M.C.; Feldman, A.L.; Villasboas, J.C.; Yang, Z.Z.; Galkin, I.; Svelolkin, V.; Postovalova, E.; Bagaev, A.; et al. Lack of intrafollicular memory CD4 + T cells is predictive of early clinical failure in newly diagnosed follicular lymphoma. Blood Cancer J. 2021, 11, 130. [Google Scholar] [CrossRef]
- Strasly, M.; Cavallo, F.; Geuna, M.; Mitola, S.; Colombo, M.P.; Forni, G.; Bussolino, F. IL-12 inhibition of endothelial cell functions and angiogenesis depends on lymphocyte-endothelial cell cross-talk. J. Immunol. 2001, 166, 3890–3899. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.E.; Harney, A.S.; Pollard, J.W. The Multifaceted Role of Perivascular Macrophages in Tumors. Cancer Cell 2016, 30, 18–25. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Barreto, J.B.; Andreu, P.; Vasquez, L.; Tawfik, D.; Kolhatkar, N.; Coussens, L.M. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 2009, 16, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Song, Y.; Wang, Y.; Huang, Y.; Li, Z.; Cui, Y.; Yi, M.; Xia, L.; Zhuang, W.; Wu, X.; et al. PD-1/PD-L1 blockade rescue exhausted CD8+ T cells in gastrointestinal stromal tumours via the PI3K/Akt/mTOR signalling pathway. Cell Prolif. 2019, 52, e12571. [Google Scholar] [CrossRef]
- Mimura, K.; Teh, J.L.; Okayama, H.; Shiraishi, K.; Kua, L.F.; Koh, V.; Smoot, D.T.; Ashktorab, H.; Oike, T.; Suzuki, Y.; et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018, 109, 43–53. [Google Scholar] [CrossRef]
- Yi, M.; Zhang, J.; Li, A.; Niu, M.; Yan, Y.; Jiao, Y.; Luo, S.; Zhou, P.; Wu, K. The construction, expression, and enhanced anti-tumor activity of YM101: A bispecific antibody simultaneously targeting TGF-beta and PD-L1. J. Hematol. Oncol. 2021, 14, 27. [Google Scholar] [CrossRef]
- Chu, Y.; Zhou, X.; Wang, X. Antibody-drug conjugates for the treatment of lymphoma: Clinical advances and latest progress. J. Hematol. Oncol. 2021, 14, 88. [Google Scholar] [CrossRef]
- Wight, J.C.; Hawkes, E.A.; Berlangieri, S.U.; Khor, R.; Grigg, A.P. An abscopal effect may augment PD-1 inhibition in refractory classical Hodgkin lymphoma. Leuk. Lymphoma 2018, 59, 2749–2751. [Google Scholar] [CrossRef]
- Zhang, J.; Medeiros, L.J.; Young, K.H. Cancer Immunotherapy in Diffuse Large B-Cell Lymphoma. Front. Oncol. 2018, 8, 351. [Google Scholar] [CrossRef]
- Lesokhin, A.M.; Ansell, S.M.; Armand, P.; Scott, E.C.; Halwani, A.; Gutierrez, M.; Millenson, M.M.; Cohen, A.D.; Schuster, S.J.; Lebovic, D.; et al. Nivolumab in Patients with Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J. Clin. Oncol. 2016, 34, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Hurvitz, S.A.; Koenig, P.A.; LaPlant, B.R.; Kabat, B.F.; Fernando, D.; Habermann, T.M.; Inwards, D.J.; Verma, M.; Yamada, R.; et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin. Cancer Res. 2009, 15, 6446–6453. [Google Scholar] [CrossRef]
- Westin, J.R.; Chu, F.; Zhang, M.; Fayad, L.E.; Kwak, L.W.; Fowler, N.; Romaguera, J.; Hagemeister, F.; Fanale, M.; Samaniego, F.; et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: A single group, open-label, phase 2 trial. Lancet Oncol. 2014, 15, 69–77. [Google Scholar] [CrossRef]
- Maskey, N.; Chen, Q.; Liu, F.; Liu, S.; Tian, S. A rare face of follicular lymphoma: Reverse variant of follicular lymphoma. Diagn. Pathol. 2020, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Freedman, A. Follicular lymphoma: 2018 update on diagnosis and management. Am. J. Hematol. 2018, 93, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Horning, S.J. Natural history of and therapy for the indolent non-Hodgkin’s lymphomas. Semin. Oncol. 1993, 20, 75–88. [Google Scholar] [PubMed]
- Grillo-Lopez, A.J.; White, C.A.; Dallaire, B.K.; Varns, C.L.; Shen, C.D.; Wei, A.; Leonard, J.E.; McClure, A.; Weaver, R.; Cairelli, S.; et al. Rituximab: The first monoclonal antibody approved for the treatment of lymphoma. Curr. Pharm. Biotechnol. 2000, 1, 1–9. [Google Scholar] [CrossRef]
- Pham, L.V.; Pogue, E.; Ford, R.J. The Role of Macrophage/B-Cell Interactions in the Pathophysiology of B-Cell Lymphomas. Front. Oncol. 2018, 8, 147. [Google Scholar] [CrossRef]
- Taskinen, M.; Karjalainen-Lindsberg, M.L.; Leppa, S. Prognostic influence of tumor-infiltrating mast cells in patients with follicular lymphoma treated with rituximab and CHOP. Blood 2008, 111, 4664–4667. [Google Scholar] [CrossRef]
- Canioni, D.; Salles, G.; Mounier, N.; Brousse, N.; Keuppens, M.; Morchhauser, F.; Lamy, T.; Sonet, A.; Rousselet, M.C.; Foussard, C.; et al. High numbers of tumor-associated macrophages have an adverse prognostic value that can be circumvented by rituximab in patients with follicular lymphoma enrolled onto the GELA-GOELAMS FL-2000 trial. J. Clin. Oncol. 2008, 26, 440–446. [Google Scholar] [CrossRef]
- Dave, S.S.; Wright, G.; Tan, B.; Rosenwald, A.; Gascoyne, R.D.; Chan, W.C.; Fisher, R.I.; Braziel, R.M.; Rimsza, L.M.; Grogan, T.M.; et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N. Engl. J. Med. 2004, 351, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Kridel, R.; Xerri, L.; Gelas-Dore, B.; Tan, K.; Feugier, P.; Vawda, A.; Canioni, D.; Farinha, P.; Boussetta, S.; Moccia, A.A.; et al. The Prognostic Impact of CD163-Positive Macrophages in Follicular Lymphoma: A Study from the BC Cancer Agency and the Lymphoma Study Association. Clin. Cancer Res. 2015, 21, 3428–3435. [Google Scholar] [CrossRef] [PubMed]
- Andjelic, B.; Mihaljevic, B.; Todorovic, M.; Bila, J.; Jakovic, L.; Jovanovic, M.P. The number of lymphoma-associated macrophages in tumor tissue is an independent prognostic factor in patients with follicular lymphoma. Appl. Immunohistochem. Mol. Morphol. 2012, 20, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Hajjar, K.; Rafii, S.; Leonard, J.P. Angiogenesis and antiangiogenic therapy in non-Hodgkin’s lymphoma. Ann. Oncol. 2009, 20, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Farinha, P.; Kyle, A.H.; Minchinton, A.I.; Connors, J.M.; Karsan, A.; Gascoyne, R.D. Vascularization predicts overall survival and risk of transformation in follicular lymphoma. Haematologica 2010, 95, 2157–2160. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.M.; Sorensen, F.B.; Bendix, K.; Nielsen, J.L.; Olsen, M.L.; Funder, A.M.; d’Amore, F. Angiogenesis in non-Hodgkin’s lymphoma: Clinico-pathological correlations and prognostic significance in specific subtypes. Leuk. Lymphoma 2007, 48, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Crivellato, E.; Nico, B.; Vacca, A.; Ribatti, D. Ultrastructural analysis of mast cell recovery after secretion by piecemeal degranulation in B-cell non-Hodgkin’s lymphoma. Leuk. Lymphoma 2003, 44, 517–521. [Google Scholar] [CrossRef]
- Marinaccio, C.; Ingravallo, G.; Gaudio, F.; Perrone, T.; Nico, B.; Maoirano, E.; Specchia, G.; Ribatti, D. Microvascular density, CD68 and tryptase expression in human diffuse large B-cell lymphoma. Leuk. Res. 2014, 38, 1374–1377. [Google Scholar] [CrossRef]
- Jayasingam, S.D.; Citartan, M.; Thang, T.H.; Mat Zin, A.A.; Ang, K.C.; Ch’ng, E.S. Evaluating the Polarization of Tumor-Associated Macrophages into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front. Oncol. 2019, 9, 1512. [Google Scholar] [CrossRef]
- Maltby, S.; Khazaie, K.; McNagny, K.M. Mast cells in tumor growth: Angiogenesis, tissue remodelling and immune-modulation. Biochim. Biophys. Acta 2009, 1796, 19–26. [Google Scholar] [CrossRef]
- Wilkins, B.S.; Buchan, S.L.; Webster, J.; Jones, D.B. Tryptase-positive mast cells accompany lymphocytic as well as lymphoplasmacytic lymphoma infiltrates in bone marrow trephine biopsies. Histopathology 2001, 39, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Nico, B.; Vacca, A.; Marzullo, A.; Calvi, N.; Roncali, L.; Dammacco, F. Do mast cells help to induce angiogenesis in B-cell non-Hodgkin’s lymphomas? Br. J. Cancer 1998, 77, 1900–1906. [Google Scholar] [CrossRef] [PubMed]
- Duse, A.O.; Ceausu, R.A.; Mezei, T.; Cimpean, A.M.; Gaje, P.; Ionita, H.; Jung, I. Mast cells contribute to the angiogenesis in non-Hodgkin lymphoma. An immunohistochemical study based on the relationship with microvessel density. Rom. J. Morphol. Embryol. 2011, 52 (Suppl. 3), 1091–1096. [Google Scholar] [PubMed]
- Alvaro, T.; Lejeune, M.; Salvado, M.T.; Lopez, C.; Jaen, J.; Bosch, R.; Pons, L.E. Immunohistochemical patterns of reactive microenvironment are associated with clinicobiologic behavior in follicular lymphoma patients. J. Clin. Oncol. 2006, 24, 5350–5357. [Google Scholar] [CrossRef] [PubMed]
- Wahlin, B.E.; Sander, B.; Christensson, B.; Kimby, E. CD8+ T-cell content in diagnostic lymph nodes measured by flow cytometry is a predictor of survival in follicular lymphoma. Clin. Cancer Res. 2007, 13 Pt 1, 388–397. [Google Scholar] [CrossRef][Green Version]
- Lee, A.M.; Clear, A.J.; Calaminici, M.; Davies, A.J.; Jordan, S.; MacDougall, F.; Matthews, J.; Norton, A.J.; Gribben, J.G.; Lister, T.A.; et al. Number of CD4+ cells and location of forkhead box protein P3-positive cells in diagnostic follicular lymphoma tissue microarrays correlates with outcome. J. Clin. Oncol. 2006, 24, 5052–5059. [Google Scholar] [CrossRef]
- Byers, R.J.; Sakhinia, E.; Joseph, P.; Glennie, C.; Hoyland, J.A.; Menasce, L.P.; Radford, J.A.; Illidge, T. Clinical quantitation of immune signature in follicular lymphoma by RT-PCR-based gene expression profiling. Blood 2008, 111, 4764–4770. [Google Scholar] [CrossRef]
- De Jong, D.; Koster, A.; Hagenbeek, A.; Raemaekers, J.; Veldhuizen, D.; Heisterkamp, S.; de Boer, J.P.; van Glabbeke, M. Impact of the tumor microenvironment on prognosis in follicular lymphoma is dependent on specific treatment protocols. Haematologica 2009, 94, 70–77. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Dong, H.; Zhu, G.; Tamada, K.; Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999, 5, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Charmpi, K.; Gravelle, P.; Tosolini, M.; Franchet, C.; Ysebaert, L.; Brousset, P.; Bidaut, A.; Ycart, B.; Fournie, J.J. Several immune escape patterns in non-Hodgkin’s lymphomas. Oncoimmunology 2015, 4, e1026530. [Google Scholar] [CrossRef] [PubMed]
- Menter, T.; Bodmer-Haecki, A.; Dirnhofer, S.; Tzankov, A. Evaluation of the diagnostic and prognostic value of PDL1 expression in Hodgkin and B-cell lymphomas. Hum. Pathol. 2016, 54, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Myklebust, J.H.; Irish, J.M.; Brody, J.; Czerwinski, D.K.; Houot, R.; Kohrt, H.E.; Timmerman, J.; Said, J.; Green, M.R.; Delabie, J.; et al. High PD-1 expression and suppressed cytokine signaling distinguish T cells infiltrating follicular lymphoma tumors from peripheral T cells. Blood 2013, 121, 1367–1376. [Google Scholar] [CrossRef]



| FL Patients | ||
|---|---|---|
| Gender (%) | Male Female | 60 40 |
| Age (years) | Median Range | 62 39–80 |
| Ki67% | Ave Range | 27 10–70 |
| Stage (number) | I II IIIA | 20 20 20 |
| First diagnosis (%) | I II IIIA | 100 100 100 |
| Extranodal sites (%) | I II IIIA | 0 0 0 |
| FLIPI (%) | 1 2 3 | 26 60 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamma, R.; Ingravallo, G.; Annese, T.; Gaudio, F.; Perrone, T.; Musto, P.; Specchia, G.; Ribatti, D. Tumor Microenvironment and Microvascular Density in Follicular Lymphoma. J. Clin. Med. 2022, 11, 1257. https://doi.org/10.3390/jcm11051257
Tamma R, Ingravallo G, Annese T, Gaudio F, Perrone T, Musto P, Specchia G, Ribatti D. Tumor Microenvironment and Microvascular Density in Follicular Lymphoma. Journal of Clinical Medicine. 2022; 11(5):1257. https://doi.org/10.3390/jcm11051257
Chicago/Turabian StyleTamma, Roberto, Giuseppe Ingravallo, Tiziana Annese, Francesco Gaudio, Tommasina Perrone, Pellegrino Musto, Giorgina Specchia, and Domenico Ribatti. 2022. "Tumor Microenvironment and Microvascular Density in Follicular Lymphoma" Journal of Clinical Medicine 11, no. 5: 1257. https://doi.org/10.3390/jcm11051257
APA StyleTamma, R., Ingravallo, G., Annese, T., Gaudio, F., Perrone, T., Musto, P., Specchia, G., & Ribatti, D. (2022). Tumor Microenvironment and Microvascular Density in Follicular Lymphoma. Journal of Clinical Medicine, 11(5), 1257. https://doi.org/10.3390/jcm11051257









