Profoundly Disturbed Lipoproteins in Cirrhotic Patients: Role of Lipoprotein-Z, a Hepatotoxic LDL-like Lipoprotein
Abstract
1. Introduction
2. Materials and Methods
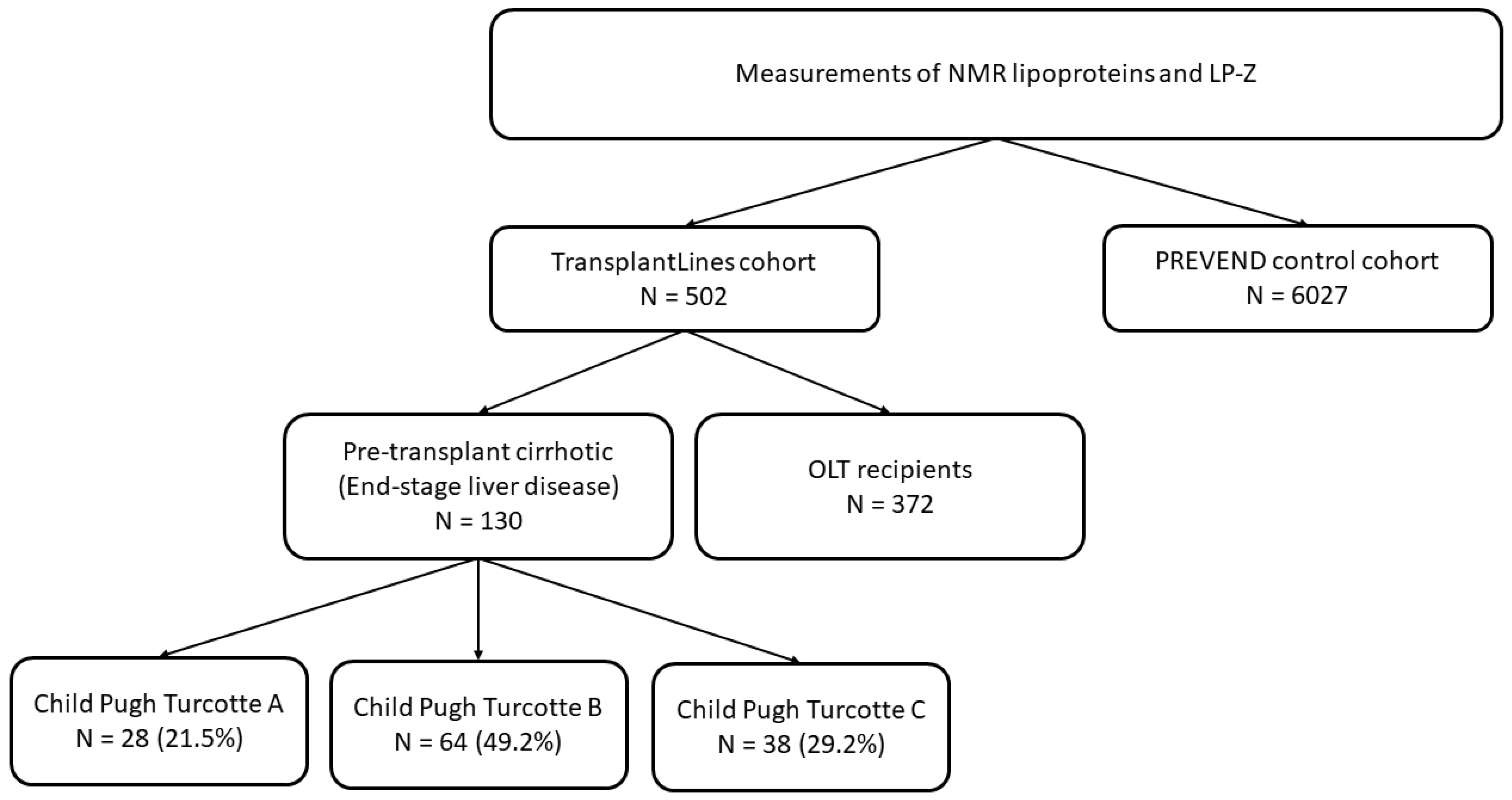
2.1. Study Population
2.2. Data Collection and Measurements
2.3. Laboratory Analysis
2.4. Statistical Analysis
3. Results
3.1. Comparison of Clinical and Laboratory Characteristics in Pre-Transplant Cirrhotic Patients and OLT Recipients
3.2. Comparison of Pre-Transplant Cirrhotic Patients and OLT Recipients with PREVEND Population
3.3. Determinants of the Presence of LP-Z
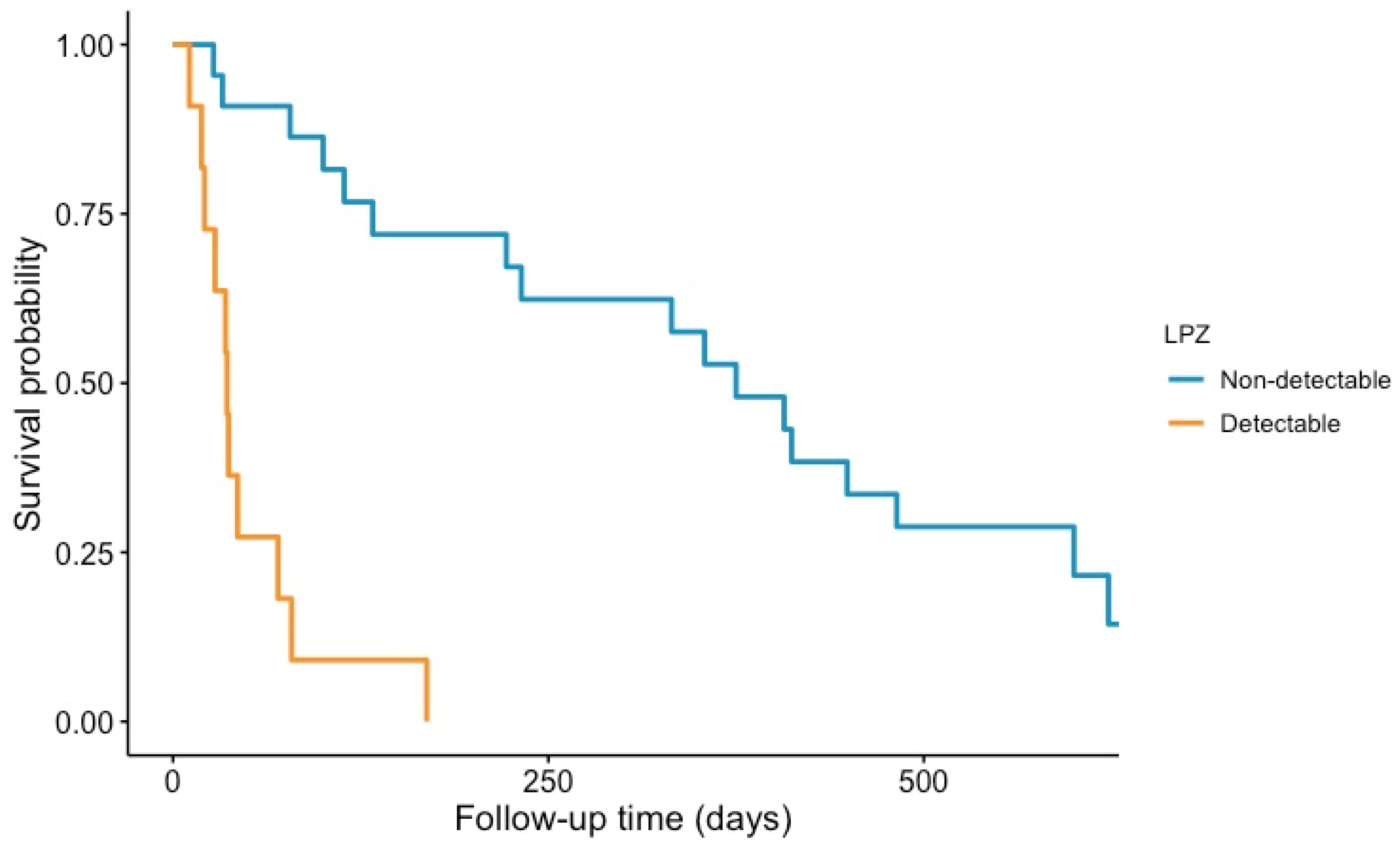
3.4. Presence of LP-Z and Mortality in Pre-Transplant Cirrhotic Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McIntyre, N. Plasma Lipids and Lipoproteins in Liver Disease. Gut 1978, 19, 526. [Google Scholar] [CrossRef] [PubMed]
- Day, R.C.; Harry, D.S.; Owen, J.S.; Foo, A.Y.; McIntyre, N. Lecithin—Cholesterol Acyltransferase and the Lipoprotein Abnormalities of Parenchymal Liver Disease. Clin. Sci. 1979, 56, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Privitera, G.; Spadaro, L.; Marchisello, S.; Fede, G.; Purrello, F. Abnormalities of Lipoprotein Levels in Liver Cirrhosis: Clinical Relevance. Digest. Dis. Sci. 2018, 63, 16–26. [Google Scholar] [CrossRef]
- Jonas, A. Lecithin Cholesterol Acyltransferase. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2000, 1529, 245–256. [Google Scholar] [CrossRef]
- Chatterjee, C.; Sparks, D.L. Hepatic Lipase, High Density Lipoproteins, and Hypertriglyceridemia. Am. J. Pathol. 2011, 178, 1429–1433. [Google Scholar] [CrossRef]
- McIntyre, N.; Calandra, S.; Pearson, A.J.G. Lipid and Lipoprotein Abnormalities in Liver Disease: The Possible Role of Lecithin: Cholesterol Acyltransferase Deficiency. Scand. J. Clin. Lab. Investig. 2009, 33, 115–120. [Google Scholar] [CrossRef]
- Tahara, D.; Nakanishi, T.; Akazawa, S.; Yamaguchi, Y.; Yamamoto, H.; Akashi, M.; Chikuba, N.; Okuno, S.; Maeda, Y.; Kusumoto, Y.; et al. Lecithin-Cholesterol Acyltransferase and Lipid Transfer Protein Activities in Liver Disease. Metabolis 1993, 42, 19–23. [Google Scholar] [CrossRef]
- Simon, J.B.; Scheig, R. Serum Cholesterol Esterification in Liver Disease—Importance of Lecithin-Cholesterol Acyltransferase. N. Engl. J. Med. 1970, 283, 841–846. [Google Scholar] [CrossRef]
- Agorastos, J.; Fox, C.; Harry, D.S.; McIntyre, N. Lecithin—Cholesterol Acyltransferase and the Lipoprotein Abnormalities of Obstructive Jaundice. Clin. Sci. 1978, 54, 369–379. [Google Scholar] [CrossRef]
- Ghadir, M.R.; Riahin, A.A.; Havaspour, A.; Nooranipour, M.; Habibinejad, A.A. The Relationship between Lipid Profile and Severity of Liver Damage in Cirrhotic Patients. Hepat. Mon. 2010, 10, 285–288. [Google Scholar]
- Chrostek, L.; Supronowicz, L.; Panasiuk, A.; Cylwik, B.; Gruszewska, E.; Flisiak, R. The Effect of the Severity of Liver Cirrhosis on the Level of Lipids and Lipoproteins. Clin. Exp. Med. 2014, 14, 417–421. [Google Scholar] [CrossRef]
- Varghese, J.S.; Krishnaprasad, K.; Upadhuyay, R.; Revathy, M.S.; Jayanthi, V. Lipoprotein Profile in Cirrhosis of Liver. Eur. J. Gastroenterol. Hepatol. 2007, 19, 521–522. [Google Scholar] [CrossRef] [PubMed]
- Sepanlou, S.G.; Safiri, S.; Bisignano, C.; Ikuta, K.S.; Merat, S.; Saberifiroozi, M.; Poustchi, H.; Tsoi, D.; Colombara, D.V.; et al.; GBD 2017 Cirrhosis Collaborators The Global, Regional, and National Burden of Cirrhosis by Cause in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef]
- Johnston, S.D.; Morris, J.K.; Cramb, R.; Gunson, B.K.; Neuberger, J. Cardiovascular Morbidity and Mortality after Orthotopic Liver Transplantation. Transplantation 2002, 73, 901–906. [Google Scholar] [CrossRef]
- Fernández-Miranda, C.; Guijarro, C.; Calle, A.; Loinaz, C.; Gónzalez-Pinto, I.; Gómez-Izquierdo, T.; Larumbe, S.; Moreno, E.; Palacio, A. Lipid Abnormalities in Stable Liver Transplant Recipients—Effects of Cyclosporin, Tacrolimus, and Steroids. Transpl. Int. 1998, 11, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Charco, R.; Cantarell, C.; Vargas, V.; Capdevila, L.; Lázaro, J.L.; Hidalgo, E.; Murio, E.; Margarit, C. Serum Cholesterol Changes in Long-term Survivors of Liver Transplantation: A Comparison between Cyclosporine and Tacrolimus Therapy. Liver Transplant. Surg. 1999, 5, 204–208. [Google Scholar] [CrossRef]
- Lucey, M.R.; Terrault, N.; Ojo, L.; Hay, J.E.; Neuberger, J.; Blumberg, E.; Teperman, L.W. Long-term Management of the Successful Adult Liver Transplant: 2012 Practice Guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013, 19, 3–26. [Google Scholar] [CrossRef]
- Laish, I.; Braun, M.; Mor, E.; Sulkes, J.; Harif, Y.; Ari, Z.B. Metabolic Syndrome in Liver Transplant Recipients: Prevalence, Risk Factors, and Association with Cardiovascular Events. Liver Transpl. 2011, 17, 15–22. [Google Scholar] [CrossRef]
- Xanthopoulos, A.; Starling, R.C.; Kitai, T.; Triposkiadis, F. Heart Failure and Liver Disease Cardiohepatic Interactions. JACC Heart Fail. 2019, 7, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Stegall, M.D.; Everson, G.; Schroter, G.; Bilir, B.; Karrer, F.; Kam, I. Metabolic Complications after Liver Transplantation. Diabetes, Hypercholesterolemia, Hypertension, and Obesity. Transplantation 1995, 60, 1057–1060. [Google Scholar]
- McCaughan, G.W.; O’Brien, E.; Sheil, A.G.R. A Follow up of 53 Adult Patients Alive beyond 2 Years Following Liver Transplantation. J. Gastroenterol. Hepatol. 1993, 8, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Jindal, R.M.; Sidner, R.A.; Hughes, D.; Pescovitz, M.D.; Leapman, S.B.; Milgrom, M.L.; Lumeng, L.; Filo, R.S. Metabolic Problems in Recipients of Liver Transplants. Clin. Transplant. 1996, 10, 213–217. [Google Scholar]
- McDiarmid, S.V.; Farmer, D.A.; Goldstein, L.I.; Martin, P.; Vargas, J.; Tipton, J.R.; Simmons, F.; Busuttil, R.W. A Randomized Prospective Trial of Steroid Withdrawal after Liver Transplantation. Transplantation 1995, 60, 1443–1450. [Google Scholar] [CrossRef]
- Punch, J.D.; Shieck, V.L.; Campbell, D.A.; Bromberg, J.S.; Turcotte, J.G.; Merion, R.M. Corticosteroid Withdrawal after Liver Transplantation. Surgery 1995, 118, 783–788. [Google Scholar] [CrossRef]
- Holdaas, H.; Potena, L.; Saliba, F. MTOR Inhibitors and Dyslipidemia in Transplant Recipients: A Cause for Concern? Transplant. Rev. 2015, 29, 93–102. [Google Scholar] [CrossRef]
- Varbo, A.; Benn, M.; Tybjærg-Hansen, A.; Jørgensen, A.B.; Frikke-Schmidt, R.; Nordestgaard, B.G. Remnant Cholesterol as a Causal Risk Factor for Ischemic Heart Disease. J. Am. Coll. Cardiol. 2013, 61, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Duran, E.K.; Aday, A.W.; Cook, N.R.; Buring, J.E.; Ridker, P.M.; Pradhan, A.D. Triglyceride-Rich Lipoprotein Cholesterol, Small Dense LDL Cholesterol, and Incident Cardiovascular Disease. J. Am. Coll. Cardiol. 2020, 75, 2122–2135. [Google Scholar] [CrossRef]
- Singh, K.; Chandra, A.; Sperry, T.; Joshi, P.H.; Khera, A.; Virani, S.S.; Ballantyne, C.M.; Otvos, J.D.; Dullaart, R.P.F.; Gruppen, E.G.; et al. Associations Between HDL Particles and Ischemic Events by Vascular Domain, Gender, and Ethnicity: A Pooled Cohort Analysis. Circulation 2020, 142, 657–669. [Google Scholar] [CrossRef]
- Sokooti, S.; Flores-Guerrero, J.L.; Kieneker, L.M.; Heerspink, H.J.L.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. HDL Particle Subspecies and Their Association with Incident Type 2 Diabetes: The PREVEND Study. J. Clin. Endocrinol. Metab. 2021, 106, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Sokooti, S.; Szili-Torok, T.; Flores-Guerrero, J.L.; Osté, M.C.J.; Gomes-Neto, A.W.; Kootstra-Ros, J.E.; Heerspink, H.J.L.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. High-Density Lipoprotein Particles and Their Relationship to Posttransplantation Diabetes Mellitus in Renal Transplant Recipients. Biomolecules 2020, 10, 481. [Google Scholar] [CrossRef]
- Sokooti, S.; Flores-Guerrero, J.L.; Heerspink, H.J.L.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. Triglyceride-Rich Lipoprotein and LDL Particle Subfractions and Their Association with Incident Type 2 Diabetes: The PREVEND Study. Cardiovasc. Diabetol. 2021, 20, 156. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Corpeleijn, E.; Gansevoort, R.T.; Gans, R.O.B.; Hillege, H.L.; Stolk, R.P.; Navis, G.; Bakker, S.J.L.; Dullaart, R.P.F. Role of HDL Cholesterol and Estimates of HDL Particle Composition in Future Development of Type 2 Diabetes in the General Population: The PREVEND Study. J. Clin. Endocrinol. Metab. 2013, 98, E1352–E1359. [Google Scholar] [CrossRef]
- Bedi, S.; Garcia, E.; Jeyarajah, E.J.; Shalaurova, I.; Perez-Matos, M.C.; Jiang, Z.G.; Dullaart, R.P.F.; Matyus, S.P.; Kirk, W.J.; Otvos, J.D.; et al. Characterization of LP-Z Lipoprotein Particles and Quantification in Subjects with Liver Disease Using a Newly Developed NMR-Based Assay. J. Clin. Med. 2020, 9, 2915. [Google Scholar] [CrossRef] [PubMed]
- Kostner, G.M.; Laggner, P.; Prexl, H.J.; Holasek, A. Investigation of the Abnormal Low-Density Lipoproteins Occurring in Patients with Obstructive Jaundice. Biochem. J. 1976, 157, 401–407. [Google Scholar] [CrossRef]
- Eisenga, M.F.; Gomes-Neto, A.W.; van Londen, M.; Ziengs, A.L.; Douwes, R.M.; Stam, S.P.; Osté, M.C.J.; Knobbe, T.J.; Hessels, N.R.; Buunk, A.M.; et al. Rationale and Design of TransplantLines: A Prospective Cohort Study and Biobank of Solid Organ Transplant Recipients. BMJ Open 2018, 8, e024502. [Google Scholar] [CrossRef]
- Borggreve, S.E.; Hillege, H.L.; Wolffenbuttel, B.H.R.; de Jong, P.E.; Bakker, S.J.L.; van der Steege, G.; van Tol, A.; Dullaart, R.P.F.; on behalf of the PREVEND Study Group. The Effect of Cholesteryl Ester Transfer Protein -629C->A Promoter Polymorphism on High-Density Lipoprotein Cholesterol Is Dependent on Serum Triglycerides. J. Clin. Endocrinol. Metab. 2005, 90, 4198–4204. [Google Scholar] [CrossRef]
- Kappelle, P.J.W.H.; Gansevoort, R.T.; Hillege, J.L.; Wolffenbuttel, B.H.R.; Dullaart, R.P.F.; on behalf of the PREVEND study group. Apolipoprotein B/A-I and Total Cholesterol/High-Density Lipoprotein Cholesterol Ratios Both Predict Cardiovascular Events in the General Population Independently of Nonlipid Risk Factors, Albuminuria and C-Reactive Protein. J. Intern. Med. 2011, 269, 232–242. [Google Scholar] [CrossRef]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.; Therneau, T.M.; Kosberg, C.L.; D’Amico, G.; Dickson, E.R.; Kim, W.R. A Model to Predict Survival in Patients with End-Stage Liver Disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef]
- Wiesner, R.; Edwards, E.; Freeman, R.; Harper, A.; Kim, R.; Kamath, P.; Kremers, W.; Lake, J.; Howard, T.; Merion, R.M.; et al. Model for End-Stage Liver Disease (MELD) and Allocation of Donor Livers. Gastroenterology 2003, 124, 91–96. [Google Scholar] [CrossRef]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the Oesophagus for Bleeding Oesophageal Varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef]
- van den Berg, E.H.; Flores-Guerrero, J.L.; Gruppen, E.G.; de Borst, M.H.; Wolak-Dinsmore, J.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. Non-Alcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: Role of Circulating Branched-Chain Amino Acids. Nutrients 2019, 11, 705. [Google Scholar] [CrossRef] [PubMed]
- Wolak-Dinsmore, J.; Gruppen, E.G.; Shalaurova, I.; Matyus, S.P.; Grant, R.P.; Gegen, R.; Bakker, S.J.L.; Otvos, J.D.; Connelly, M.A.; Dullaart, R.P.F. A Novel NMR-Based Assay to Measure Circulating Concentrations of Branched-Chain Amino Acids: Elevation in Subjects with Type 2 Diabetes Mellitus and Association with Carotid Intima Media Thickness. Clin. Biochem. 2018, 54, 92–99. [Google Scholar] [CrossRef]
- Matyus, S.P.; Braun, P.J.; Wolak-Dinsmore, J.; Jeyarajah, E.J.; Shalaurova, I.; Xu, Y.; Warner, S.M.; Clement, T.S.; Connelly, M.A.; Fischer, T.J. NMR Measurement of LDL Particle Number Using the Vantera® Clinical Analyzer. Clin. Biochem. 2014, 47, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Matyus, S.P.; Braun, P.J.; Wolak-Dinsmore, J.; Saenger, A.K.; Jeyarajah, E.J.; Shalaurova, I.; Warner, S.M.; Fischer, T.J.; Connelly, M.A. HDL Particle Number Measured on the Vantera®, the First Clinical NMR Analyzer. Clin. Biochem. 2015, 48, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Jeyarajah, E.J.; Cromwell, W.C.; Otvos, J.D. Lipoprotein Particle Analysis by Nuclear Magnetic Resonance Spectroscopy. Clin. Lab. Med. 2006, 26, 847–870. [Google Scholar] [CrossRef]
- Garcia, E.; Bennett, D.W.; Connelly, M.A.; Jeyarajah, E.J.; Warf, F.C.; Shalaurova, I.; Matyus, S.P.; Wolak-Dinsmore, J.; Oskardmay, D.N.; Young, R.M.; et al. The Extended Lipid Panel Assay: A Clinically-Deployed High-Throughput Nuclear Magnetic Resonance Method for the Simultaneous Measurement of Lipids and Apolipoprotein B. Lipids Health Dis. 2020, 19, 247. [Google Scholar] [CrossRef]
- Freeman, L.A.; Shamburek, R.D.; Sampson, M.L.; Neufeld, E.B.; Sato, M.; Karathanasis, S.K.; Remaley, A.T. Plasma Lipoprotein-X Quantification on Filipin-Stained Gels: Monitoring Recombinant LCAT Treatment Ex Vivo. J. Lipid Res. 2019, 60, 1050–1057. [Google Scholar] [CrossRef]
- Newson, R.B. Comparing the Predictive Powers of Survival Models Using Harrell’s C or Somers’ D. Stata J. 2010, 10, 339–358. [Google Scholar] [CrossRef]
- Seidel, D. Lipoproteins in Liver Disease. Clin. Chem. Lab. Med. 1987, 25, 541–552. [Google Scholar] [CrossRef][Green Version]
- Müller, P.; Fellin, R.; Lambrecht, J.; Agostini, B.; Wieland, H.; Rost, W.; Seidel, D. Hypertriglyceridaemia Secondary to Liver Disease. Eur. J. Clin. Investig. 1974, 4, 419–428. [Google Scholar] [CrossRef]
- Jiang, X.-C.; Qin, S.; Qiao, C.; Kawano, K.; Lin, M.; Skold, A.; Xiao, X.; Tall, A.R. Apolipoprotein B Secretion and Atherosclerosis Are Decreased in Mice with Phospholipid-Transfer Protein Deficiency. Nat. Med. 2001, 7, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Swenson, T.; Simmons, J.; Hesler, C.; Bisgaier, C.; Tall, A. Cholesteryl Ester Transfer Protein Is Secreted by Hep G2 Cells and Contains Asparagine-Linked Carbohydrate and Sialic Acid. J. Biol. Chem. 1987, 262, 16271–16274. [Google Scholar] [CrossRef]
- Vergeer, M.; Boekholdt, S.M.; Sandhu, M.S.; Ricketts, S.L.; Wareham, N.J.; Brown, M.J.; de Faire, U.; Leander, K.; Gigante, B.; Kavousi, M.; et al. Genetic Variation at the Phospholipid Transfer Protein Locus Affects Its Activity and High-Density Lipoprotein Size and Is a Novel Marker of Cardiovascular Disease Susceptibility. Circulation 2010, 122, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, P.R.; Abdulle, A.E.; Bulthuis, M.L.C.; Perton, F.G.; Connelly, M.A.; van Goor, H.; Dullaart, R.P.F. The Systemic Redox Status Is Maintained in Non-Smoking Type 2 Diabetic Subjects without Cardiovascular Disease: Association with Elevated Triglycerides and Large VLDL. J. Clin. Med. 2019, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Lagrost, L. Plasma Phospholipid Transfer Protein: A Multifaceted Protein with a Key Role in the Assembly and Secretion of Apolipoprotein B–Containing Lipoproteins by the Liver. Hepatology 2012, 56, 415–418. [Google Scholar] [CrossRef]
- Bassani, L.; Fernandes, S.A.; Raimundo, F.V.; Harter, D.L.; Gonzalez, M.C.; Marroni, C.A. Lipid Profile Of Cirrhotic Patients And Its Association with Prognostic Scores: A Cross-Sectional Study. Arq. Gastroenterol. 2015, 52, 210–215. [Google Scholar] [CrossRef]
- Borggreve, S.E.; Vries, R.D.; Dullaart, R.P.F. Alterations in High-density Lipoprotein Metabolism and Reverse Cholesterol Transport in Insulin Resistance and Type 2 Diabetes Mellitus: Role of Lipolytic Enzymes, Lecithin: Cholesterol Acyltransferase and Lipid Transfer Proteins. Eur. J. Clin Investig. 2003, 33, 1051–1069. [Google Scholar] [CrossRef]
- Eppinga, R.N.; Hartman, M.H.T.; van Veldhuisen, D.J.; Lexis, C.P.H.; Connelly, M.A.; Lipsic, E.; van der Horst, I.C.C.; van der Harst, P.; Dullaart, R.P.F. Effect of Metformin Treatment on Lipoprotein Subfractions in Non-Diabetic Patients with Acute Myocardial Infarction: A Glycometabolic Intervention as Adjunct to Primary Coronary Intervention in ST Elevation Myocardial Infarction (GIPS-III) Trial. PLoS ONE 2016, 11, e0145719. [Google Scholar] [CrossRef]
- Hu, K.; Perez-Matos, M.C.; Argemi, J.; Vilar, E.; Shalaurova, I.; Bullitt, E.; Landeen, L.; Sugahara, G.; Deng, H.; Mathur, K.; et al. Lipoprotein Z, A Novel Hepatotoxic Lipoprotein, Predicts Outcome in Alcoholic Hepatitis. Hepatology, 2021; online ahead of print. [Google Scholar] [CrossRef]
- Pavanello, C.; Calabresi, L. Genetic, Biochemical, and Clinical Features of LCAT Deficiency: Update for 2020. Curr. Opin. Lipidol. 2020, 31, 232–237. [Google Scholar] [CrossRef]
| Pre-Transplant Cirrhotic Patients N = 130 | OLT Recipients N = 372 | p-Value | Adjusted p-Value * | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Sex: men/women, n (%) | 85 (65.4)/45 (34.6) | 218 (58.6)/154 (41.4) | 0.174 | n.a. |
| Age (years), median (IQR) | 59.5 (52.0–65.0) | 59.0 (47.0–67.0) | 0.652 | n.a. |
| BMI (kg/m2), median (IQR) | 28.1 (24.3–30.9) | 25.9 (22.9–29.6) | 0.006 | 0.726 |
BMI
| 28 (30.8) 32 (35.2) 31 (34.1) | 135 (41.5) 117 (36.0) 73 (22.5) | 0.063 0.883 0.024 | 0.928 0.605 0.616 |
| Smoking, n (%) | 16 (18.8) | 34 (11.7) | 0.088 | 0.132 |
Child Pugh Turcotte classification
| 28 (21.5) 64 (49.2) 38 (29.2) | - - - | n.a. n.a. n.a. | n.a. n.a. n.a. |
| MELD score, median (IQR) | 15.0 (10.0–19.0) | - | n.a. | n.a. |
| Mortality on waiting list, n (%) | 29 (22.3) | - | n.a. | n.a. |
| History of cardiovascular disease, n (%) | 6 (4.8) | 28 (7.6) | 0.282 | 0.067 |
| History of diabetes, n (%) | 36 (28.6) | 106 (28.6) | 0.987 | 0.055 |
| Glucose lowering drugs, n (%) | 35 (31.8) | 79 (21.8) | 0.031 | 0.697 |
| Lipid lowering drugs, n (%) | 19 (17.3) | 87 (24.0) | 0.140 | 0.019 |
| Blood tests | ||||
| ALT (U/L), median (IQR) | 40.0 (28.0–60.0) | 25.0 (18.0–36.5) | <0.001 | 0.002 |
| AST (U/L), median (IQR) | 54.0 (44.0–84.0) | 25.0 (20.0–33.0) | <0.001 | <0.001 |
| GGT (U/L), median (IQR) | 95.5 (48.3–150.8) | 39.0 (21.0–89.0) | <0.001 | 0.483 |
| ALP (U/L), mean ± SD | 140.5 (98.3–215.3) | 87.0 (69.3–129.8) | <0.001 | 0.003 |
| Bilirubin direct, median (IQR) | 27.0 (16.0–85.0) | 9.0 (7.0–12.0) | <0.001 | 0.002 |
| Bilirubin total, median (IQR) | 40.0 (23.0–99.0) | 10.0 (7.0–14.0) | <0.001 | <0.001 |
| Albumin (g/L), median (IQR) | 31.0 (27.0–36.0) | 44.0 (42.0–46.0) | <0.001 | <0.001 |
| HbA1c (mmol/mol), median (IQR) | 32.0 (26.0–39.5) | 36.0 (32.0–43.0) | 0.001 | 0.001 |
| HbA1c (%), median (IQR) | 5.1 (4.5–5.8) | 5.4 (5.1–6.1) | 0.001 | 0.001 |
| Fasting glucose (mmol/L), median (IQR) | 6.4 (5.0–8.5) | 5.7 (5.2–6.8) | 0.133 | 0.712 |
| Lipids and lipoproteins | ||||
| Total cholesterol (mmol/L), median (IQR) | 3.2 (2.5–4.1) | 4.2 (3.6–4.9) | <0.001 | <0.001 |
| Non-HDL cholesterol (mmol/L), median (IQR) | 2.2 (1.7–3.0) | 2.8 (2.2–3.4) | <0.001 | 0.003 |
| HDL cholesterol (mmol/L), median (IQR) | 0.9 (0.4–1.2) | 1.3 (1.1–1.7) | <0.001 | <0.001 |
| LDL cholesterol (mmol/L), median (IQR) | 1.8 (1.2–2.3) | 2.1 (1.7–2.5) | <0.001 | 0.031 |
| Triglycerides (mmol/L), median (IQR) | 0.7 (0.5–1.1) | 1.3 (1.0–1.8) | <0.001 | <0.001 |
| ApoB (g/L), median (IQR) | 63.0 (48.0–83.8) | 74.0 (59.0–89.0) | <0.001 | 0.182 |
| ApoA-I (g/L), median (IQR) | 57.5 (31.8–82.3) | 128.0 (111.0–146.0) | <0.001 | <0.001 |
| TRLP (nmol/L), median (IQR) | 94.3 (39.6–147.0) | 127.7 (85.4–182.6) | <0.001 | 0.001 |
| Very large TRLP (nmol/L), median (IQR) | 0.0 (0.0–0.0) | 0.0 (0.0–0.1) | <0.001 | 0.173 |
| Large TRLP (nmol/L), median (IQR) | 0.3 (0.0–1.4) | 2.0 (0.4–5.8) | <0.001 | <0.001 |
| Medium TRLP (nmol/L), median (IQR) | 2.1 (0.0–8.0) | 15.4 (7.0–26.3) | <0.001 | <0.001 |
| Small TRLP (nmol/L), median (IQR) | 17.3 (1.6–34.0) | 33.4 (17.0–57.5) | <0.001 | <0.001 |
| Very small TRLP (nmol/L), median (IQR) | 62.3 (18.2–117.2) | 65.5 (35.7–107.4) | 0.370 | 0.836 |
| TRL size (nm), median (IQR) | 41.5 (35.4–49.4) | 45.9 (41.6–51.8) | <0.001 | <0.001 |
| LDLP (nmol/L), median (IQR) | 1026.5 (745.3–1477.3) | 1234.5 (1013.5–1494.8) | <0.001 | 0.316 |
| Large LDLP (nmol/L), median (IQR) | 438.0 (208.8.3–671.8) | 292.5 (139.8–496.0) | <0.001 | <0.001 |
| Medium LDLP (nmol/L), median (IQR) | 0.0 (0.0–155.3) | 139.5 (0.0–396.0) | <0.001 | <0.001 |
| Small LDLP (nmol/L), median (IQR) | 256.0 (98.8–398.5) | 637.0 (403.3–860.5) | <0.001 | <0.001 |
| LDL size (nm), median (IQR) | 21.7 (21.1–22.0) | 21.1 (20.7–21.4) | <0.001 | <0.001 |
| Total HDLP (µmol/L), median (IQR) | 6.5 (4.0–10.0) | 19.5 (17.1–21.6) | <0.001 | <0.001 |
| Large HDLP (µmol/L), median (IQR) | 2.3 (1.0–3.8) | 2.1 (1.2–3.6) | 0.415 | 0.930 |
| Medium HDLP (µmol/L), median (IQR) | 0.5 (0.0–2.0) | 4.8 (3.6–6.3) | <0.001 | <0.001 |
| Small HDLP (µmol/L), median (IQR) | 3.0 (1.2–5.5) | 12.1 (9.6–14.1) | <0.001 | <0.001 |
| HDL size (nm), median (IQR) | 10.4 (9.3–11.3) | 9.2 (8.8–9.7) | <0.001 | <0.001 |
HDL subspecies
| 0.0 (0.0–0.2) 2.8 (0.9–5.1) 0.1 (0.0–0.8) 0.2 (0.0–0.9 )0.1 (0.0–0.6) 0.4 (0.0–1.1) 0.8 (0.0–2.5) | 1.9 (0.5–3.4) 9.6 (7.8–11.5) 3.1 (1.6–4.3) 1.7 (1.0–2.4) 0.5 (0.2–1.0) 0.7 (0.3–1.7) 0.4 (0.1–1.0) | <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 0.044 | <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 |
| LP-Z present, n (%) LP-Z (nmol/L), median (IQR) | 40 (30.8%) 0 (0.0–666.4) | 3 (0.8%) 0 (0.0–0.0) | <0.001 <0.001 | <0.001 <0.001 |
| Primary liver diseases | ||||
| Storage disease, n (%) | 4 (3.1) | 33 (8.9) | 0.031 | n.a. |
| Autoimmune hepatitis, n (%) | 11 (8.5) | 19 (5.1) | 0.165 | n.a. |
| Cholestatic liver disease (PSC/PBC), n (%) | 34 (26.2) | 99 (26.6) | 0.919 | n.a. |
| Viral, n (%) | 12 (9.2) | 39 (10.5) | 0.684 | n.a. |
| Alcohol, n (%) | 29 (22.3) | 49 (13.2) | 0.013 | n.a. |
| MAFLD, n (%) | 33 (25.4) | 34 (9.1) | <0.001 | n.a. |
| Vascular, n (%) | 2 (1.5) | 1 (0.3) | 0.166 | n.a. |
| Malignancy, n (%) | 0 (0) | 5 (1.3) | 0.334 | n.a. |
| Other, n (%) | 5 (3.8) | 93 (25.0) | <0.001 | n.a. |
| Comparison of Pre-Transplant Cirrhotic Patients (N = 130) with the PREVEND Population (N = 6027) | Comparison of OLT Recipients (N = 372) with the PREVEND Population (N = 6027) | |
|---|---|---|
| Lipids and lipoproteins | ||
| Total cholesterol (mmol/L), median (IQR) | −1.827 (−1.989 to −1.665) * | −1.000 (−1.097 to −0.904) * |
| Non-HDL cholesterol (mmol/L), median (IQR) | −1.514 (−1.676 to −1.352) * | −1.157 (−1.253 to −1.060) * |
| HDL cholesterol (mmol/L), median (IQR) | −1.096 (−1.249 to −0.944) * | 0.508 (0.415 to 0.600) * |
| LDL cholesterol (mmol/L), median (IQR) | −1.684 (−1.844 to −1.525) * | −1.426 (−1.521 to −1.331) * |
| Triglycerides (mmol/L), median (IQR) | −0.552 (−0.724 to −0.379) * | 0.141 (0.038 to 0.245) * |
| ApoB (mg/dL), median (IQR) | −0.803 (−0.971 to −0.635) * | −0.600 (−0.699 to −0.501) * |
| ApoA-I (mg/dL), median (IQR) | −2.815 (−2.961 to −2.669) * | 0.001 (−0.086 to 0.089) |
| TRLP (nmol/L), median (IQR) | −0.644 (−0.813 to −0.476) * | −0.235 (−0.335 to −0.134) * |
| Very large TRLP (nmol/L), median (IQR) | −0.088 (−0.263 to 0.087) | 0.025 (−0.079 to 0.130) |
| Large TRLP (nmol/L), median (IQR) | −0.609 (−0.779 to −0.439) * | −0.023 (−0.127 to 0.081) |
| Medium TRLP (nmol/L), median (IQR) | −0.732 (−0.901 to −0.564) * | 0.149 (0.047 to 0.251) * |
| Small TRLP (nmol/L), median (IQR) | −0.740 (−0.913 to −0.567) * | −0.353 (−0.457 to −0.249) * |
| Very small TRLP (nmol/L), median (IQR) | 0.056 (−0.117 to 0.228) | −0.046 (−0.149 to 0.056) |
| TRL size (nm), median (IQR) | −0.423 (−0.596 to −0.250) * | 0.116 (0.013 to 0.219) ** |
| LDLP (nmol/L), median (IQR) | −0.781 (−0.949 to −0.612) * | −0.606 (−0.705 to −0.507) * |
| Large LDLP (nmol/L), median (IQR) | 0.628 (0.463 to 0.792) * | −0.089 (−0.186 to 0.009) |
| Medium LDLP (nmol/L), median (IQR) | −0.950 (−1.121 to −0.778) * | −0.531 (−0.634 to −0.428) * |
| Small LDLP (nmol/L), median (IQR) | −0.833 (−1.006 to −0.661) * | −0.014 (−0.118 to 0.090) |
| LDL size (nm), median (IQR) | 0.833 (0.667 to 1.000) * | −0.051 (−0.150 to 0.048) |
| Total HDLP (µmol/L), median (IQR) | −3.990 (−4.129 to −3.851) * | −0.473 (−0.556 to −0.389) * |
| Large HDLP (µmol/L), median (IQR) | 0.518 (0.362 to 0.674) * | 0.631 (0.536 to 0.727) * |
| Medium HDLP (µmol/L), median (IQR) | −1.619 (−1.777 to −1.460) * | −0.030 (−0.126 to 0.065) |
| Small HDLP (µmol/L), median (IQR) | −3.289 (−3.435 to −3.143) * | −0.731 (−0.820 to −0.642) * |
| HDL size (nm), median (IQR) | 0.371 (2.436 to 2.733) * | 0.655 (0.571 to 0.738) * |
| HDL subspecies | ||
| −1.719 (−1.881 to −1.557) * −2.839 (−2.995 to −2.682) * −1.353 (−1.517 to −1.189) * −1.026 (−1.190 to −0.862) * −0.098 (−0.264 to 0.069) −0.217 (−0.379 to −0.055) * 1.686 (1.536 to 1.836) * | −0.681 (−0.780 to −0.582) * −0.424 (−0.519 to −0.330) * −0.102 (−0.202 to −0.002) ** 0.108 (0.008 to 0.208) ** 0.596 (0.492 to 0.699) * 0.239 (0.140 to 0.338) * 0.610 (0.520 to 0.700) * |
| LP-Z (nmol/L), median (IQR) | 3.176 (3.017 to 3.336) * | 0.031 (0.008 to 0.055) * |
| LP-Z Not Detectable N = 90 (69.2%) | LP-Z Detectable N = 40 (30.8%) | p-Value | Adjusted p-Value * | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Sex: men/women, n (%) | 60 (66.7)/30 (33.3) | 25 (62.5)/15 (37.5) | 0.645 | n.a. |
| Age (years), median (IQR) | 60.0 (54.0–65.3) | 58.5 (51.0–64.0) | 0.296 | n.a. |
| BMI (kg/m2), median (IQR) | 28.2 (24.6–31.1) | 26.4 (23.2–31.0) | 0.239 | 0.932 |
| BMI | ||||
| 17 (26.2) 25 (38.5) 23 (35.4) | 11 (42.3) 7 (26.9) 8 (30.8) | 0.131 0.298 0.675 | 0.494 0.132 0.255 |
| Smoking, n (%) | 13 (20.0) | 3 (15.0) | 0.617 | 0.418 |
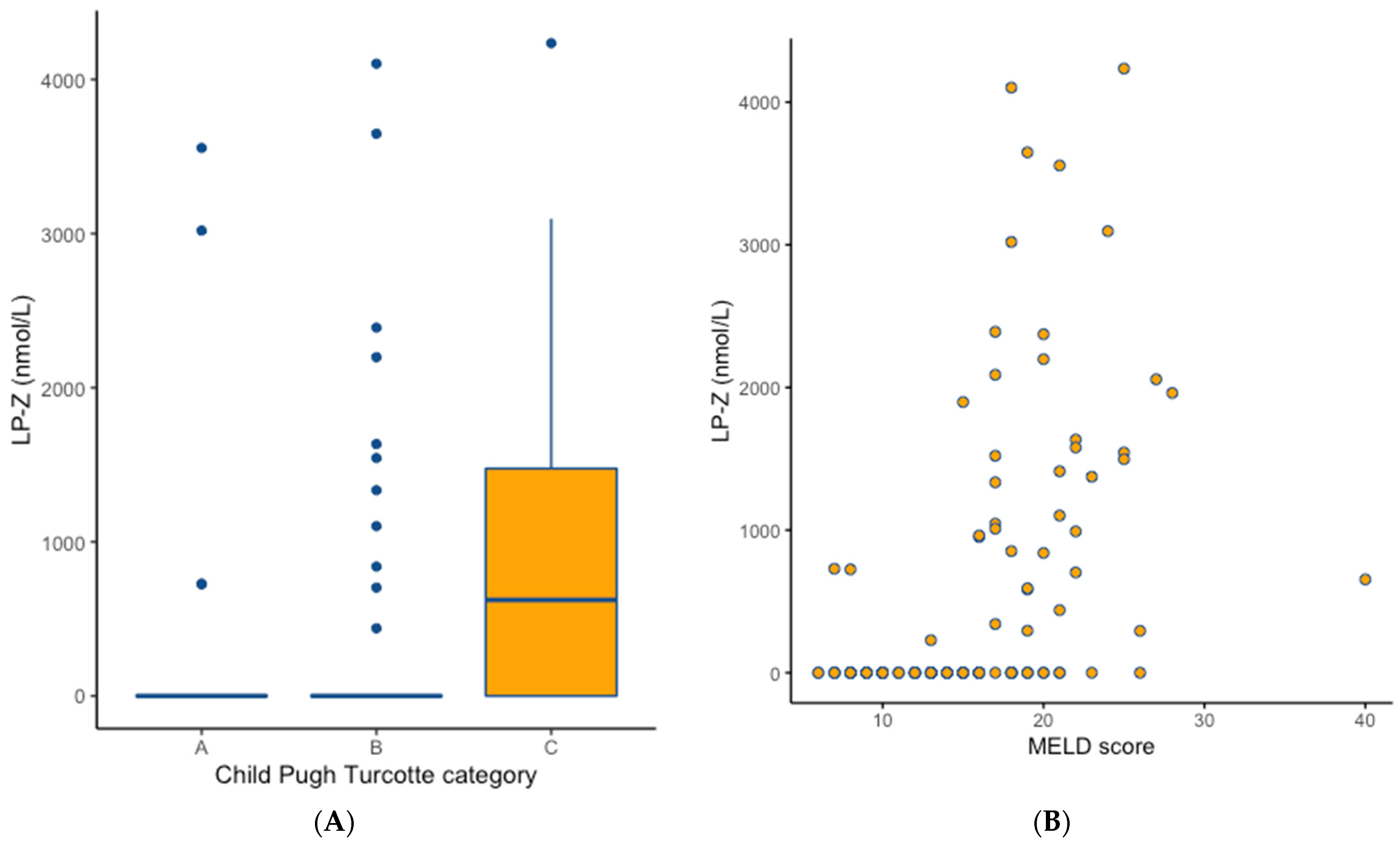
Child Pugh Turcotte classification
| 24 (26.7) 53 (58.9) 13 (14.4) | 4 (10.0) 11 (27.5) 25 (62.5) | 0.038 0.001 <0.001 | 0.035 0.001 <0.001 |
| MELD score, median (IQR) | 13.0 (9.8–16.0) | 19.5 (17.0–22.0) | <0.001 | <0.001 |
| Mortality on waiting list, n (%) | 18 (20.0) | 11 (27.5) | 0.150 | 0.999 |
| History of cardiovascular disease, n (%) | 6 (6.9) | 0 (0.0) | 0.176 | 0.999 |
| History of diabetes, n (%) | 28 (32.2) | 8 (20.5) | 0.180 | 0.252 |
| Glucose lowering drugs, n (%) | 28 (37.8) | 7 (19.4) | 0.052 | 0.069 |
| Lipid lowering drugs, n (%) | 13 (17.6) | 6 (16.7) | 0.907 | 0.775 |
| Blood tests | ||||
| ALT (U/L), median (IQR) | 36.0 (28.0–49.5) | 53.5 (33.5–112.3) | 0.006 | 0.031 |
| AST (U/L), median (IQR) | 50.0 (37.0–62.3) | 88.0 (54.0–152.8) | <0.001 | 0.069 |
| GGT (U/L), median (IQR) | 97.5 (52.5–152.0) | 69.5 (40.5–141.5) | 0.378 | 0.630 |
| ALP (U/L), mean ± SD | 130.5 (92.0–167.5) | 218.5 (104.3–298.0) | 0.005 | 0.004 |
| Bilirubin direct, median (IQR) | 18.0 (13.0–29.0) | 162.0 (51.0–228.0) | <0.001 | 0.002 |
| Bilirubin total, median (IQR) | 28.0 (18.0–52.5) | 162.5 (75.5–257.3) | <0.001 | <0.001 |
| Albumin (g/L), median (IQR) | 32.0 (27.3–36.0) | 28.0 (25.3–36.0) | 0.111 | 0.169 |
| HbA1c (mmol/mol), median (IQR) | 34.0 (27.0–46.0) | 27.0 (18.0–33.0) | 0.018 | 0.095 |
| HbA1c (%), median (IQR) | 5.3 (4.7–6.4) | 4.6 (3.8–5.2) | 0.017 | 0.093 |
| Fasting glucose (mmol/L), median (IQR) | 6.4 (5.0–8.0) | 6.6 (4.9–9.8) | 0.554 | 0.326 |
| Lipids and lipoproteins | ||||
| Total cholesterol (mmol/L), median (IQR) | 3.4 (2.8–4.1) | 2.4 (1.6–4.2) | 0.001 | 0.044 |
| Non-HDL cholesterol (mmol/L), median (IQR) | 2.3 (1.9–3.0) | 2.1 (1.4–3.9) | 0.504 | 0.356 |
| HDL cholesterol (mmol/L), median (IQR) | 1.1 (0.8–1.3) | 0.2 (0.1–0.5) | <0.001 | <0.001 |
| LDL cholesterol (mmol/L), median (IQR) | 1.9 (1.4–2.2) | 1.7 (1.1–2.3) | 0.409 | 0.915 |
| Triglycerides (mmol/L), median (IQR) | 0.6 (0.5–1.0) | 0.9 (0.5–1.3) | 0.101 | 0.152 |
| ApoB (mg/dL), median (IQR) | 57.0 (46.8–73.5) | 94.0 (50.5–123.0) | <0.001 | <0.001 |
| ApoA-I (mg/dL), median (IQR) | 68.5 (53.0–88.0) | 20.5 (11.3–34.0) | <0.001 | <0.001 |
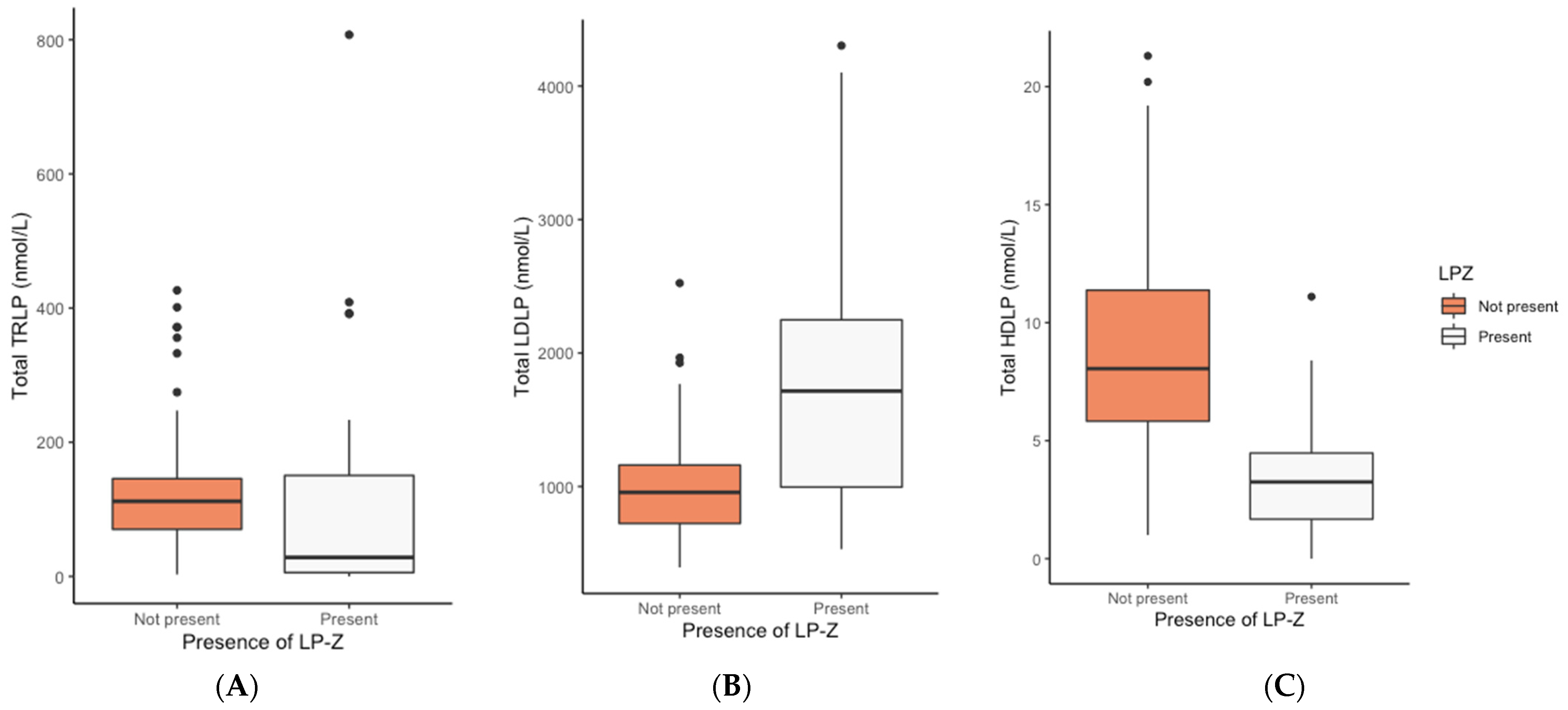
| TRLP (nmol/L), median (IQR) | 112.0 (69.6–147.0) | 28.4 (4.7–163.6) | 0.001 | 0.220 |
| Very large TRLP (nmol/L), median (IQR) | 0.0 (0.0–0.1) | 0.0 (0.0–0.0) | 0.010 | 0.310 |
| Large TRLP (nmol/L), median (IQR) | 0.3 (0.0–1.6) | 0.1 (0.0–1.3) | 0.208 | 0.358 |
| Medium TRLP (nmol/L), median (IQR) | 2.0 (0.0–6.8) | 2.7 (0.0–13.3) | 0.215 | 0.138 |
| Small TRLP (nmol/L), median (IQR) | 20.3 (11.2–33.8) | 0.6 (0.0–29.3) | 0.001 | 0.859 |
| Very small TRLP (nmol/L), median (IQR) | 74.6 (38.8–118.4) | 4.6 (0.0–110.6) | <0.001 | 0.100 |
| TRL size (nm), median (IQR) | 40.9 (34.7–48.4) | 45.6 (36.2–55.7) | 0.154 | 0.756 |
| LDLP (nmol/L), median (IQR) | 956.0 (721.5–1166.3) | 1715.0 (968.5–2256.3) | <0.001 | <0.001 |
| Large LDLP (nmol/L), median (IQR) | 472.5 (337.8–671.8) | 235.5 (20.5–727.3) | 0.007 | 0.274 |
| Medium LDLP (nmol/L), median (IQR) | 16.5 (0.0–234.5) | 0.0 (0.0–0.0) | <0.001 | 0.004 |
| Small LDLP (nmol/L), median (IQR) | 277.0 (113.0–425.0) | 182.0 (26.8–394.0) | 0.108 | 0.298 |
| LDL size (nm), median (IQR) | 21.7 (21.3–22.0) | 21.3 (20.0–22.1) | 0.033 | <0.001 |
| Total HDLP (µmol/L), median (IQR) | 8.1 (5.8–11.5) | 3.3 (1.6–4.8) | <0.001 | <0.001 |
| Large HDLP (µmol/L), median (IQR) | 3.1 (1.9–3.9) | 0.7 (0.0–1.5) | <0.001 | <0.001 |
| Medium HDLP (µmol/L), median (IQR) | 0.9 (0.2–3.0) | 0.0 (0.0–0.3) | <0.001 | <0.001 |
| Small HDLP (µmol/L), median (IQR) | 3.4 (1.8–6.1) | 2.1 (0.4–3.2) | 0.001 | 0.001 |
| HDL size (nm), median (IQR) | 10.8 (9.7–11.5) | 9.5 (8.1–10.3) | <0.001 | <0.001 |
HDL subspecies
| 0.0 (0.0–0.4) 3.3 (1.3–5.6) 0.4 (0.0–1.2) 0.5 (0.0–1.3) 0.1 (0.0–0.6) 0.6 (0.0–1.3) 1.8 (0.3–2.9) | 0.0 (0.0–0.1) 1.7 (0.0–2.8) 0.0 (0.0–0.1) 0.0 (0.0–0.1) 0.0 (0.0–0.6) 0.0 (0.0–0.5) 0.0 (0.0–0.3) | 0.264 <0.001 <0.001 <0.001 0.699 <0.001 <0.001 | 0.962 <0.001 0.004 0.001 0.377 <0.001 <0.001 |
| LP-Z (nmol/L), median (IQR) | - | 1354.5 (725.7–2080.9) | n.a. | n.a. |
| Primary liver diseases | ||||
| Storage disease, n (%) | 4 (4.4) | 0 (0) | 0.311 | n.a. |
| Autoimmune hepatitis, n (%) | 8 (8.9) | 3 (7.5) | 1.00 | n.a. |
| Cholestatic liver disease (PSC/PBC), n (%) | 18 (20.0) | 16 (40.0) | 0.017 | n.a. |
| Viral, n (%) | 9 (10.0) | 3 (7.5) | 0.754 | n.a. |
| Alcohol, n (%) | 22 (24.4) | 7 (17.5) | 0.380 | n.a. |
| MAFLD, n (%) | 24 (26.7) | 9 (22.5) | 0.614 | n.a. |
| Vascular, n (%) | 2 (2.2) | 0 (0.0) | 1.00 | n.a. |
| Malignancy, n (%) | 0 (0) | 0 (0) | n.a. | n.a. |
| Other, n (%) | 3 (3.3) | 2 (5.0) | 0.643 | n.a. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Berg, E.H.; Flores-Guerrero, J.L.; Gruppen, E.G.; Garcia, E.; Connelly, M.A.; de Meijer, V.E.; Bakker, S.J.L.; Blokzijl, H.; Dullaart, R.P.F. Profoundly Disturbed Lipoproteins in Cirrhotic Patients: Role of Lipoprotein-Z, a Hepatotoxic LDL-like Lipoprotein. J. Clin. Med. 2022, 11, 1223. https://doi.org/10.3390/jcm11051223
van den Berg EH, Flores-Guerrero JL, Gruppen EG, Garcia E, Connelly MA, de Meijer VE, Bakker SJL, Blokzijl H, Dullaart RPF. Profoundly Disturbed Lipoproteins in Cirrhotic Patients: Role of Lipoprotein-Z, a Hepatotoxic LDL-like Lipoprotein. Journal of Clinical Medicine. 2022; 11(5):1223. https://doi.org/10.3390/jcm11051223
Chicago/Turabian Stylevan den Berg, Eline H., Jose L. Flores-Guerrero, Eke G. Gruppen, Erwin Garcia, Margery A. Connelly, Vincent E. de Meijer, Stephan J. L. Bakker, Hans Blokzijl, and Robin P. F. Dullaart. 2022. "Profoundly Disturbed Lipoproteins in Cirrhotic Patients: Role of Lipoprotein-Z, a Hepatotoxic LDL-like Lipoprotein" Journal of Clinical Medicine 11, no. 5: 1223. https://doi.org/10.3390/jcm11051223
APA Stylevan den Berg, E. H., Flores-Guerrero, J. L., Gruppen, E. G., Garcia, E., Connelly, M. A., de Meijer, V. E., Bakker, S. J. L., Blokzijl, H., & Dullaart, R. P. F. (2022). Profoundly Disturbed Lipoproteins in Cirrhotic Patients: Role of Lipoprotein-Z, a Hepatotoxic LDL-like Lipoprotein. Journal of Clinical Medicine, 11(5), 1223. https://doi.org/10.3390/jcm11051223








