Serum Hypoalbuminemia Is a Long-Term Prognostic Marker in Medical Hospitalized Patients, Irrespective of the Underlying Disease
Abstract
:1. Introduction
2. Patients and Methods
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Igarashi, T. Pediatric fanconi syndrome. In Pediatric Nephrology; Avner, E.D., Harmon, W.E., Niaudet, P., Yoshikawa, N., Emma, F., Goldstein, S.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1355–1388. [Google Scholar]
- Pratt, D.S. Evaluation of Liver Function. In Harrison’s Principles of Internal Medicine; Jameson, J.L., Fauci, A.S., Kasper, D.L., Hauser, S.L., Longo, D.L., Loscalzo, J., Eds.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Watanabe, H.; Maruyama, T. Albumin as a Biomarker. In Albumin in Medicine: Pathological and Clinical Applications; Otagiri, M., Chuang, V.T.G., Eds.; Springer: Singapore, 2016; pp. 51–69. [Google Scholar]
- Chen, Z.; Shao, Y.; Wang, K.; Cao, W.; Xiong, Y.; Wu, R.; Luo, S.; Xu, X.; He, X. Prognostic role of pretreatment serum albumin in renal cell carcinoma: A systematic review and meta-analysis. Onco Targets Ther. 2016, 9, 6701–6710. [Google Scholar] [CrossRef] [Green Version]
- Corti, M.C.; Guralnik, J.M.; Salive, M.E.; Sorkin, J.D. Serum albumin level and physical disability as predictors of mortality in older persons. JAMA 1994, 272, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, F.; Lecleire, S.; Pop, D.; Rigal, O.; Hamidou, H.; Paillot, B.; Ducrotte, P.; Lerebours, E.; Michel, P. Baseline nutritional status is predictive of response to treatment and survival in patients treated by definitive chemoradiotherapy for a locally advanced esophageal cancer. Am. J. Gastroenterol. 2007, 102, 2557–2563. [Google Scholar] [CrossRef] [PubMed]
- Don, B.R.; Kaysen, G. Serum albumin: Relationship to inflammation and nutrition. Semin. Dial. 2004, 17, 432–437. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Herrmann, F.R.; Safran, C.; Levkoff, S.E.; Minaker, K.L. Serum albumin level on admission as a predictor of death, length of stay, and readmission. Arch. Intern. Med. 1992, 152, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Knaus, W.A.; Wagner, D.P.; Draper, E.A.; Zimmerman, J.E.; Bergner, M.; Bastos, P.G.; Sirio, C.A.; Murphy, D.J.; Lotring, T.; Damiano, A.; et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 1991, 100, 1619–1636. [Google Scholar] [CrossRef] [Green Version]
- Gupta, D.; Vashi, P.G.; Lammersfeld, C.A.; Braun, D.P. Role of nutritional status in predicting the length of stay in cancer: A systematic review of the epidemiological literature. Ann. Nutr. Metab. 2011, 59, 96–106. [Google Scholar] [CrossRef]
- Novack, V.; Pencina, M.; Zahger, D.; Fuchs, L.; Nevzorov, R.; Jotkowitz, A.; Porath, A. Routine laboratory results and thirty day and one-year mortality risk following hospitalization with acute decompensated heart failure. PLoS ONE 2010, 5, e12184. [Google Scholar] [CrossRef]
- Asher, V.; Lee, J.; Bali, A. Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Med. Oncol. 2012, 29, 2005–2009. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, K.; Liang, Z.; Guo, S.; Zhang, P.; Xu, Y.; Zhou, H. Prognostic role of pre-treatment serum albumin in patients with nasopharyngeal carcinoma: A meta-analysis and systematic review. Clin. Otolaryngol. 2020, 45, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, J.; Zheng, H.; Wang, X.; Liu, Z.; Sun, T. Prognostic Role of Serum Albumin, Total Lymphocyte Count, and Mini Nutritional Assessment on Outcomes After Geriatric Hip Fracture Surgery: A Meta-Analysis and Systematic Review. J. Arthroplast. 2019, 34, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Owen, W.F., Jr.; Lew, N.L.; Liu, Y.; Lowrie, E.G.; Lazarus, J.M. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N. Engl. J. Med. 1993, 329, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Law, M.R.; Morris, J.K.; Wald, N.J.; Hale, A.K. Serum albumin and mortality in the BUPA study. British United Provident Association. Int. J. Epidemiol. 1994, 23, 38–41. [Google Scholar] [CrossRef]
- Schalk, B.W.; Visser, M.; Bremmer, M.A.; Penninx, B.W.; Bouter, L.M.; Deeg, D.J. Change of serum albumin and risk of cardiovascular disease and all-cause mortality: Longitudinal Aging Study Amsterdam. Am. J. Epidemiol. 2006, 164, 969–977. [Google Scholar] [CrossRef] [Green Version]
- Djousse, L.; Rothman, K.J.; Cupples, L.A.; Levy, D.; Ellison, R.C. Serum albumin and risk of myocardial infarction and all-cause mortality in the Framingham Offspring Study. Circulation 2002, 106, 2919–2924. [Google Scholar] [CrossRef]
- Muller, C.; Stift, A.; Argeny, S.; Bergmann, M.; Gnant, M.; Marolt, S.; Unger, L.; Riss, S. Delta albumin is a better prognostic marker for complications following laparoscopic intestinal resection for Crohn’s disease than albumin alone–A retrospective cohort study. PLoS ONE 2018, 13, e0206911. [Google Scholar] [CrossRef]
- Jones, C.H.; Newstead, C.G.; Wills, E.J.; Davison, A.M. Serum albumin and survival in CAPD patients: The implications of concentration trends over time. Nephrol. Dial. Transplant. 1997, 12, 554–558. [Google Scholar] [CrossRef]
- Chien, S.-C.; Chen, C.-Y.; Lin, C.-F.; Yeh, H.-I. Critical appraisal of the role of serum albumin in cardiovascular disease. Biomark. Res. 2017, 5, 31. [Google Scholar] [CrossRef]
- Nicholson, J.P.; Wolmarans, M.R.; Park, G.R. The role of albumin in critical illness. Br. J. Anaesth. 2000, 85, 599–610. [Google Scholar] [CrossRef] [Green Version]
- Sort, P.; Navasa, M.; Arroyo, V.; Aldeguer, X.; Planas, R.; Ruiz-del-Arbol, L.; Castells, L.; Vargas, V.; Soriano, G.; Guevara, M.; et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N. Engl. J. Med. 1999, 341, 403–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucsics, T.; Krones, E. Renal dysfunction in cirrhosis: Acute kidney injury and the hepatorenal syndrome. Gastroenterol. Rep. 2017, 5, 127–137. [Google Scholar] [CrossRef] [PubMed]
- China, L.; Maini, A.; Skene, S.S.; Shabir, Z.; Sylvestre, Y.; Colas, R.A.; Ly, L.; Becares Salles, N.; Belloti, V.; Dalli, J.; et al. Albumin Counteracts Immune-Suppressive Effects of Lipid Mediators in Patients with Advanced Liver Disease. Clin. Gastroenterol. Hepatol. 2018, 16, 738–747.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, J.; Claria, J.; Amoros, A.; Aguilar, F.; Castro, M.; Casulleras, M.; Acevedo, J.; Duran-Guell, M.; Nunez, L.; Costa, M.; et al. Effects of Albumin Treatment on Systemic and Portal Hemodynamics and Systemic Inflammation in Patients with Decompensated Cirrhosis. Gastroenterology 2019, 157, 149–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caironi, P.; Tognoni, G.; Masson, S.; Fumagalli, R.; Pesenti, A.; Romero, M.; Fanizza, C.; Caspani, L.; Faenza, S.; Grasselli, G.; et al. Albumin replacement in patients with severe sepsis or septic shock. N. Engl. J. Med. 2014, 370, 1412–1421. [Google Scholar] [CrossRef] [Green Version]
- Finfer, S.; Bellomo, R.; Boyce, N.; French, J.; Myburgh, J.; Norton, R. Investigators SS: A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N. Engl. J. Med. 2004, 350, 2247–2256. [Google Scholar]
- Golub, R.; Sorrento, J.J., Jr.; Cantu, R., Jr.; Nierman, D.M.; Moideen, A.; Stein, H.D. Efficacy of albumin supplementation in the surgical intensive care unit: A prospective, randomized study. Crit. Care Med. 1994, 22, 613–619. [Google Scholar] [CrossRef]
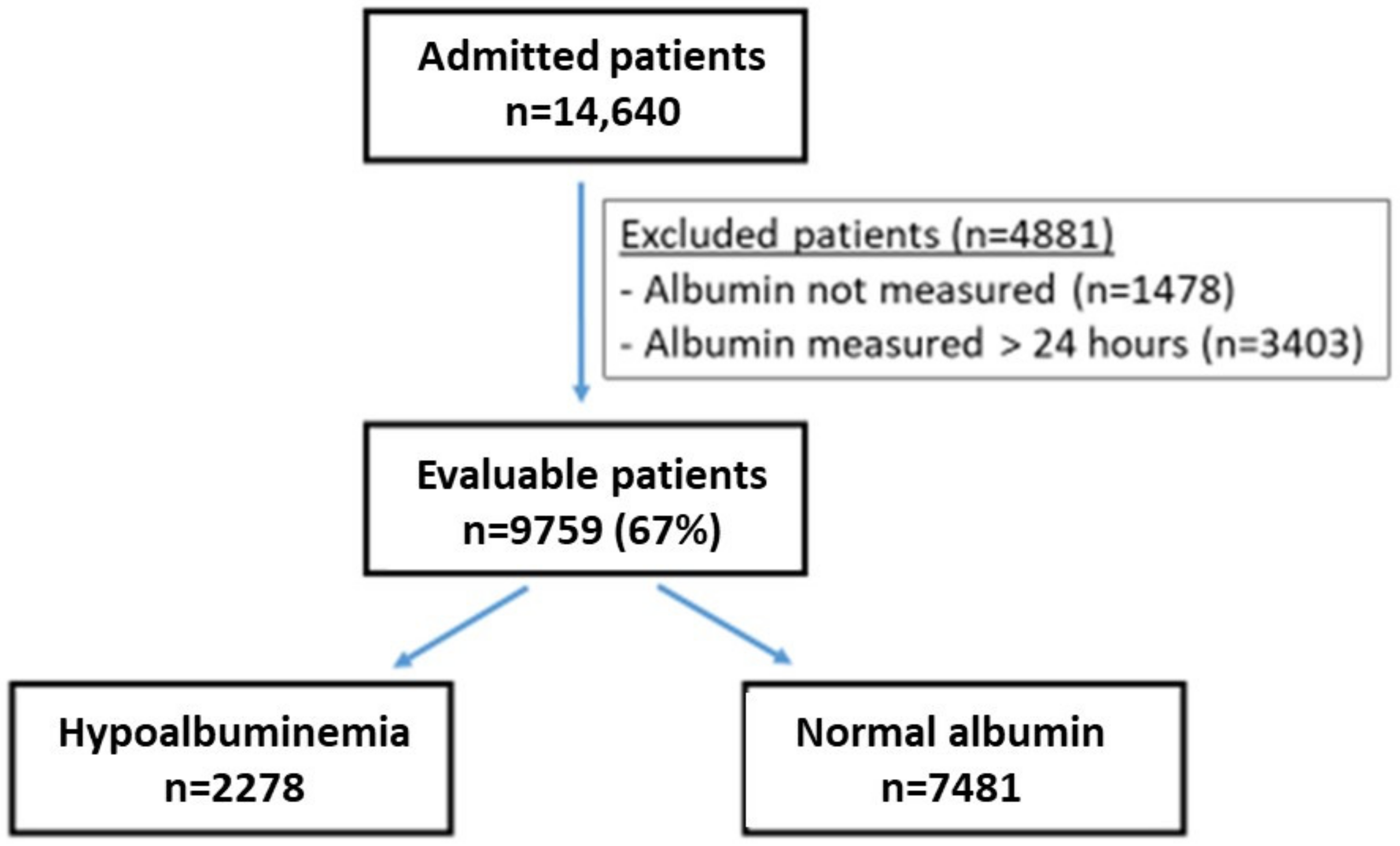

| All Patients | Hypoalbuminemia | Normal Albumin | p | |
|---|---|---|---|---|
| n | 9759 | 2278 | 7481 | - |
| Age median (IQR *) | 74.04 (60.0–84.1) | 78.8 (65.4–87.6) | 72.4 (58.6–83.0) | p < 0.001 |
| Gender: Males | 50.9% | 50.53% | 51.08% | - |
| Serum albumin g/L median; (IQR) | 38 (3.6–4.1) | 30.5 (2.9–3.3) | 40 (3.7–4.2) | p < 0.001 |
| Hb g/L, median; (IQR) | 125 (109–138) | 109 (95–124) | 128 (115–134) | p < 0.001 |
| Creatinine µmol/L; median; (IQR) | 94.6 (76.9–123.8) | 136.1 (74.3–151.2) | 93.7 (79.6–117.6) | p < 0.001 |
| CCI median (IQR) | 4 (2–6) | 5 (4–7) | 4 (2–5) | p < 0.001 |
| Hypoalbuminemia | Normal Albumin | p | |
|---|---|---|---|
| n | 2278 | 7481 | |
| LOS (median) (IQR *) | 5d (3–9) | 3d (2–6) | p < 0.001 |
| Prolonged stay (>7 days) | 31.3% | 15.2% | p < 0.001 |
| Re-admission in 1 yr (%) | 51.5% | 42.4% | p < 0.001 |
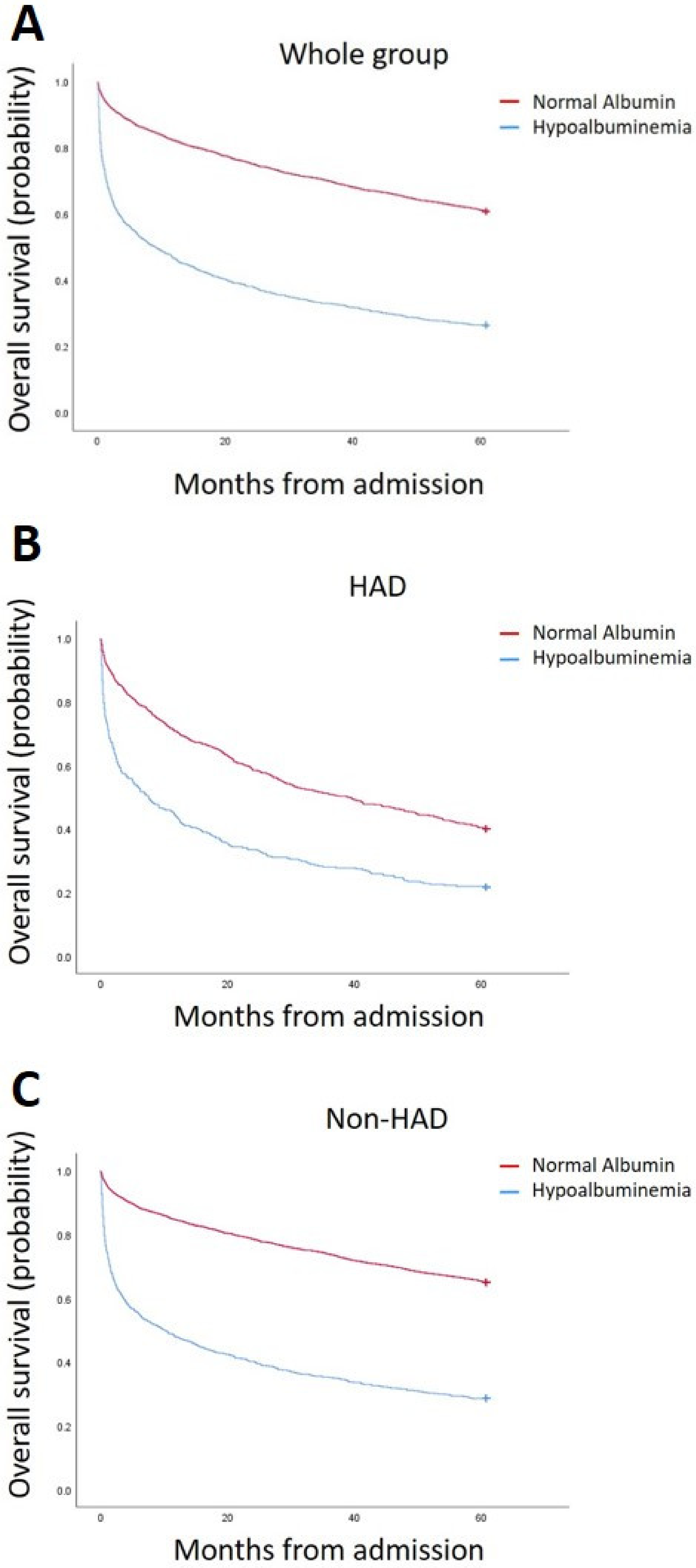
| 1 yr Mortality (%) | 50.97% | 17.4% | p < 0.001 |
| 3 Parameters | OR | 95% CI | p-Value | Prediction Value |
| Age | 1.02 | 1.02–1.03 | <0.01 | |
| Gender | 0.99 | 0.90–1.10 | 0.89 | |
| CCI | 1.27 | 1.24–1.31 | <0.01 | |
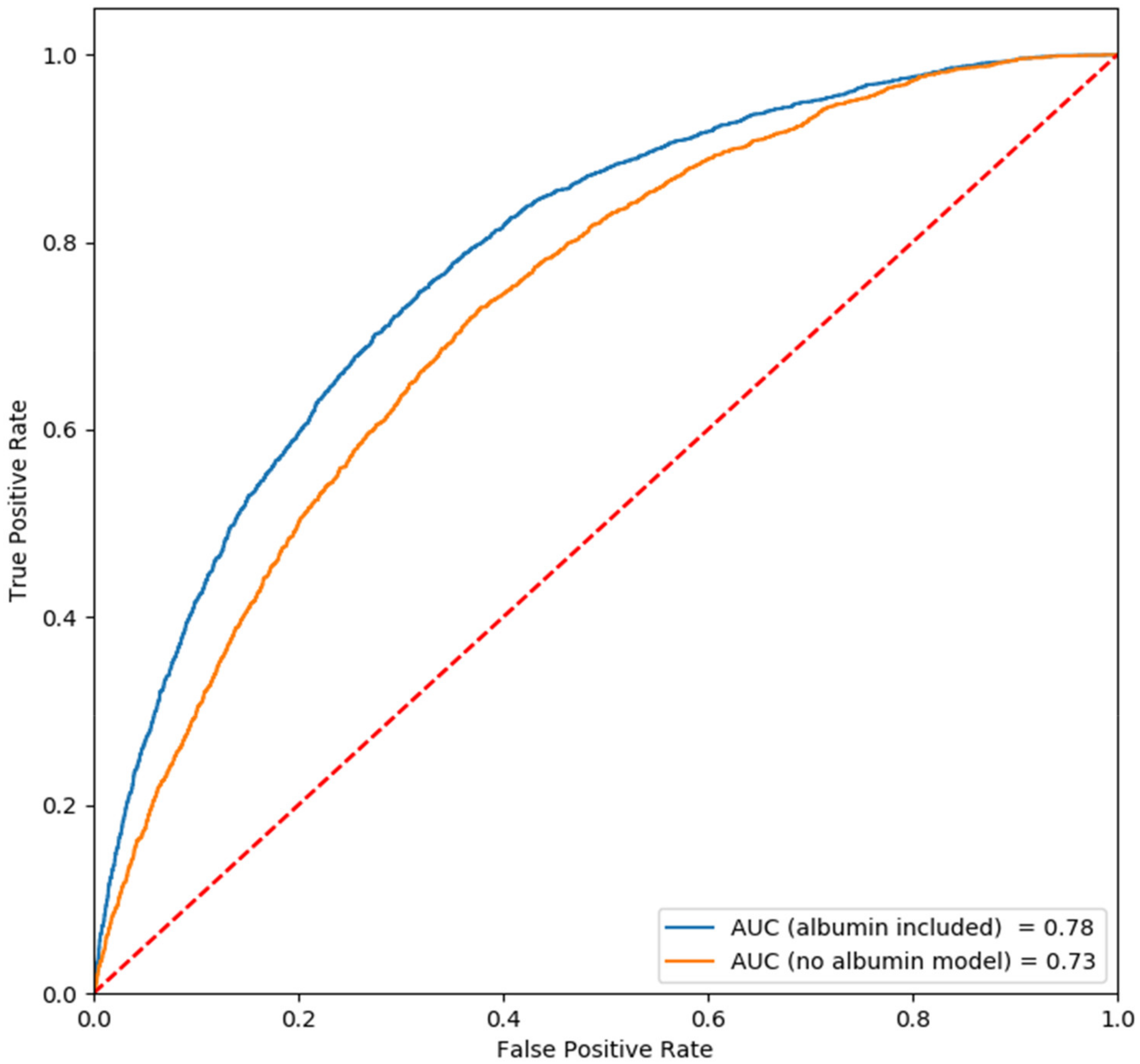
| AUC | 0.73 | |||
| 4 Parameters | OR | 95% CI | p-Value | Prediction Value |
| Age | 1.02 | 1.02–1.03 | <0.001 | |
| Gender | 1.00 | 0.90–1.11 | 0.97 | |
| CCI | 1.24 | 1.21–1.27 | <0.01 | |
| Hypoalbuminemia | 4.19 | 3.76–4.67 | <0.01 | |
| AUC | 0.78 |
| Hypoalbuminemia | Normal Albumin | p | |
|---|---|---|---|
| n | 736 | 1265 | |
| Age median (IQR *) | 79.9 (68.2–87.4) | 78.4 (67.2–85.8) | 0.07 |
| CCI median (IQR) | 6 (5–8) | 6 (5–8) | 0.14 |
| LOS median, days (IQR) | 5 (3–9) | 4 (2–7) | <0.001 |
| Prolonged stay (%) (>7 days) | 34% | 20.4% | <0.001 |
| Re-admission in 1 yr (%) | 54.9% | 55.3% | 0.89 |
| 1-year Mortality (%) | 55.2% | 27.7% | <0.001 |
| OR | 95% CI | p | Prediction Value | |
|---|---|---|---|---|
| Age | 1.02 | 1.02–1.03 | p < 0.01 | |
| Gender | 1.09 | 0.89–1.33 | p = 0.43 | |
| CCI | 1.18 | 1.13–1.24 | p < 0.01 | |
| Hypoalbuminemia | 3.40 | 2.78–4.16 | p < 0.01 | |
| AUC | 0.73 |
| Hypoalbuminemia | Normal Albumin | p | |
|---|---|---|---|
| n | 1542 | 6216 | |
| Age median (IQR *) | 78.2 (64.8–86.9) | 70.7 (56.4–82.1) | <0.001 |
| CCI median (IQR) | 5 (3–6) | 4 (2–5) | <0.001 |
| LOS median (IQR) | 5 (3–8) | 3 (2–5) | <0.001 |
| Prolonged Stay (%) (>7 days) | 30.2% | 14.1% | <0.001 |
| Re-admission in 1 yr (%) | 49.9% | 39.75% | <0.001 |
| 1 yr Mortality (%) | 48.9% | 15.3% | <0.001 |
| OR | 95% CI | p | Prediction Value | |
|---|---|---|---|---|
| Age | 1.02 | 1.02–1.03 | p < 0.01 | |
| Gender | 0.98 | 0.87–1.11 | p = 0.73 | |
| CCI | 1.26 | 1.22–1.31 | p < 0.01 | |
| Hypoalbuminemia | 4.49 | 3.95–5.12 | p < 0.01 | |
| AUC | 0.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oster, H.S.; Dolev, Y.; Kehat, O.; Weis-Meilik, A.; Mittelman, M. Serum Hypoalbuminemia Is a Long-Term Prognostic Marker in Medical Hospitalized Patients, Irrespective of the Underlying Disease. J. Clin. Med. 2022, 11, 1207. https://doi.org/10.3390/jcm11051207
Oster HS, Dolev Y, Kehat O, Weis-Meilik A, Mittelman M. Serum Hypoalbuminemia Is a Long-Term Prognostic Marker in Medical Hospitalized Patients, Irrespective of the Underlying Disease. Journal of Clinical Medicine. 2022; 11(5):1207. https://doi.org/10.3390/jcm11051207
Chicago/Turabian StyleOster, Howard S., Yardenna Dolev, Orli Kehat, Ahuva Weis-Meilik, and Moshe Mittelman. 2022. "Serum Hypoalbuminemia Is a Long-Term Prognostic Marker in Medical Hospitalized Patients, Irrespective of the Underlying Disease" Journal of Clinical Medicine 11, no. 5: 1207. https://doi.org/10.3390/jcm11051207
APA StyleOster, H. S., Dolev, Y., Kehat, O., Weis-Meilik, A., & Mittelman, M. (2022). Serum Hypoalbuminemia Is a Long-Term Prognostic Marker in Medical Hospitalized Patients, Irrespective of the Underlying Disease. Journal of Clinical Medicine, 11(5), 1207. https://doi.org/10.3390/jcm11051207






