Advance Directives in Oncology and Haematology: A Long Way to Go—A Narrative Review
Abstract
1. Introduction
2. Method
3. Patients
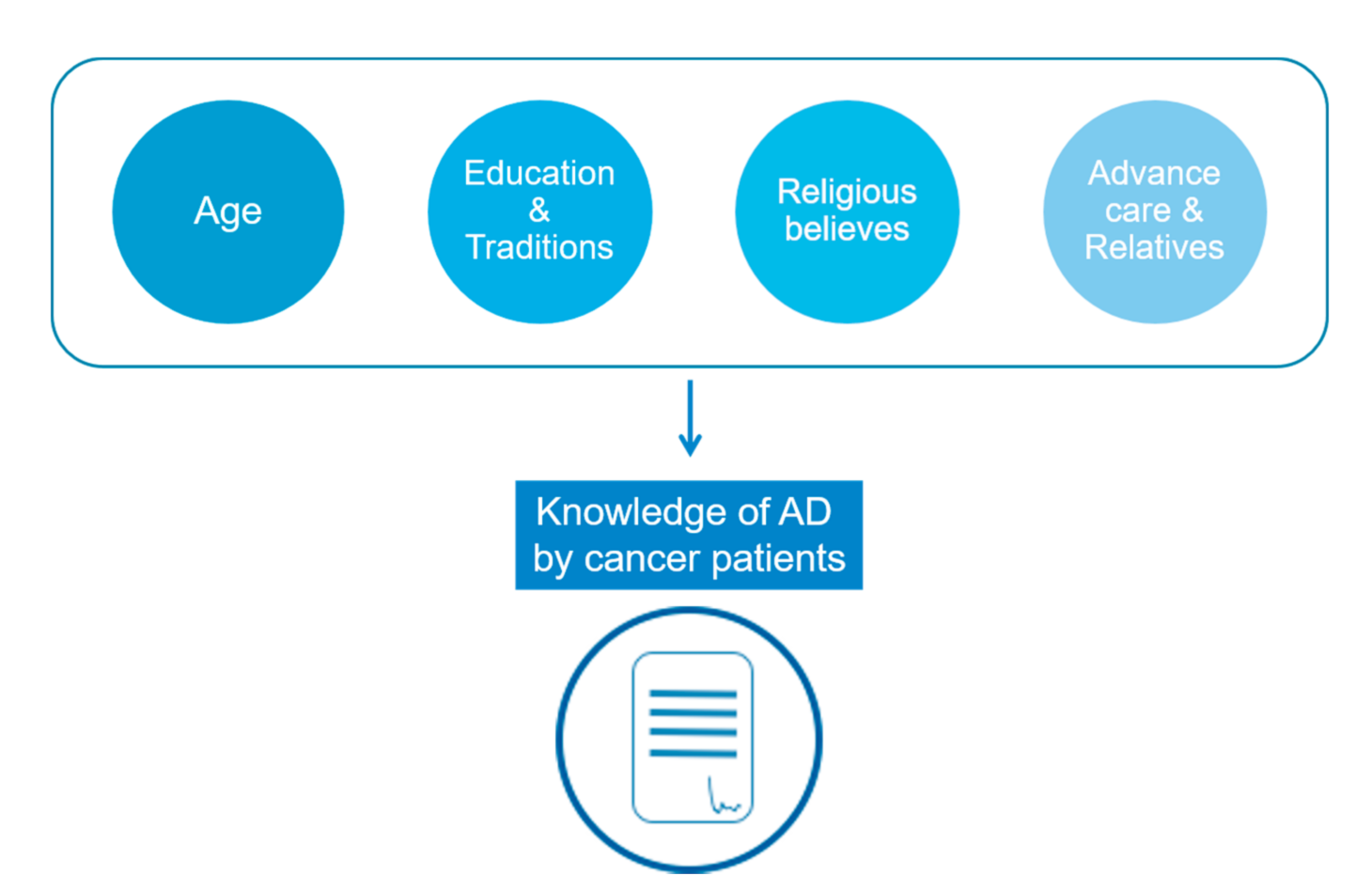
3.1. Demographic Characteristics
3.2. Education and Social Characteristics
3.3. Religious Beliefs and Traditions
3.4. AD to Relieve Relatives
3.5. Awareness of AD
4. Sickness
4.1. The Importance of Prognosis
4.2. Mental Stunning
4.3. Link between End-of-Life Care and Anticipated Discussion
5. Healthcare Workers: Reluctance to Provide Information on AD
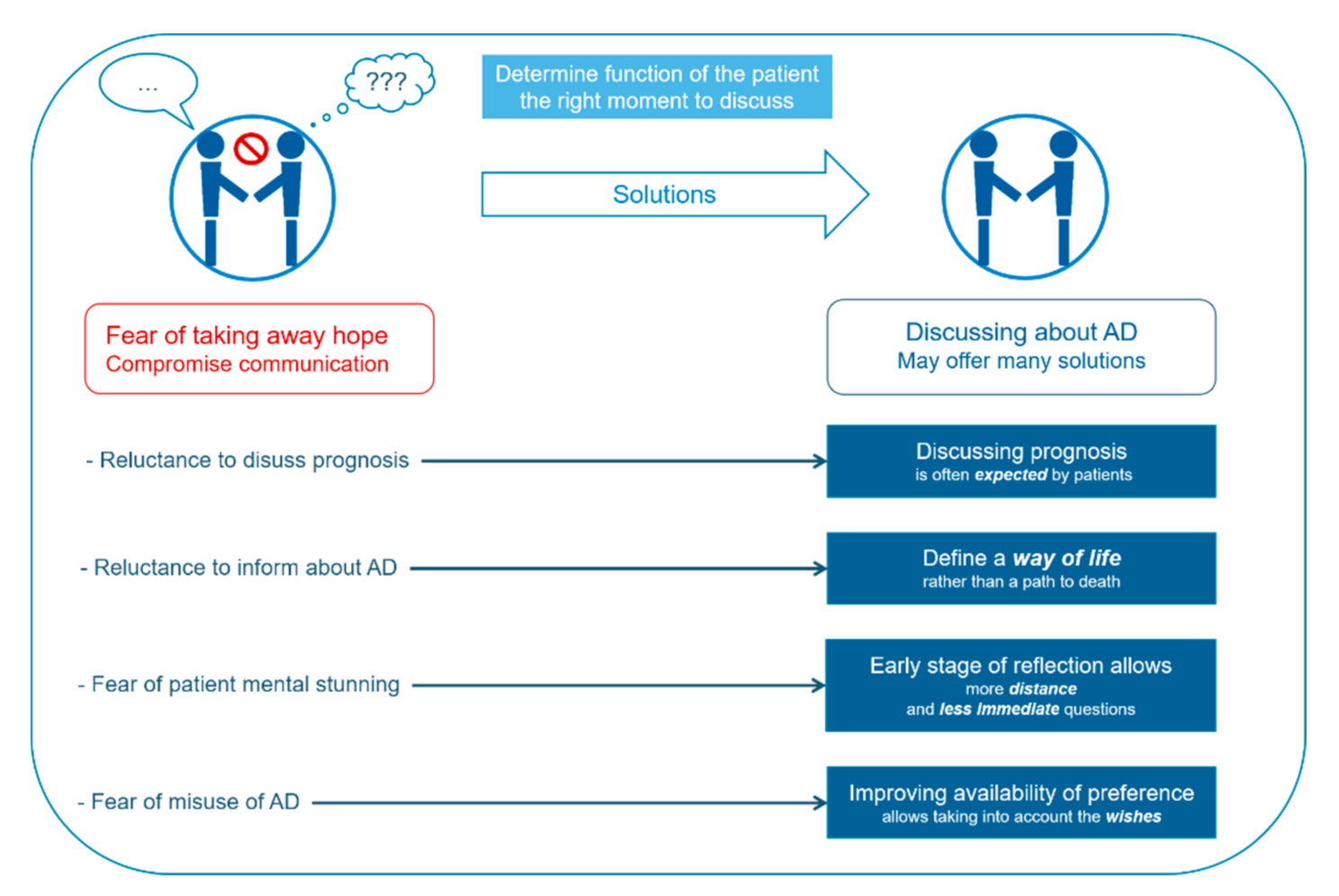
5.1. Reluctance to Discuss Prognosis
5.2. Fear of Taking Away Hope
5.3. Finding the Right Timing
5.4. Lack of Time
6. Fear of Misuse
7. Discussion: How to Improve AD’s Generalization?
7.1. AD for Who?
7.2. When to Discuss about AD
7.3. Discussing AD: With Who?
7.4. What to Discuss and How to Write?
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer Treatment and Survivorship Statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Quaresma, M.; Coleman, M.P.; Rachet, B. 40-Year Trends in an Index of Survival for All Cancers Combined and Survival Adjusted for Age and Sex for Each Cancer in England and Wales, 1971-2011: A Population-Based Study. Lancet 2015, 385, 1206–1218. [Google Scholar] [CrossRef]
- Soerjomataram, I.; Lortet-Tieulent, J.; Parkin, D.M.; Ferlay, J.; Mathers, C.; Forman, D.; Bray, F. Global Burden of Cancer in 2008: A Systematic Analysis of Disability-Adjusted Life-Years in 12 World Regions. Lancet 2012, 380, 1840–1850. [Google Scholar] [CrossRef]
- Meert, A.-P.; Grigoriu, B.; Licker, M.; Van Schil, P.E.; Berghmans, T. Intensive Care in Thoracic Oncology. Eur. Respir. J. 2017, 49. [Google Scholar] [CrossRef]
- Falanga, A.; Russo, L.; Milesi, V.; Vignoli, A. Mechanisms and Risk Factors of Thrombosis in Cancer. Crit. Rev. Oncol. Hematol. 2017, 118, 79–83. [Google Scholar] [CrossRef]
- Maschmeyer, G.; Bertschat, F.-L.; Moesta, K.T.; Häusler, E.; Held, T.K.; Nolte, M.; Osterziel, K.-J.; Papstein, V.; Peters, M.; Reich, G.; et al. Outcome Analysis of 189 Consecutive Cancer Patients Referred to the Intensive Care Unit as Emergencies during a 2-Year Period. Eur. J. Cancer 2003, 39, 783–792. [Google Scholar] [CrossRef]
- Soares, M.; Caruso, P.; Silva, E.; Teles, J.M.M.; Lobo, S.M.A.; Friedman, G.; Dal Pizzol, F.; Mello, P.V.C.; Bozza, F.A.; Silva, U.V.A.; et al. Characteristics and Outcomes of Patients with Cancer Requiring Admission to Intensive Care Units: A Prospective Multicenter Study. Crit. Care Med. 2010, 38, 9–15. [Google Scholar] [CrossRef]
- Normilio-Silva, K.; de Figueiredo, A.C.; Pedroso-de-Lima, A.C.; Tunes-da-Silva, G.; Nunes da Silva, A.; Delgado Dias Levites, A.; de-Simone, A.T.; Lopes Safra, P.; Zancani, R.; Tonini, P.C.; et al. Long-Term Survival, Quality of Life, and Quality-Adjusted Survival in Critically Ill Patients with Cancer. Crit. Care Med. 2016, 44, 1327–1337. [Google Scholar] [CrossRef]
- Lecuyer, L.; Chevret, S.; Thiery, G.; Darmon, M.; Schlemmer, B.; Azoulay, E. The ICU Trial: A New Admission Policy for Cancer Patients Requiring Mechanical Ventilation. Crit. Care Med. 2007, 35, 808–814. [Google Scholar] [CrossRef]
- Azoulay, E.; Mokart, D.; Pène, F.; Lambert, J.; Kouatchet, A.; Mayaux, J.; Vincent, F.; Nyunga, M.; Bruneel, F.; Laisne, L.-M.; et al. Outcomes of Critically Ill Patients with Hematologic Malignancies: Prospective Multicenter Data from France and Belgium--a Groupe de Recherche Respiratoire En Réanimation Onco-Hématologique Study. J. Clin. Oncol. 2013, 31, 2810–2818. [Google Scholar] [CrossRef]
- Vincent, F.; Soares, M.; Mokart, D.; Lemiale, V.; Bruneel, F.; Boubaya, M.; Gonzalez, F.; Cohen, Y.; Azoulay, E.; Darmon, M. In-Hospital and Day-120 Survival of Critically Ill Solid Cancer Patients after Discharge of the Intensive Care Units: Results of a Retrospective Multicenter Study—A Groupe de Recherche Respiratoire En Réanimation En Onco–Hématologie (Grrr-OH) Study. Ann. Intensive Care 2018, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.D.; Nallagangula, A.; Nalamalapu, S.; Nunna, K.; Nausran, U.; Robinson, K.A.; Dinglas, V.D.; Needham, D.M.; Eakin, M.N. Patient Outcomes after Critical Illness: A Systematic Review of Qualitative Studies Following Hospital Discharge. Crit. Care 2016, 20, 345. [Google Scholar] [CrossRef] [PubMed]
- Oeyen, S.G.; Benoit, D.D.; Annemans, L.; Depuydt, P.O.; Van Belle, S.J.; Troisi, R.I.; Noens, L.A.; Pattyn, P.; Decruyenaere, J.M. Long-Term Outcomes and Quality of Life in Critically Ill Patients with Hematological or Solid Malignancies: A Single Center Study. Intensive Care Med. 2013, 39, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Hofhuis, J.G.M.; Spronk, P.E.; van Stel, H.F.; Schrijvers, G.J.P.; Rommes, J.H.; Bakker, J. The Impact of Critical Illness on Perceived Health-Related Quality of Life during ICU Treatment, Hospital Stay, and after Hospital Discharge: A Long-Term Follow-up Study. Chest 2008, 133, 377–385. [Google Scholar] [CrossRef]
- Hofhuis, J.; Hautvast, J.L.A.; Schrijvers, A.J.P.; Bakker, J. Quality of Life on Admission to the Intensive Care: Can We Query the Relatives? Intensive Care Med. 2003, 29, 974–979. [Google Scholar] [CrossRef]
- Garrouste-Orgeas, M.; Tabah, A.; Vesin, A.; Philippart, F.; Kpodji, A.; Bruel, C.; Grégoire, C.; Max, A.; Timsit, J.F.; Misset, B. The ETHICA Study (Part II): Simulation Study of Determinants and Variability of ICU Physician Decisions in Patients Aged 80 or Over. Intensive Care Med. 2013, 39, 1574–1583. [Google Scholar] [CrossRef]
- Mack, J.W.; Cronin, A.; Keating, N.L.; Taback, N.; Huskamp, H.A.; Malin, J.L.; Earle, C.C.; Weeks, J.C. Associations between End-of-Life Discussion Characteristics and Care Received near Death: A Prospective Cohort Study. J. Clin. Oncol. 2012, 30, 4387–4395. [Google Scholar] [CrossRef]
- Sato, T.; Soejima, K.; Fujisawa, D.; Takeuchi, M.; Arai, D.; Nakachi, I.; Naoki, K.; Kawada, I.; Yasuda, H.; Ishioka, K.; et al. Prognostic Understanding at Diagnosis and Associated Factors in Patients with Advanced Lung Cancer and Their Caregivers. Oncologist 2018, 23, 1218–1229. [Google Scholar] [CrossRef]
- Kutner, L. The Living Will: Coping with the Historical Event of Death. Bayl. Law Rev. 1975, 27, 39–53. [Google Scholar]
- Silveira, M.J.; Kim, S.Y.H.; Langa, K.M. Advance Directives and Outcomes of Surrogate Decision Making before Death. N. Engl. J. Med. 2010, 362, 1211–1218. [Google Scholar] [CrossRef]
- Guyon, G.; Garbacz, L.; Baumann, A.; Bohl, E.; Maheut-Bosser, A.; Coudane, H.; Kanny, G.; Gillois, P.; Claudot, F. Trusted person and living will: Information and implementation defect. Rev. Med. Interne 2014, 35, 643–648. [Google Scholar] [CrossRef]
- Zhang, Q.; Xie, C.; Xie, S.; Liu, Q. The Attitudes of Chinese Cancer Patients and Family Caregivers toward Advance Directives. Int. J. Environ. Res. Public Health 2016, 13, 816. [Google Scholar] [CrossRef]
- Sahm, S.; Will, R.; Hommel, G. Attitudes towards and Barriers to Writing Advance Directives amongst Cancer Patients, Healthy Controls, and Medical Staff. J. Med. Ethics 2005, 31, 437–440. [Google Scholar] [CrossRef]
- Dow, L.A.; Matsuyama, R.K.; Ramakrishnan, V.; Kuhn, L.; Lamont, E.B.; Lyckholm, L.; Smith, T.J. Paradoxes in Advance Care Planning: The Complex Relationship of Oncology Patients, Their Physicians, and Advance Medical Directives. J. Clin. Oncol. 2010, 28, 299–304. [Google Scholar] [CrossRef]
- Mercadante, S.; Costanzi, A.; Marchetti, P.; Casuccio, A. Attitudes among Patients with Advanced Cancer toward Euthanasia and Living Wills. J. Pain. Symptom Manag. 2016, 51, e3–e6. [Google Scholar] [CrossRef]
- De Vleminck, A.; Pardon, K.; Houttekier, D.; Van den Block, L.; Vander Stichele, R.; Deliens, L. The Prevalence in the General Population of Advance Directives on Euthanasia and Discussion of End-of-Life Wishes: A Nationwide Survey. BMC Palliat. Care 2015, 14, 71. [Google Scholar] [CrossRef]
- Pennec, S.; Monnier, A.; Pontone, S.; Aubry, R. End-of-Life Medical Decisions in France: A Death Certificate Follow-up Survey 5 Years after the 2005 Act of Parliament on Patients’ Rights and End of Life. BMC Palliat. Care 2012, 11, 25. [Google Scholar] [CrossRef]
- McDonald, J.C.; du Manoir, J.M.; Kevork, N.; Le, L.W.; Zimmermann, C. Advance Directives in Patients with Advanced Cancer Receiving Active Treatment: Attitudes, Prevalence, and Barriers. Support. Care Cancer 2017, 25, 523–531. [Google Scholar] [CrossRef]
- Obel, J.; Brockstein, B.; Marschke, M.; Robicsek, A.; Konchak, C.; Sefa, M.; Ziomek, N.; Benfield, T.; Peterson, C.; Gustafson, C.; et al. Outpatient Advance Care Planning for Patients with Metastatic Cancer: A Pilot Quality Improvement Initiative. J. Palliat. Med. 2014, 17, 1231–1237. [Google Scholar] [CrossRef]
- Ganti, A.K.; Lee, S.J.; Vose, J.M.; Devetten, M.P.; Bociek, R.G.; Armitage, J.O.; Bierman, P.J.; Maness, L.J.; Reed, E.C.; Loberiza, F.R. Outcomes after Hematopoietic Stem-Cell Transplantation for Hematologic Malignancies in Patients with or without Advance Care Planning. J. Clin. Oncol. 2007, 25, 5643–5648. [Google Scholar] [CrossRef]
- Tan, T.S.; Jatoi, A. An Update on Advance Directives in the Medical Record: Findings from 1186 Consecutive Patients with Unresectable Exocrine Pancreas Cancer. J. Gastrointest. Cancer 2008, 39, 100–103. [Google Scholar] [CrossRef]
- Ho, G.W.K.; Skaggs, L.; Yenokyan, G.; Kellogg, A.; Johnson, J.A.; Lee, M.C.; Heinze, K.; Hughes, M.T.; Sulmasy, D.P.; Kub, J.; et al. Patient and Caregiver Characteristics Related to Completion of Advance Directives in Terminally Ill Patients. Palliat. Support. Care 2017, 15, 12–19. [Google Scholar] [CrossRef]
- Clark, M.A.; Ott, M.; Rogers, M.L.; Politi, M.C.; Miller, S.C.; Moynihan, L.; Robison, K.; Stuckey, A.; Dizon, D. Advance Care Planning as a Shared Endeavor: Completion of ACP Documents in a Multidisciplinary Cancer Program. Psychooncology 2017, 26, 67–73. [Google Scholar] [CrossRef]
- Hubert, E.; Schulte, N.; Belle, S.; Gerhardt, A.; Merx, K.; Hofmann, W.-K.; Stein, A.; Burkholder, I.; Hofheinz, R.-D.; Kripp, M. Cancer Patients and Advance Directives: A Survey of Patients in a Hematology and Oncology Outpatient Clinic. Onkologie 2013, 36, 398–402. [Google Scholar] [CrossRef]
- Ha, F.J.; Weickhardt, A.J.; Parakh, S.; Vincent, A.D.; Glassford, N.J.; Warrillow, S.; Jones, D. Survival and Functional Outcomes of Patients with Metastatic Solid Organ Cancer Admitted to the Intensive Care Unit of a Tertiary Centre. Crit. Care Resusc. 2017, 19, 159–166. [Google Scholar] [CrossRef]
- Roger, C.; Morel, J.; Molinari, N.; Orban, J.C.; Jung, B.; Futier, E.; Desebbe, O.; Friggeri, A.; Silva, S.; Bouzat, P.; et al. Practices of End-of-Life Decisions in 66 Southern French ICUs 4 Years after an Official Legal Framework: A 1-Day Audit. Anaesth. Crit. Care Pain Med. 2015, 34, 73–77. [Google Scholar] [CrossRef]
- Brown, A.J.; Shen, M.J.; Urbauer, D.; Taylor, J.; Parker, P.A.; Carmack, C.; Prescott, L.; Kolawole, E.; Rosemore, C.; Sun, C.; et al. Room for Improvement: An Examination of Advance Care Planning Documentation among Gynecologic Oncology Patients. Gynecol. Oncol. 2016, 142, 525–530. [Google Scholar] [CrossRef]
- Van Oorschot, B.; Schuler, M.; Simon, A.; Flentje, M. Advance Directives: Prevalence and Attitudes of Cancer Patients Receiving Radiotherapy. Support. Care Cancer 2012, 20, 2729–2736. [Google Scholar] [CrossRef]
- Zheng, R.-J.; Fu, Y.; Xiang, Q.-F.; Yang, M.; Chen, L.; Shi, Y.-K.; Yu, C.-H.; Li, J.-Y. Knowledge, Attitudes, and Influencing Factors of Cancer Patients toward Approving Advance Directives in China. Support. Care Cancer 2016, 24, 4097–4103. [Google Scholar] [CrossRef]
- Sudore, R.L.; Lum, H.D.; You, J.J.; Hanson, L.C.; Meier, D.E.; Pantilat, S.Z.; Matlock, D.D.; Rietjens, J.A.C.; Korfage, I.J.; Ritchie, C.S.; et al. Defining Advance Care Planning for Adults: A Consensus Definition from a Multidisciplinary Delphi Panel. J. Pain Symptom Manag. 2017. [Google Scholar] [CrossRef]
- Keam, B.; Yun, Y.H.; Heo, D.S.; Park, B.W.; Cho, C.-H.; Kim, S.; Lee, D.H.; Lee, S.N.; Lee, E.S.; Kang, J.H.; et al. The Attitudes of Korean Cancer Patients, Family Caregivers, Oncologists, and Members of the General Public toward Advance Directives. Support Care Cancer 2013, 21, 1437–1444. [Google Scholar] [CrossRef]
- Kelley, C.G.; Lipson, A.R.; Daly, B.J.; Douglas, S.L. Advance Directive Use and Psychosocial Characteristics: An Analysis of Patients Enrolled in a Psychosocial Cancer Registry. Cancer Nurs. 2009, 32, 335–341. [Google Scholar] [CrossRef]
- Lee, J.E.; Shin, D.W.; Son, K.Y.; Park, H.J.; Lim, J.-Y.; Song, M.S.; Park, Y.-H.; Cho, B. Factors Influencing Attitudes toward Advance Directives in Korean Older Adults. Arch. Gerontol. Geriatr. 2018, 74, 155–161. [Google Scholar] [CrossRef]
- Chakraborty, A.; Boer, J.C.; Selomulya, C.; Plebanski, M.; Royce, S.G. Insights into Endotoxin-Mediated Lung Inflammation and Future Treatment Strategies. Expert Rev. Respir. Med. 2018, 12, 941–955. [Google Scholar] [CrossRef]
- Alano, G.J.; Pekmezaris, R.; Tai, J.Y.; Hussain, M.J.; Jeune, J.; Louis, B.; El-Kass, G.; Ashraf, M.S.; Reddy, R.; Lesser, M.; et al. Factors Influencing Older Adults to Complete Advance Directives. Palliat. Support. Care 2010, 8, 267–275. [Google Scholar] [CrossRef]
- Hoe, D.F.; Enguidanos, S. So Help Me, God: Religiosity and End-of-Life Choices in a Nationally Representative Sample. J. Palliat. Med. 2020, 23, 563–567. [Google Scholar] [CrossRef]
- Sharp, S.; Carr, D.; Macdonald, C. Religion and End-of-Life Treatment Preferences: Assessing the Effects of Religious Denomination and Beliefs. Soc. Forces 2012, 91, 275–298. [Google Scholar] [CrossRef]
- Karches, K.E.; Chung, G.S.; Arora, V.; Meltzer, D.O.; Curlin, F.A. Religiosity, Spirituality, and End-of-Life Planning: A Single-Site Survey of Medical Inpatients. J. Pain Symptom. Manag. 2012, 44, 843–851. [Google Scholar] [CrossRef]
- Maciejewski, P.K.; Phelps, A.C.; Kacel, E.L.; Balboni, T.A.; Balboni, M.; Wright, A.A.; Pirl, W.; Prigerson, H.G. Religious Coping and Behavioral Disengagement: Opposing Influences on Advance Care Planning and Receipt of Intensive Care near Death. Psychooncology 2012, 21, 714–723. [Google Scholar] [CrossRef]
- Wallace, C.L. Family Communication and Decision Making at the End of Life: A Literature Review. Palliat. Support. Care 2015, 13, 815–825. [Google Scholar] [CrossRef]
- Wong, S.Y.; Lo, S.H.; Chan, C.H.; Chui, H.S.; Sze, W.K.; Tung, Y. Is It Feasible to Discuss an Advance Directive with a Chinese Patient with Advanced Malignancy? A Prospective Cohort Study. Hong Kong Med. J. 2012, 18, 178–185. [Google Scholar]
- McPherson, C.J.; Wilson, K.G.; Murray, M.A. Feeling like a Burden to Others: A Systematic Review Focusing on the End of Life. Palliat. Med. 2007, 21, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Puchalski, C.M.; Zhong, Z.; Jacobs, M.M.; Fox, E.; Lynn, J.; Harrold, J.; Galanos, A.; Phillips, R.S.; Califf, R.; Teno, J.M. Patients Who Want Their Family and Physician to Make Resuscitation Decisions for Them: Observations from SUPPORT and HELP. Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatment. Hospitalized Elderly Longitudinal Project. J. Am. Geriatr. Soc. 2000, 48, S84-90. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.M.; Butow, P.N.; Tattersall, M.H.N.; Devine, R.J.; Simpson, J.M.; Aggarwal, G.; Clark, K.J.; Currow, D.C.; Elliott, L.M.; Lacey, J.; et al. Randomized Controlled Trial of a Prompt List to Help Advanced Cancer Patients and Their Caregivers to Ask Questions about Prognosis and End-of-Life Care. J. Clin. Oncol. 2007, 25, 715–723. [Google Scholar] [CrossRef]
- Dimoska, A.; Tattersall, M.H.N.; Butow, P.N.; Shepherd, H.; Kinnersley, P. Can a “Prompt List” Empower Cancer Patients to Ask Relevant Questions? Cancer 2008, 113, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Pfirstinger, J.; Kattner, D.; Edinger, M.; Andreesen, R.; Vogelhuber, M. The Impact of a Tumor Diagnosis on Patients’ Attitudes toward Advance Directives. Oncology 2014, 87, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Enzinger, A.C.; Zhang, B.; Schrag, D.; Prigerson, H.G. Outcomes of Prognostic Disclosure: Associations with Prognostic Understanding, Distress, and Relationship with Physician among Patients with Advanced Cancer. J. Clin. Oncol. 2015, 33, 3809–3816. [Google Scholar] [CrossRef]
- Weeks, J.C.; Cook, E.F.; O’Day, S.J.; Peterson, L.M.; Wenger, N.; Reding, D.; Harrell, F.E.; Kussin, P.; Dawson, N.V.; Connors, A.F.; et al. Relationship between Cancer Patients’ Predictions of Prognosis and Their Treatment Preferences. JAMA 1998, 279, 1709–1714. [Google Scholar] [CrossRef]
- Sborov, K.; Giaretta, S.; Koong, A.; Aggarwal, S.; Aslakson, R.; Gensheimer, M.F.; Chang, D.T.; Pollom, E.L. Impact of Accuracy of Survival Predictions on Quality of End-of-Life Care Among Patients with Metastatic Cancer Who Receive Radiation Therapy. J. Oncol. Pract. 2019, 15, e262–e270. [Google Scholar] [CrossRef]
- Derry, H.M.; Reid, M.C.; Prigerson, H.G. Advanced Cancer Patients’ Understanding of Prognostic Information: Applying Insights from Psychological Research. Cancer Med. 2019, 8, 4081–4088. [Google Scholar] [CrossRef]
- George, L.S.; Maciejewski, P.K.; Epstein, A.S.; Shen, M.; Prigerson, H.G. Advanced Cancer Patients’ Changes in Accurate Prognostic Understanding and Their Psychological Well-Being. J. Pain Symptom Manag. 2020, 59, 983–989. [Google Scholar] [CrossRef]
- Yoo, S.H.; Lee, J.; Kang, J.H.; Maeng, C.H.; Kim, Y.J.; Song, E.-K.; Koh, Y.; Yun, H.-J.; Shim, H.-J.; Kwon, J.H.; et al. Association of Illness Understanding with Advance Care Planning and End-of-Life Care Preferences for Advanced Cancer Patients and Their Family Members. Support. Care Cancer 2020, 28, 2959–2967. [Google Scholar] [CrossRef] [PubMed]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F.; et al. Early Palliative Care for Patients with Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.A.; Pirl, W.F.; Jackson, V.A.; Muzikansky, A.; Lennes, I.T.; Heist, R.S.; Gallagher, E.R.; Temel, J.S. Effect of Early Palliative Care on Chemotherapy Use and End-of-Life Care in Patients with Metastatic Non–Small-Cell Lung Cancer. JCO 2011, 30, 394–400. [Google Scholar] [CrossRef]
- Wright, A.A.; Zhang, B.; Ray, A.; Mack, J.W.; Trice, E.; Balboni, T.; Mitchell, S.L.; Jackson, V.A.; Block, S.D.; Maciejewski, P.K.; et al. Associations between End-of-Life Discussions, Patient Mental Health, Medical Care near Death, and Caregiver Bereavement Adjustment. JAMA 2008, 300, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Granek, L.; Krzyzanowska, M.K.; Tozer, R.; Mazzotta, P. Oncologists’ Strategies and Barriers to Effective Communication about the End of Life. J. Oncol. Pract. 2013, 9, e129–e135. [Google Scholar] [CrossRef]
- Robinson, C.A.; Fyles, G.; McKenzie, M. Oncologist Experience Implementing Goals of Care Discussions in Everyday Ambulatory Oncology Practice: Implications for Education. J. Cancer Educ. 2017, 32, 301–307. [Google Scholar] [CrossRef]
- Daugherty, C.K.; Hlubocky, F.J. What Are Terminally Ill Cancer Patients Told about Their Expected Deaths? A Study of Cancer Physicians’ Self-Reports of Prognosis Disclosure. J. Clin. Oncol. 2008, 26, 5988–5993. [Google Scholar] [CrossRef][Green Version]
- Sullivan, A.M.; Lakoma, M.D.; Matsuyama, R.K.; Rosenblatt, L.; Arnold, R.M.; Block, S.D. Diagnosing and Discussing Imminent Death in the Hospital: A Secondary Analysis of Physician Interviews. J. Palliat. Med. 2007, 10, 882–893. [Google Scholar] [CrossRef]
- Ledoux, M.; Rhondali, W.; Monnin, L.; Thollet, C.; Gabon, P.; Filbet, M. Advanced directives: Nurses’ and physicians’ representations in 2012. Bull. Cancer 2013, 100, 941–945. [Google Scholar] [CrossRef]
- Keating, N.L.; Landrum, M.B.; Rogers, S.O.; Baum, S.K.; Virnig, B.A.; Huskamp, H.A.; Earle, C.C.; Kahn, K.L. Physician Factors Associated with Discussions about End-of-Life Care. Cancer 2010, 116, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Hubert, S.; Wainschtein, S.; Hugues, A.; Schimpf, C.; Degroote, T.; Tiercelet, K.; Tran, M.; Bruel, C.; Philippart, F.; REQUIEM group (REQUIEM: Research/Reflexion on end of life support QUality In Everyday Medical practice). Advance Directives in France: Do Junior General Practitioners Want to Improve Their Implementation and Usage? A Nationwide Survey. BMC Med. Ethics 2019, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Almack, K.; Cox, K.; Moghaddam, N.; Pollock, K.; Seymour, J. After You: Conversations between Patients and Healthcare Professionals in Planning for End of Life Care. BMC Palliat. Care 2012, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- The, A.M.; Hak, T.; Koëter, G.; van der Wal, G. Collusion in Doctor-Patient Communication about Imminent Death: An Ethnographic Study. West. J. Med. 2001, 174, 247–253. [Google Scholar] [CrossRef]
- Barnes, K.A.; Barlow, C.A.; Harrington, J.; Ornadel, K.; Tookman, A.; King, M.; Jones, L. Advance Care Planning Discussions in Advanced Cancer: Analysis of Dialogues between Patients and Care Planning Mediators. Palliat. Support. Care 2011, 9, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B. Offering Truth: One Ethical Approach to the Uninformed Cancer Patient. Arch. Intern. Med. 1993, 153, 572–576. [Google Scholar] [CrossRef]
- Lamont, E.B.; Siegler, M. Paradoxes in Cancer Patients’ Advance Care Planning. J. Palliat. Med. 2000, 3, 27–35. [Google Scholar] [CrossRef]
- Boyd, K.; Mason, B.; Kendall, M.; Barclay, S.; Chinn, D.; Thomas, K.; Sheikh, A.; Murray, S.A. Advance Care Planning for Cancer Patients in Primary Care: A Feasibility Study. Br. J. Gen. Pract. 2010, 60, e449–e458. [Google Scholar] [CrossRef]
- Fu, S.; Barber, F.D.; Naing, A.; Wheler, J.; Hong, D.; Falchook, G.; Piha-Paul, S.; Tsimberidou, A.; Howard, A.; Kurzrock, R. Advance Care Planning in Patients with Cancer Referred to a Phase I Clinical Trials Program: The MD Anderson Cancer Center Experience. J. Clin. Oncol. 2012, 30, 2891–2896. [Google Scholar] [CrossRef]
- Robinson, C.A. “Our Best Hope Is a Cure.” Hope in the Context of Advance Care Planning. Palliat. Support. Care 2012, 10, 75–82. [Google Scholar] [CrossRef]
- Murray, L.; Butow, P.N.; White, K.; Kiernan, M.C.; D’Abrew, N.; Herz, H. Advance Care Planning in Motor Neuron Disease: A Qualitative Study of Caregiver Perspectives. Palliat. Med. 2016, 30, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Temel, J.S.; Shaw, A.T.; Greer, J.A. Challenge of Prognostic Uncertainty in the Modern Era of Cancer Therapeutics. J. Clin. Oncol. 2016, 34, 3605–3608. [Google Scholar] [CrossRef] [PubMed]
- Helft, P.R. Necessary Collusion: Prognostic Communication with Advanced Cancer Patients. J. Clin. Oncol. 2005, 23, 3146–3150. [Google Scholar] [CrossRef] [PubMed]
- Laryionava, K.; Heußner, P.; Hiddemann, W.; Winkler, E.C. Framework for Timing of the Discussion about Forgoing Cancer-Specific Treatment Based on a Qualitative Study with Oncologists. Support. Care Cancer 2015, 23, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Barnes, K.; Jones, L.; Tookman, A.; King, M. Acceptability of an Advance Care Planning Interview Schedule: A Focus Group Study. Palliat. Med. 2007, 21, 23–28. [Google Scholar] [CrossRef]
- Díaz-Montes, T.P.; Johnson, M.K.; Giuntoli, R.L.; Brown, A.J. Importance and Timing of End-of-Life Care Discussions among Gynecologic Oncology Patients. Am. J. Hosp. Palliat. Care 2013, 30, 59–67. [Google Scholar] [CrossRef]
- Fried, T.R.; O’Leary, J.R. Using the Experiences of Bereaved Caregivers to Inform Patient- and Caregiver-Centered Advance Care Planning. J. Gen. Intern. Med. 2008, 23, 1602–1607. [Google Scholar] [CrossRef]
- Walczak, A.; Henselmans, I.; Tattersall, M.H.N.; Clayton, J.M.; Davidson, P.M.; Young, J.; Bellemore, F.A.; Epstein, R.M.; Butow, P.N. A Qualitative Analysis of Responses to a Question Prompt List and Prognosis and End-of-Life Care Discussion Prompts Delivered in a Communication Support Program. Psychooncology 2015, 24, 287–293. [Google Scholar] [CrossRef]
- Goelz, T.; Wuensch, A.; Stubenrauch, S.; Ihorst, G.; de Figueiredo, M.; Bertz, H.; Wirsching, M.; Fritzsche, K. Specific Training Program Improves Oncologists’ Palliative Care Communication Skills in a Randomized Controlled Trial. J. Clin. Oncol. 2011, 29, 3402–3407. [Google Scholar] [CrossRef]
- Fournier, V.; Berthiau, D.; Kempf, E.; d’Haussy, J. Are advance directives useful for doctors and what for? Presse Med. 2013, 42, e159–e169. [Google Scholar] [CrossRef]
- Biola, H.; Sloane, P.D.; Williams, C.S.; Daaleman, T.P.; Zimmerman, S. Preferences versus Practice: Life-Sustaining Treatments in Last Months of Life in Long-Term Care. J. Am. Med. Dir. Assoc. 2010, 11, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Bomba, P.A.; Kemp, M.; Black, J.S. POLST: An Improvement over Traditional Advance Directives. Clevel. Clin. J. Med. 2012, 79, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Lamas, D.; Panariello, N.; Henrich, N.; Hammes, B.; Hanson, L.C.; Meier, D.E.; Guinn, N.; Corrigan, J.; Hubber, S.; Luetke-Stahlman, H.; et al. Advance Care Planning Documentation in Electronic Health Records: Current Challenges and Recommendations for Change. J. Palliat. Med. 2018, 21, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Brinkman-Stoppelenburg, A.; Witkamp, F.E.; van Zuylen, L.; van der Rijt, C.C.D.; van der Heide, A. Palliative Care Team Consultation and Quality of Death and Dying in a University Hospital: A Secondary Analysis of a Prospective Study. PLoS ONE 2018, 13, e0201191. [Google Scholar] [CrossRef]
- Johnstone, M.-J.; Kanitsaki, O. Ethics and Advance Care Planning in a Culturally Diverse Society. J. Transcult. Nurs. 2009, 20, 405–416. [Google Scholar] [CrossRef]
- Johnson, S.; Butow, P.; Kerridge, I.; Tattersall, M. Advance Care Planning for Cancer Patients: A Systematic Review of Perceptions and Experiences of Patients, Families, and Healthcare Providers. Psychooncology 2016, 25, 362–386. [Google Scholar] [CrossRef]
- Michael, N.; O’Callaghan, C.; Baird, A.; Gough, K.; Krishnasamy, M.; Hiscock, N.; Clayton, J. A Mixed Method Feasibility Study of a Patient- and Family-Centred Advance Care Planning Intervention for Cancer Patients. BMC Palliat Care 2015, 14, 27. [Google Scholar] [CrossRef]
- Huang, H.-L.; Tsai, J.-S.; Yao, C.-A.; Cheng, S.-Y.; Hu, W.-Y.; Chiu, T.-Y. Shared Decision Making with Oncologists and Palliative Care Specialists Effectively Increases the Documentation of the Preferences for Do Not Resuscitate and Artificial Nutrition and Hydration in Patients with Advanced Cancer: A Model Testing Study. BMC Palliat. Care 2020, 19, 17. [Google Scholar] [CrossRef]
- You, J.J.; Downar, J.; Fowler, R.A.; Lamontagne, F.; Ma, I.W.Y.; Jayaraman, D.; Kryworuchko, J.; Strachan, P.H.; Ilan, R.; Nijjar, A.P.; et al. Barriers to Goals of Care Discussions with Seriously Ill Hospitalized Patients and Their Families: A Multicenter Survey of Clinicians. JAMA Intern. Med. 2015, 175, 549–556. [Google Scholar] [CrossRef]
- Temel, J.S.; Greer, J.A.; Gallagher, E.R.; Jackson, V.A.; Lennes, I.T.; Muzikansky, A.; Park, E.R.; Pirl, W.F. Electronic Prompt to Improve Outpatient Code Status Documentation for Patients with Advanced Lung Cancer. JCO 2013, 31, 710–715. [Google Scholar] [CrossRef]
- Goswami, P.; Mistric, M.; Diane Barber, F. Advance Care Planning: Advanced Practice Provider-Initiated Discussions and Their Effects on Patient-Centered End-of-Life Care. Clin. J. Oncol. Nurs. 2020, 24, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Eliott, J.A.; Olver, I.N. Perceptions of “Good Palliative Care” Orders: A Discursive Study of Cancer Patients’ Comments. J. Palliat. Med. 2003, 6, 59–68. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serey, K.; Cambriel, A.; Pollina-Bachellerie, A.; Lotz, J.-P.; Philippart, F. Advance Directives in Oncology and Haematology: A Long Way to Go—A Narrative Review. J. Clin. Med. 2022, 11, 1195. https://doi.org/10.3390/jcm11051195
Serey K, Cambriel A, Pollina-Bachellerie A, Lotz J-P, Philippart F. Advance Directives in Oncology and Haematology: A Long Way to Go—A Narrative Review. Journal of Clinical Medicine. 2022; 11(5):1195. https://doi.org/10.3390/jcm11051195
Chicago/Turabian StyleSerey, Kevin, Amélie Cambriel, Adrien Pollina-Bachellerie, Jean-Pierre Lotz, and François Philippart. 2022. "Advance Directives in Oncology and Haematology: A Long Way to Go—A Narrative Review" Journal of Clinical Medicine 11, no. 5: 1195. https://doi.org/10.3390/jcm11051195
APA StyleSerey, K., Cambriel, A., Pollina-Bachellerie, A., Lotz, J.-P., & Philippart, F. (2022). Advance Directives in Oncology and Haematology: A Long Way to Go—A Narrative Review. Journal of Clinical Medicine, 11(5), 1195. https://doi.org/10.3390/jcm11051195







