Nonsurgical Periodontal Treatment Options and Their Impact on Subgingival Microbiota
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Clinical Periodontal Parameters
2.4. Statistical Analysis
2.5. Microbial Subgingival Sample Collection and DNA Isolation
2.6. Next Generation Sequencing
2.7. Data Analysis
2.7.1. Paired-End Read Assembly
2.7.2. Operational Taxonomic Unit (OTU) Cluster and Species Annotation
2.7.3. Bioinformatics: Alpha and Beta Diversity
3. Results
3.1. Patient Characteristics
3.2. Microbiota Structure Analysis
3.2.1. Relative Abundance in Relation to Periodontal Therapy
3.2.2. Alpha Diversity of Subgingival Microbiota Related to Periodontal Treatment
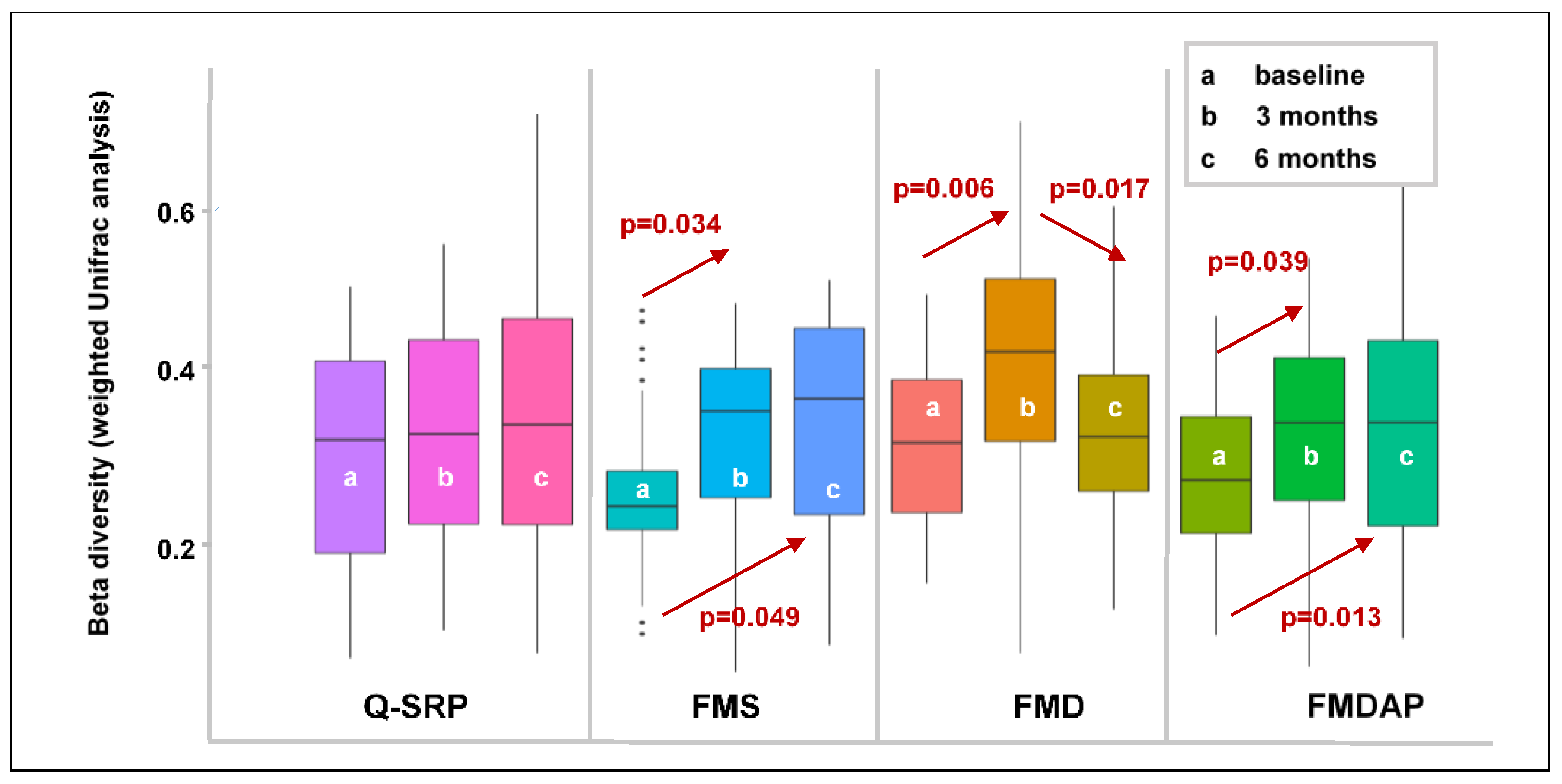
3.2.3. Effects of Periodontal Treatment on Beta Diversity
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hajishengallis, G.; Liang, S.; Payne, M.A.; Hashim, A.; Jotwani, R.; Eskan, M.A.; McIntosh, M.L.; Alsam, A.; Kirkwood, K.L.; Lambris, J.D.; et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 2011, 10, 497–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajishengallis, G.; Darveau, R.P.; Curtis, M.A. The keystone pathogen hypothesis. Nat. Rev. Microbiol. 2011, 10, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Khalid, T.; Bettiol, S.; Crocombe, L.A. Non-surgical periodontal therapy effectively improves patient-reported outcomes: A systematic review. Int. J. Dent. Hyg. 2021, 19, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Beikler, T.; Abdeen, G.; Schnitzer, S.; Sälzer, S.; Ehmke, B.; Heinecke, A.; Fleming, T.F. Microbiological shifts in intra- and extraoral habitats following mechanical periodontal therapy. J. Clin. Periodontol. 2004, 31, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Bollen, C.M.; Vandekerckhove, B.N.; Dekeyser, C.; Papaioannou, W.; Eyssen, H. Full- vs. partial-mouth disinfection in the treatment of periodontal infections: Short-term clinical and microbiological observations. J. Dent. Res. 1995, 74, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Mongardini, C.; van Steenberghe, D. The effect of a 1-stage full-mouth disinfection on oral malodor and microbial colonization of the tongue in periodontitis. A pilot study. J. Periodontol. 1998, 69, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; De Soete, M.; Boschmans, G.; Pauwels, M.; Coucke, W.; Teughels, W.; van Steenberghe, D. Benefit of “one-stage full-mouth disinfection” is explained by disinfection and root planing within 24 hours: A randomized controlled trial. J. Clin. Periodontol. 2006, 33, 639–647. [Google Scholar] [CrossRef]
- Fang, H.; Han, M.; Li, Q.L.; Cao, C.Y.; Xia, R.; Zhang, Z.H. Comparison of full-mouth disinfection and quadrant-wise scaling in the treatment of adult chronic periodontitis: A systematic review and meta-analysis. J. Periodontal. Res. 2016, 51, 417–430. [Google Scholar] [CrossRef]
- Santuchi, C.C.; Cortelli, J.R.; Cortelli, S.C.; Cota, L.O.; Fonseca, D.C.; Alencar, C.O.; Costa, F.O. Scaling and Root Planing per Quadrant Versus One-Stage Full-Mouth Disinfection: Assessment of the Impact of Chronic Periodontitis Treatment on Quality of Life-A Clinical Randomized, Controlled Trial. J. Periodontol. 2016, 87, 114–123. [Google Scholar] [CrossRef]
- Fonseca, D.C.; Cortelli, J.R.; Cortelli, S.C.; Miranda Cota, L.O.; Machado Costa, L.C.; Moreira Castro, M.V.; Oliveira Azevedo, A.M.; Costa, F.O. Clinical and Microbiologic Evaluation of Scaling and Root Planing per Quadrant and One-Stage Full-Mouth Disinfection Associated with Azithromycin or Chlorhexidine: A Clinical Randomized Controlled Trial. J. Periodontol. 2015, 86, 1340–1351. [Google Scholar] [CrossRef]
- Eberhardt, J.; Jepsen, S.; Jervøe-Storm, P.M.; Needleman, I.; Worthington, H.V. Full-mouth treatment modalities (within 24 hours) for chronic periodontitis in adults. Cochrane Database Syst. Rev. 2015, 17, CD004622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jentsch, H.F.R.; Flechsig, C.; Kette, B.; Eick, S. Adjunctive air-polishing with erythritol in nonsurgical periodontal therapy: A randomized clinical trial. BMC Oral Health 2020, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.M.; Michael, S.; Schittenhelm, F.; Reichert, S.; Kupitz, D.; Dommisch, H.; Kasaj, A.; Wied, S.; Vela, O.; Stratul, S. Comparison of three full-mouth concepts for the nonsurgical treatment of stage III and IV periodontitis—A randomized controlled trial. J. Clin. Periodontol. 2021, 48, 1516–1527. [Google Scholar] [CrossRef] [PubMed]
- Doungudomdacha, S.; Rawlinson, A.; Walsh, T.F.; Douglas, C.W.I. Effect of non-surgical periodontal treatment on clinical parameters and the numbers of Porphyromonas gingivalis, Prevotella intermedia and Actinobacillus actinomycetemcomitans at adult periodontitis sites. J. Clin. Periodontol. 2001, 28, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.S.; Lourenção, D.S.; Neto, L.G.L.; Pannuti, C.M.; Hirata, R.D.C.; Hirata, M.H.; Lotufo, R.F.M.; De Micheli, G. Clinical and Microbiologic Evaluation, by Real-Time Polymerase Chain Reaction, of Non-Surgical Treatment of Aggressive Periodontitis Associated with Amoxicillin and Metronidazole. J. Periodontol. 2012, 83, 744–752. [Google Scholar] [CrossRef]
- Finoti, L.S.; Corbi, S.C.T.; Anovazzi, G.; Teixeira, S.R.L.; Capela, M.V.; Tanaka, M.H.; Kim, Y.J.; Orrico, S.R.P.; Cirelli, J.A.; Mayer, M.P.A.; et al. Pathogen levels and clinical response to periodontal treatment in patients with Interleukin 8 haplotypes. Pathog. Dis. 2013, 69, 21–28. [Google Scholar]
- Gul, S.S.; Griffiths, G.S.; Stafford, G.P.; Al-Zubidi, M.I.; Rawlinson, A.; Douglas, C.W.I. Investigation of a Novel Predictive Biomarker Profile for the Outcome of Periodontal Treatment. J. Periodontol. 2017, 88, 1135–1144. [Google Scholar] [CrossRef]
- Rosalem, W.; Rescala, B.; Teles, R.P.; Fischer, R.G.; Gustafsson, A.; Figueredo, C.M. Effect of non-surgical treatment on chronic and aggressive periodontitis: Clinical, immunologic, and microbiologic findings. J. Periodontol. 2011, 82, 979–989. [Google Scholar] [CrossRef]
- Chen, C.; Hemme, C.; Beleno, J.; Shi, Z.J.; Ning, D.; Qin, Y.; Tu, Q.; Jorgensen, M.; He, Z.; Wu, L.; et al. Oral microbiota of periodontal health and disease and their changes after nonsurgical periodontal therapy. ISME J. 2018, 12, 1210–1224. [Google Scholar] [CrossRef]
- Johnston, W.; Rosier, B.T.; Artacho, A.; Paterson, M.; Piela, K.; Delaney, C.; Brown, J.L.; Ramage, G.; Mira, A.; Culshaw, S. Mechanical biofilm disruption causes microbial and immunological shifts in periodontitis patients. Sci. Rep. 2021, 11, 9796. [Google Scholar] [CrossRef]
- Feres, M.; Retamal-Valdes, B.; Fermiano, D.; Faveri, M.; Figueiredo, L.C.; Mayer, M.P.A.; Lee, J.J.; Bittinger, K.; Teles, F. Microbiome changes in young periodontitis patients treated with adjunctive metronidazole and amoxicillin. J. Periodontol. 2021, 92, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. 1), S173–S182. [Google Scholar] [CrossRef] [Green Version]
- Quirynen, M.; Mongardini, C.; De Soete, M.; Pauwels, M.; Coucke, W.; Van Eldere, J.; van Steenberghe, D. The role of chlorhexidine in the one-stage full-mouth disinfection treatment of patients with advanced adult periodontitis. Long-term clinical and microbiological observations. J. Clin. Periodontol. 2000, 27, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Radvar, M. The effect of smoking on mechanical and antimicrobial periodontal therapy. J. Periodontol. 1997, 68, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Löe, H. The gingival index, the plaque index and the retention index systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIJME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OUT sequences from microbial amplicons reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Kwon, T.; Lamster, I.B.; Levin, L. Current concepts in the management of periodontitis. Intern. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Liu, G.; Luan, Q.; Chen, F.; Chen, Z.; Zhang, Q.; Yu, X. Shift in the subgingival microbiome following scaling and root planing in generalized aggressive periodontitis. J. Clin. Periodontol. 2018, 45, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Park, H.; Cheng, B.; Kokaras, A.; Paster, B.; Burkett, S.; Watson, C.W.; Annavajhala, M.K.; Uhlemann, A.C.; Noble, J.M. Subgingival microbiome and clinical periodontal status in an elderly cohort: The WHICAP ancillary study of oral health. J. Periodontol. 2020, 91 (Suppl. 1), S56–S67. [Google Scholar] [CrossRef] [PubMed]
- Sawant, S.; Dugad, J.; Parikh, D.; Singh, H. Absolute quantification of oral bacteria involved in oral cancer by real-time PCR. Med. Microecol. 2021, 7, 100034. [Google Scholar] [CrossRef]
- Abdelbary, M.M.H.; Schittenhelm, F.; Yekta-Michael, S.S.; Reichert, S.; Schulz, S.; Kasaj, A.; Braun, A.; Conrads, G.; Stein, J.M. Impact of three nonsurgical, full-mouth periodontal treatments on total bacterial load and selected pathobionts. Antibiotics 2022. under revision. [Google Scholar]
- Wagner, B.D.; Grunwald, G.K.; Zerbe, G.O.; Mikulich-Gilbertson, S.K.; Robertson, C.E.; Zemanick, E.T.; Harris, J.K. On the use of diversity measures in longitudinal sequencing studies of microbial communities. Front. Microbiol. 2018, 9, 1037. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; We, Y.; Hu, W.; Nie, Y.; Wu, X.; Lu, R. The subgingival microbiome of periodontal pockets with different probing depths in chronic and aggressive periodontitis: A pilot study. Front. Cell. Infect. Microbiol. 2018, 8, 124. [Google Scholar] [CrossRef]
- Abusleme, L.; Dupuy, A.K.; Dutzan, N.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D.; Gamonal, J.; Diaz, P.I. The subgingival microbiome in health and periodontitis and its relationship with community biomass and infammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef] [Green Version]
- Schulz, S.; Porsch, M.; Große, I.; Hoffmann, K.; Schaller, H.G.; Reichert, S. Comparison of the oral microbiome of patients with aggressive periodontitis and periodontitisfree subjects. Arch. Oral. Biol. 2019, 99, 169–176. [Google Scholar] [CrossRef]
- Deng, Z.L.; Szafranski, S.P.; Jarek, M.; Bhuju, S.; Wagner-Döbler, I. Dysbiosis in chronic periodontitis: Key microbial players and interactions with the human host. Sci. Rep. 2017, 7, 3703. [Google Scholar] [CrossRef] [Green Version]
- Schwarzberg, K.; Le, R.; Bharti, B.; Lindsay, S.; Casaburi, G.; Salvatore, F.; Saber, M.H.; Alonaizan, F.; Slots, J.; Gottlieb, R.A.; et al. The personal human oral microbiome obscures the effects of treatment on periodontal disease. PLoS ONE 2014, 9, e86708. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, M.; Kiani, F.; Sayehmiri, F.; Sayehmiri, K.; Sheikhi, A.; Azodi, M.Z. Study of Porphyromonas gingivalis in periodontal diseases: A systematic review and meta-analysis. Med. J. Islam. Repub. Iran 2017, 31, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haffajee, A.D.; Teles, R.P.; Socransky, S.S. Association of Eubacterium nodatum and Treponema denticola with human periodontitis lesions. Oral. Microbiol. Immunol. 2006, 21, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Subramanian, A.; Anishetty, S. Comparative pan genome analysis of oral Prevotella species implicated in periodontitis. Funct. Integr. Genom. 2017, 17, 513–536. [Google Scholar] [CrossRef] [PubMed]
- Nibali, L.; Sousa, V.; Davrandi, M.; Spratt, D.; Alyahya, Q.; Dopico, J.; Donos, N. Differences in the periodontal microbiome of successfully treated and persistent aggressive periodontitis. J. Clin. Periodontol. 2020, 47, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Teles, R.; Wennstrom, J.L.; Lindhe, J.; Bogren, A.; Hasturk, H.; van Dyke, T.; Wang, X.; Goodson, J.M. Effect of periodontal therapy on the subgingival microbiota over a 2-year monitoring period. I. Overall effect and kinetics of change. J. Clin. Periodontol. 2013, 40, 771–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchard, P.; Carra, M.C.; Boillot, A.; Mora, F.; Rangé, H. Risk factors in periodontology: A conceptual framework. J. Clin. Periodontol. 2017, 44, 125–131. [Google Scholar] [CrossRef]
- Leite, F.R.M.; Nascimento, G.G.; Scheutz, F.; López, R. Effect of Smoking on Periodontitis: A Systematic Review and Meta-regression. Am. J. Prev. Med. 2018, 54, 831–841. [Google Scholar] [CrossRef]
- Ioannidou, E. The Sex and Gender Intersection in Chronic Periodontitis. Front. Public Health 2017, 5, 189. [Google Scholar] [CrossRef] [Green Version]
- Bartold, P.M. Lifestyle and periodontitis: The emergence of personalized periodontics. Periodontology 2000 2018, 78, 7–11. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Ballini, A.; Di Cosola, M.; Saini, R.; Benincasa, C.; Aiello, E.; Marrelli, B.; Rajiv Saini, S.; Ceruso, F.M.; Nocini, R.; Topi, S.; et al. A Comparison of Manual Nylon Bristle Toothbrushes versus Thermoplastic Elastomer Toothbrushes in Terms of Cleaning Efficacy and the Biological Potential Role on Gingival Health. Appl. Sci. 2021, 11, 7180. [Google Scholar] [CrossRef]
- Inchingolo, F.; Martelli, F.S.; Gargiulo Isacco, C.; Borsani, E.; Cantore, S.; Corcioli, F.; Boddi, A.; Nguyễn, K.C.D.; De Vito, D.; Aityan, S.K.; et al. Chronic Periodontitis and Immunity, Towards the Implementation of a Personalized Medicine: A Translational Research on Gene Single Nucleotide Polymorphisms (SNPs) Linked to Chronic Oral Dysbiosis in 96 Caucasian Patients. Biomedicines 2020, 8, 115. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Maiorani, C.; Milone, A.; Alovisi, M.; Scribante, A. Paraprobiotics in Non-Surgical Periodontal Therapy: Clinical and Microbiological Aspects in a 6-Month Follow-Up Domiciliary Protocol for Oral Hygiene. Microorganisms 2022, 10, 337. [Google Scholar] [CrossRef]
- Furquim Dos Santos Cardoso, V.; Amaral Roppa, R.H.; Antunes, C.; Silva Moraes, A.N.; Santi, L.; Konrath, E.L. Efficacy of medicinal plant extracts as dental and periodontal antibiofilm agents: A systematic review of randomized clinical trials. J. Ethnopharmacol. 2021, 281, 114541. [Google Scholar] [CrossRef] [PubMed]
- Wiatrak, K.; Morawiec, T.; Rój, R.; Kownacki, P.; Nitecka-Buchta, A.; Niedzielski, D.; Wychowański, P.; Machorowska-Pieniążek, A.; Cholewka, A.; Baldi, D.; et al. Evaluation of Effectiveness of a Toothpaste Containing Tea Tree Oil and Ethanolic Extract of Propolis on the Improvement of Oral Health in Patients Using Removable Partial Dentures. Molecules 2021, 26, 4071. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, A.R.; Agarwal, E.; Naik, S.B. Clinical and microbiologic effects of commercially available dentifrice containing aloe vera: A randomized controlled clinical trial. J. Periodontol. 2012, 83, 797–804. [Google Scholar] [CrossRef]
- Hayakumo, S.; Arakawa, S.; Takahashi, M.; Kondo, K.; Mano, Y.; Izumi, Y. Effects of ozone nano-bubble water on periodontopathic bacteria and oral cells-in vitro studies. Sci. Technol. Adv. Mater. 2014, 15, 55003. [Google Scholar] [CrossRef] [Green Version]


| Variable | Q-SRP (n = 10) | FMS (n = 10) | FMD (n = 10) | FMDAP (n = 10) |
|---|---|---|---|---|
| Age (years) | 58.1 ± 9.7 | 55.3 ± 11.8 | 58.9 ± 14.3 | 58.0 ± 12.6 |
| Gender (male/female) | 8/2 | 6/4 | 4/6 | 5/5 |
| Smokers (n) | 2 | 4 | 4 | 1 |
| Variable | Timepoint | Q-SRP (n = 10) | FMS (n = 10) | FMD (n = 10) | FMDAP (n = 10) |
|---|---|---|---|---|---|
| PI | Baseline | 1.09 ± 0.35 | 1.35 ± 0.54 | 0.98 ± 0.60 | 1.14 ± 0.48 |
| 3 months | 0.55 ± 0.35 * | 0.78 ± 0.45 * | 0.52 ± 0.46 * | 0.49 ± 0.37 * | |
| 6 months | 0.60 ± 0.48 * | 0.92 ± 0.45 * | 0.82 ± 0.54 | 0.40 ± 0.25 * | |
| GI | Baseline | 1.28 ± 0.38 | 1.47 ± 0.68 | 1.22 ± 0.55 | 1.31 ± 0.47 |
| 3 months | 0.71 ± 0.56 * | 0.79 ± 0.58 * | 0.55 ± 0.52 * | 0.55 ± 0.45 * | |
| 6 months | 0.86 ± 0.68 * | 0.64 ± 0.51 * | 0.56 ± 0.43 * | 0.42 ± 0.32 * | |
| PPD (mm) | Baseline | 3.77 ± 0.74 | 3.92 ± 0.64 | 4.00 ± 0.49 | 3.91 ± 0.60 |
| 3 months | 3.32 ± 0.66 | 3.43 ± 0.60 * | 3.47 ± 0.47 * | 3.18 ± 0.69 * | |
| 6 months | 3.36 ± 0.62 | 3.35 ± 0.58 * | 3.30 ± 0.55 * | 3.18 ± 0.63 * | |
| CAL (mm) | Baseline | 4.36 ± 0.96 | 4.47 ± 0.52 | 5.10 ± 1.02 | 4.68 ± 0.75 |
| 3 months | 3.98 ± 0.82 * | 4.14 ± 0.49 * | 4.66 ± 0.93 * | 4.12 ± 0.65 * | |
| 6 months | 4.01 ± 0.81 * | 4.11 ± 0.64 | 4.63 ± 1.07 * | 4.15 ± 0.63 * | |
| BOP (%) | Baseline | 33.17 ± 14.82 | 49.60 ± 23.00 | 33.78 ± 14.54 | 42.36 ± 19.26 |
| 3 months | 18.06 ± 11.88 * | 24.65 ± 20.54 * | 18.73 ± 12.12 * | 15.19 ± 15.65 * | |
| 6 months | 21.07 ± 11.00 * | 17.61 ± 17.43 | 24.90 ± 17.67 * | 12.59 ± 13.78 * | |
| NBSS (%) | Baseline | 57.00 ± 17.17 | 40.50 ± 21.11 | 47.50 ± 16.88 | 48.40 ± 18.93 |
| 3 months | 71.10 ±13.61 * | 64.50 ± 17.78 * | 70.40 ± 15.31 * | 75.50 ± 19.23 * | |
| 6 months | 69.80 ± 12.39 * | 70.80 ± 17.55 * | 66.6 ± 17.27 | 79.20 ± 16.40 * |
| Treatment | Time Point | Bacterial Species | |
|---|---|---|---|
| P. gingivalis change regarding baseline | C. consius change regarding baseline | ||
| FMS | Baseline 3 months 6 months | 100% 41.7% * 75% | 100% 128.6% * 300% * |
| E. nodatum change regarding baseline | P. dentalis change regarding baseline | ||
| FMD | Baseline 3 months 6 months | 100% 33.3% * 66.7% | 100% 25% * 50% |
| Uncultured Prevotella sp. change regarding baseline | |||
| FMDAP | Baseline 3 months 6 months | 100% 29.4% * 76.5% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, S.; Stein, J.M.; Schumacher, A.; Kupietz, D.; Yekta-Michael, S.S.; Schittenhelm, F.; Conrads, G.; Schaller, H.-G.; Reichert, S. Nonsurgical Periodontal Treatment Options and Their Impact on Subgingival Microbiota. J. Clin. Med. 2022, 11, 1187. https://doi.org/10.3390/jcm11051187
Schulz S, Stein JM, Schumacher A, Kupietz D, Yekta-Michael SS, Schittenhelm F, Conrads G, Schaller H-G, Reichert S. Nonsurgical Periodontal Treatment Options and Their Impact on Subgingival Microbiota. Journal of Clinical Medicine. 2022; 11(5):1187. https://doi.org/10.3390/jcm11051187
Chicago/Turabian StyleSchulz, Susanne, Jamal M. Stein, Anne Schumacher, David Kupietz, Sareh S. Yekta-Michael, Florian Schittenhelm, Georg Conrads, Hans-Günter Schaller, and Stefan Reichert. 2022. "Nonsurgical Periodontal Treatment Options and Their Impact on Subgingival Microbiota" Journal of Clinical Medicine 11, no. 5: 1187. https://doi.org/10.3390/jcm11051187
APA StyleSchulz, S., Stein, J. M., Schumacher, A., Kupietz, D., Yekta-Michael, S. S., Schittenhelm, F., Conrads, G., Schaller, H.-G., & Reichert, S. (2022). Nonsurgical Periodontal Treatment Options and Their Impact on Subgingival Microbiota. Journal of Clinical Medicine, 11(5), 1187. https://doi.org/10.3390/jcm11051187






