1. Introduction
Milk fat globule epidermal growth factor 8 (MFGE8) is a secreted integrin-binding protein, found in mammary epithelial cell surfaces and aortic media. Physiologically, MFGE8 binds to phosphatidylserine at the surface of apoptotic cells and enables their recognition by ανβ3/β5 integrins, promoting the phagocytosis of apoptotic cells by macrophages and the development of subsequent tolerogenic immune responses. Of interest, MFGE8 deficiency in mice delays the clearance of apoptotic lymphocytes, leading to enhanced self-antigen presentation and generation of a disease reminiscent of human systemic lupus erythematosus (SLE) [
1].
Previous studies suggest that MFGE8 serum levels are associated with severe atherosclerosis, acting as an age-related proinflammatory factor [
2]. Additionally, MFGE8 gene polymorphisms have been associated with autoimmunity development in humans. A Korean study reported that SLE patients carrying the MFGE8 rs2271715 CC and rs4945 CA or AA genotypes display higher MFGE8 serum levels, while the rs2271715 and rs3743388 polymorphism a associated with increased risk for SLE development [
1].
Given that patients suffering by systemic autoimmune diseases display an excessive burden for subclinical atherosclerosis [
3,
4,
5,
6], the aim of our study was to examine whether four MFGE8 variants—previously linked to autoimmunity development in a Taiwanese study—(
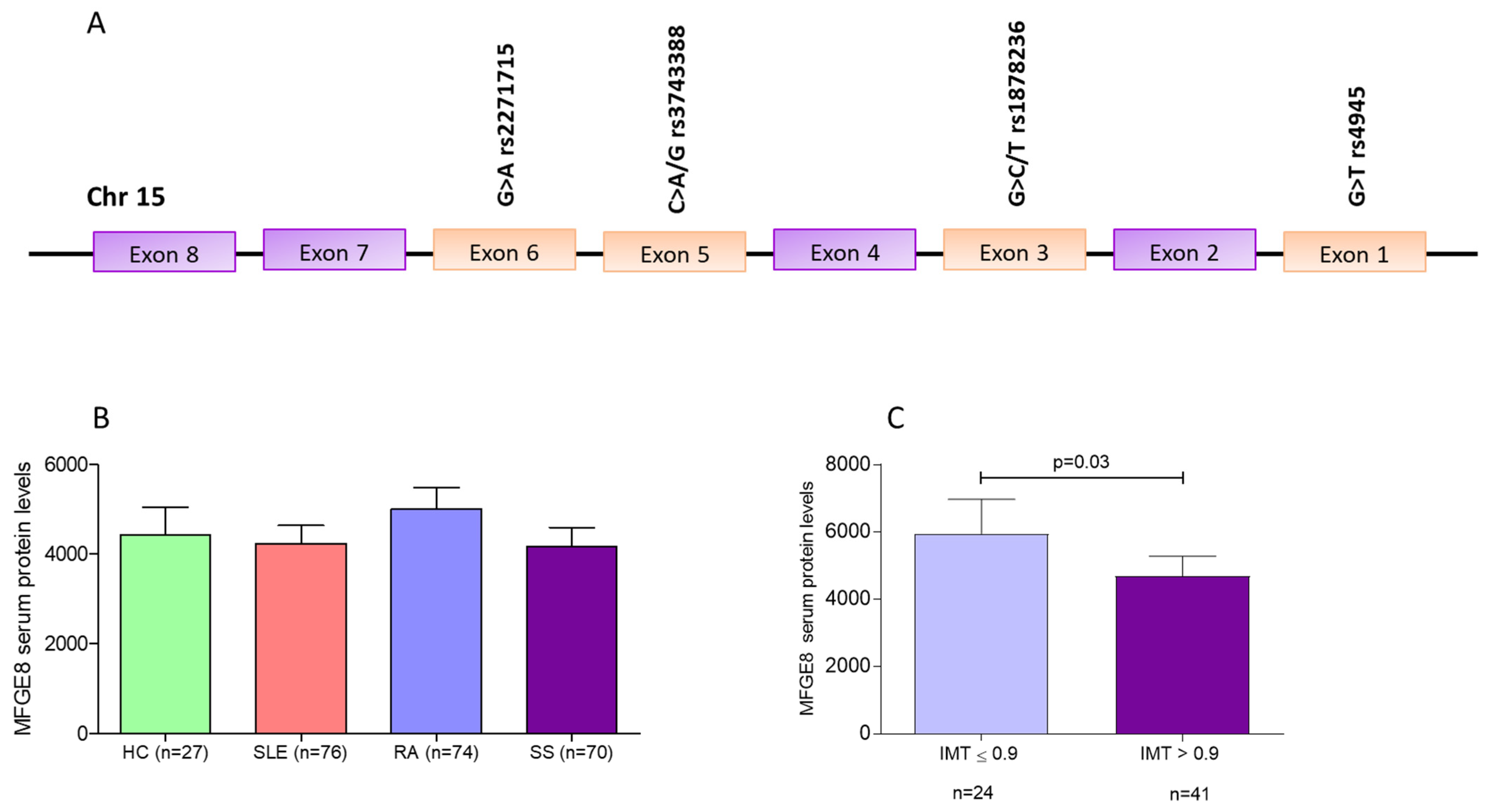
Figure 1A) and MFGE8 serum levels are associated with autoimmune disease susceptibility, as well as autoimmune-related atherosclerosis in Caucasian patients [
7].
2. Patients and Methods
In the present case-control study, DNA derived from peripheral blood of 107 Sjögren’s Syndrome (SS), 116 Rheumatoid arthritis (RA), 123 SLE patients and 199 HC, was extracted, stored and implemented for genotyping studies at the Molecular and Applied Physiology Unit, Department of Physiology, National and Kapodistrian University of Athens. All SS, SLE and RA patients fulfilled the 2016 SS [
8], 2019 SLE [
9] and 2010 RA [
10] classification criteria, respectively. Patients younger than 18 years were excluded from the study. Clinical, serological, histopathological characteristics, as well as markers for subclinical atherosclerosis (carotid plaque and arterial wall thickening, defined as intima media thickness (IMT) scores more than 0.90 mm) were collected for all patients, as previously described [
4,
5]. All study participants provided written informed consent according to the Declaration of Helsinki.
3. Restriction Fragment Length Polymorphism-Polymerase Chain Reaction (RFLP-PCR)
Patients and HC were genotyped for the MFGE8 rs2271715, rs1878326, rs4945, rs3743388 single nucleotide polymorphisms by restriction fragment length polymorphism (RFLP-PCR). Briefly, following the DNA extraction from whole peripheral blood, a genomic DNA aliquot (30–50 ng/mL) was amplified by polymerase chain reaction (PCR), using the following primers: for rs2271715 F: 5′-AAG GTA GGT CCT GTT GGG GC-3′ and R: 5′-GGC TCA GAA TGA AAC CCA GC-3′, for rs1878326 F: 5′-GGC TTA GGC AGA GGT CAT ACA G-3′ and R: 5′-CTG CAA ACC CAA GAA GGT CAC-3′, for rs4945 F: 5′-GTG CTG AGC CGC CTG ATT TA-3′ and R: 5′-TCA CCC AGG GCG ACG A-3′, and for rs3743388 F: 5′-GGT TTA TGC CCA GGC TCC AT-3′ and R: 5′-TCT CAC GTA CTG AGC CTC CA-3′. PCR amplification for all SNPs was performed in a Gene Amp PCR System 9700 (Applied Biosystems, Foster City, CA, USA) for 34 cycles (30 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C). The resulting PCR products were digested using 2.5 units of restriction endonuclease (RSAI for rs2271715, BstNI for rs1878326, BANII for rs4945 and StyI HF for rs3743388, New England Biolabs Ipswich, MA, USA) per reaction, for 55 min at 37 °C. Kits for PCR reactions were supplied by Kapa Biosystems, (Kapa ready mix, Wilmington, DE, USA). PCR products and restriction fragments were visualized on a 3% agarose gel stained with ethidium bromide. The four SNPs after digestion yielded fragments as follows: for rs2271715 CC 287 bp, TT 231 bp and 56 bp, CT 287 bp, 231 bp and 56 bp, for rs1878326 AA 197 bp, CC 126 bp and 61 bp, AC 197 bp, 126 bp and 61 bp, for rs4945 CC 182 bp, AA 111 bp and 71 bp, CA 182 bp, 111 bp, and 71 bp, and for rs3743388 GG 313 bp, CC 231 bp and 82 bp, GC 313 bp, 231 bp and 82 bp.
4. Measurement of MFGE8 Serum Levels
MFGE8 protein levels were measured in sera derived from 70 SS, 76 SLE, 74 RA and 27 HC, using a commercially available ELISA kit (Human MFGE8 Elisa Kit, ABCAM, Waltham, MA, USA), according to the manufacturer’s instructions. All measurements were performed in duplicate, at 412 nm, using a plate reader (Versamax, Molecular Devices, San Jose, CA, USA).
5. Statistics
Allele and genotype frequencies in patients and HCs were determined for the four MFGE8 variants by SHEsis and SNPStats software. Genotype frequencies in control subjects for each SNP were tested for departure from the Hardy–Weinberg equilibrium. Genotype frequencies for all variants were compared in patients and HCs using the χ2 test and ORs and corresponding 95% CIs were estimated. Adjustment for the effects of age and gender was performed. Five genetic models (codominant, dominant, recessive, over dominant, and additive) were also determined.
We assessed two-group comparisons of continuous data, using t-tests or the Mann–Whitney test, when the data distribution was not normal. The SPSS v.26 and GraphPad Prism 8 software were used. We determined the correlation between gene expression data using a non-parametric Spearman’s test. A p-value of <0.05 was considered statistically significant.
6. Results
To investigate the allele and genotype distribution of MFGE8 gene variants rs2271715, rs1878326, rs4945 and rs3743388 among SS, RA, SLE patients, and HC, allele and genotype analysis was performed. While no significant differences were found in the allele and genotype distribution of the four MFGE8 studied variants, among SS and SLE patients, compared to HC after adjustment for age and sex, in the RA patient group, the CA genotype of rs4945 variant exhibited a protective effect against disease development (OR (95% CI): 0.58 (0.34–0.99),
p = 0.043). No other significant associations were detected between the MFGE8 variants and any demographic, clinical, laboratory features or markers of subclinical atherosclerosis for any disease group. Of interest, following haplotype analysis, we found a significant protective effect of the CACG haplotype, in both RA (HC vs. RA: 0.11% vs. 0.04%, OR (95% CI): 0.26 (0.10–0.71),
p = 0.009) and SS patients (HC vs. SS: 0.11% vs. 0.05%, OR (95% CI): 0.22 (0.06–0.8),
p = 0.023) compared to HC (
Table 1). However, this observation was not confirmed in the SLE population.
Regarding MFGE8 serum levels, no significant differences were found between RA, SS, SLE and HC (
Figure 1B). Similarly, after stratification of all disease cohorts and HC, according to the genotypes of the four MFGE8 variants, no differences in MFGE8 serum levels were detected (data not shown). Of interest, SS, but not RA or SLE, patients with IMT ≤ 0.9 displayed higher MGFE8 serum levels compared to those with >0.9 (mean ± SD: 594 ± 504 ng/μL vs. 468 ± 381 ng/μL,
p = 0.03) (
Figure 1C). No significant associations between markers of subclinical atherosclerosis and MFGE8 serum levels were detected in RA or SLE groups.
7. Discussion
To our knowledge, this is the first time that the CACG MFGE8 haplotype was found to confer reduced risk for RA and SS development, with MFGE8 protein levels being reduced in SS patients with evidence of carotid arterial wall thickening. Previous studies conducted in Korean and Taiwanese SLE cohorts have reported that SLE patients carrying the MFGE8 rs2271715 and rs3743388 variants display higher MFGE8 serum levels [
1] and increased risk for SLE compared to HC [
1,
7] (findings not confirmed in the present cohort). Additionally, we observed that the CA genotype of the rs4945 variant exhibits a protective effect against RA development, a finding that was not observed in SS and SLE cohorts. A recent study conducted in a Korean SLE cohort revealed that SLE patients carrying the A allele of the rs4945 variant a display higher erythrocyte sedimentation rate (ESR) compared to non-carriers [
1]. However, no association with any clinical disease manifestation was observed in our disease cohorts. The apparently discrepant results between the different studies might reflect genetic differences between Caucasian and Asian patients.
A limitation of our study is the relatively small number of patients enrolled in the study. Due to the study design, we retrospectively included only patients with available atherosclerosis-related data. Thus, these findings should be further explored in larger multicenter cohorts.
Taken together, we report a novel association of MFGE8 genetic variations in SS and RA susceptibility, along with reduced MFGE8 serum levels in SS patients with heightened atherosclerotic risk. These findings further strengthen the concept of shared pathophysiological mechanisms between atherogenesis and autoimmune diseases.
Author Contributions
Conceptualization, A.C. and C.S.; methodology, C.S., A.N. and C.P.M.; formal analysis, C.P.M.; investigation, C.S., A.N.; resources, A.C., C.P.M.; data curation, C.P.M., C.S.; writing—original draft preparation, C.S.; writing—review and editing, C.P.M., C.S., A.C.; visualization, supervision, C.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All patients gave their informed consent, and this retrospective study is in accordance with the Helsinki Declaration.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baek, W.-Y.; Woo, J.-M.; Kim, H.-A.; Jung, J.-Y.; Suh, C.-H. Polymorphisms of MFGE8 are associated with susceptibility and clinical manifestations through gene expression modulation in Koreans with systemic lupus erythematosus. Sci. Rep. 2019, 9, 18565. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhang, H.; Qin, X. Age-related differences in serum MFG-E8, TGF-β1 and correlation to the severity of atherosclerosis determined by ultrasound. Mol. Med. Rep. 2017, 16, 9741–9748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arida, A.; Legaki, A.-I.; Kravvariti, E.; Protogerou, A.; Sfikakis, P.P.; Chatzigeorgiou, A. PCSK9/LDLR system and rheumatoid arthritis-related atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 738764. [Google Scholar] [CrossRef] [PubMed]
- Giannelou, M.; Skarlis, C.; Stamouli, A.; Antypa, E.; Moutsopoulos, H.M.; Mavragani, C.P. Atherosclerosis in SLE: A potential role for serum parathormone levels. Lupus Sci. Med. 2020, 7, e000393. [Google Scholar] [CrossRef] [PubMed]
- Gravani, F.; Papadaki, I.; Antypa, E.; Nezos, A.; Masselou, K.; Ioakeimidis, D.; Koutsilieris, M.; Moutsopoulos, H.M.; Mavragani, C.P. Subclinical atherosclerosis and impaired bone health in patients with Primary Sjogren’s Syndrome: Prevalence, clinical and laboratory associations. Arthritis Res. Ther. 2015, 17, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arida, A.; Protogerou, A.D.; Kitas, G.D.; Sfikakis, P.P. Systemic inflammatory response and atherosclerosis: The paradigm of chronic inflammatory rheumatic diseases. Int. J. Mol. Sci. 2018, 19, 1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.Y.; Wu, C.S.; Tsai, H.F.; Chang, S.K.; Tsai, W.I.; Hsu, P.N. Genetic polymorphism in milk fat globule-EGF Factor 8 (MFG-E8) is associated with systemic lupus erythematosus in human. Lupus 2009, 18, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for Primary Sjögren’s Syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann. Rheum. Dis. 2017, 76, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Aringer, M.; Costenbader, K.H.; Daikh, D.I.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.; Kamen, D.L.; et al. 2019 EULAR/ACR classification criteria for systemic lupus erythematosus. Arthritis Rheumatol. 2019, 71, 1400–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).