The Assessment of Maternal and Fetal Intima-Media Thickness in Perinatology
Abstract
:1. Introduction
2. Effectiveness of IMT Measurements
3. IMT in Pregnancy
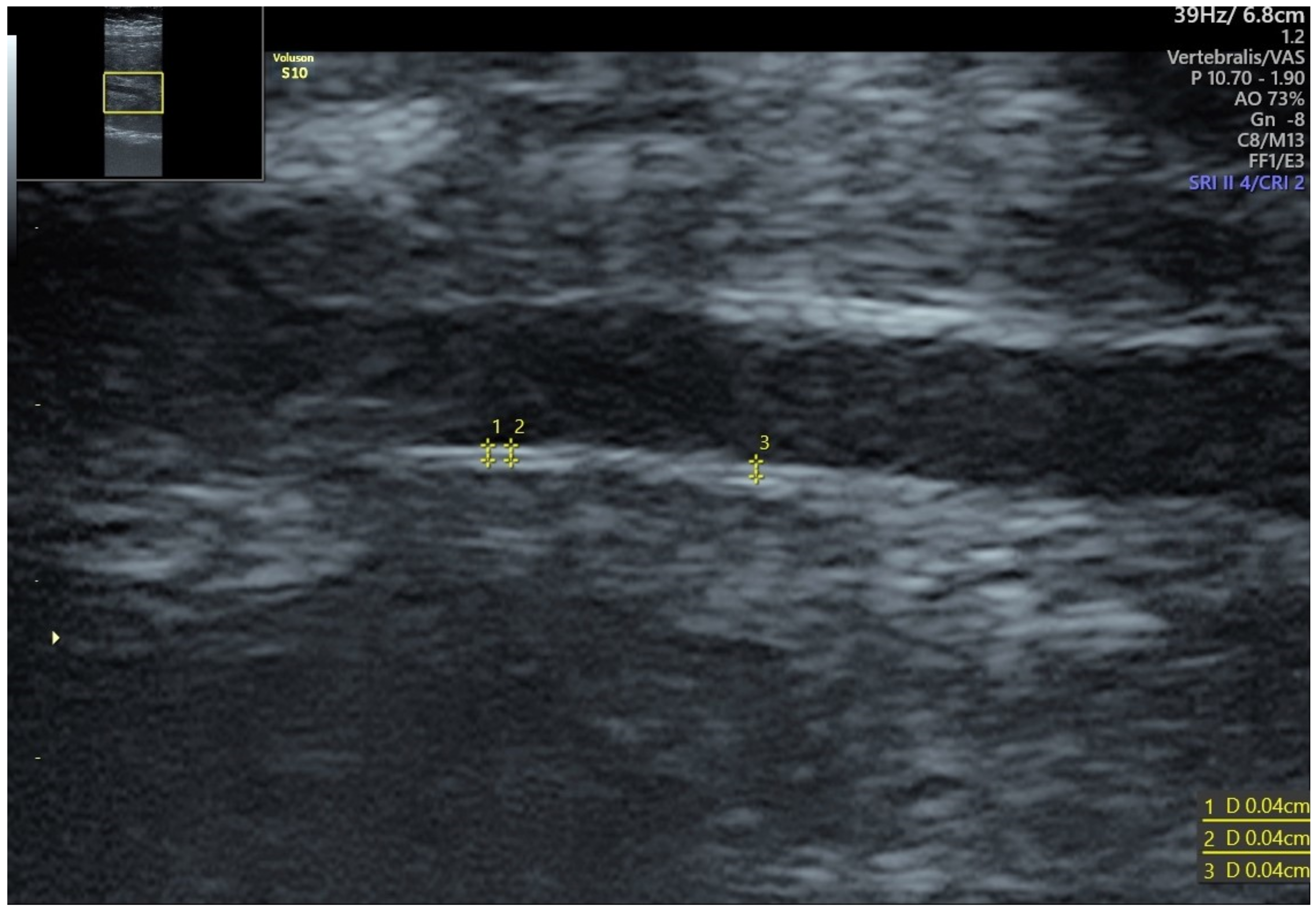
4. Fetal IMT
5. Treatment Options
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willeit, P.; Tschiderer, L.; Allara, E.; Reuber, K.; Seekircher, L.; Gao, L.; Liao, X.; Lonn, E.; Gerstein, H.C.; Yusuf, S.; et al. Carotid Intima-Media Thickness Progression as Surrogate Marker for Cardiovascular Risk: Meta-Analysis of 119 Clinical Trials Involving 100 667 Patients. Circulation 2020, 142, 621–642. [Google Scholar] [CrossRef] [PubMed]
- Prospective Study on the Prognostic Value of Repeated Carotid Intima-Media Thickness Assessment in Patients with Coronary and Extra Coronary Steno-Occlusive Arterial Disease. Available online: https://www.mp.pl/paim/issue/article/4407 (accessed on 28 November 2021).
- Ji, X.; Leng, X.-Y.; Dong, Y.; Ma, Y.-H.; Xu, W.; Cao, X.-P.; Hou, X.-H.; Dong, Q.; Tan, L.; Yu, J.-T. Modifiable Risk Factors for Carotid Atherosclerosis: A Meta-Analysis and Systematic Review. Ann. Transl. Med. 2019, 7, 632. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.W.; Markus, H.S.; Bots, M.L.; Rosvall, M.; Sitzer, M. Prediction of Clinical Cardiovascular Events with Carotid Intima-Media Thickness: A Systematic Review and Meta-Analysis. Circulation 2007, 115, 459–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Oord, S.C.H.; Sijbrands, E.J.G.; ten Kate, G.L.; van Klaveren, D.; van Domburg, R.T.; van der Steen, A.F.W.; Schinkel, A.F.L. Carotid Intima-Media Thickness for Cardiovascular Risk Assessment: Systematic Review and Meta-Analysis. Atherosclerosis 2013, 228, 1–11. [Google Scholar] [CrossRef]
- Lamotte, C.; Iliescu, C.; Libersa, C.; Gottrand, F. Increased Intima-Media Thickness of the Carotid Artery in Childhood: A Systematic Review of Observational Studies. Eur. J. Pediatr. 2011, 170, 719–729. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Iliadis, F.; Liapis, C.D. Exercise and Carotid Atherosclerosis. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Markopoulou, P.; Papanikolaou, E.; Analytis, A.; Zoumakis, E.; Siahanidou, T. Preterm Birth as a Risk Factor for Metabolic Syndrome and Cardiovascular Disease in Adult Life: A Systematic Review and Meta-Analysis. J. Pediatr. 2019, 210, 69–80.e5. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, X.; Yin, H.; Sun, Z.; Zügel, M.; Steinacker, J.M.; Schumann, U. Exercise Training and Endothelial Function in Patients with Type 2 Diabetes: A Meta-Analysis. Cardiovasc. Diabetol. 2018, 17, 64. [Google Scholar] [CrossRef]
- Lespagnol, E.; Dauchet, L.; Pawlak-Chaouch, M.; Balestra, C.; Berthoin, S.; Feelisch, M.; Roustit, M.; Boissière, J.; Fontaine, P.; Heyman, E. Early Endothelial Dysfunction in Type 1 Diabetes Is Accompanied by an Impairment of Vascular Smooth Muscle Function: A Meta-Analysis. Front. Endocrinol. 2020, 11, 203. [Google Scholar] [CrossRef]
- Ryckman, K.K.; Spracklen, C.N.; Smith, C.J.; Robinson, J.G.; Saftlas, A.F. Maternal Lipid Levels during Pregnancy and Gestational Diabetes: A Systematic Review and Meta-Analysis. BJOG 2015, 122, 643–651. [Google Scholar] [CrossRef]
- Lain, K.Y.; Catalano, P.M. Metabolic Changes in Pregnancy. Clin. Obstet. Gynecol. 2007, 50, 938–948. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, F.; Li, S. Metabolic Adaptations in Pregnancy: A Review. ANM 2017, 70, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Sitruk-Ware, R. Progestins and Cardiovascular Risk Markers. Steroids 2000, 65, 651–658. [Google Scholar] [CrossRef]
- Barbour, L.A.; McCurdy, C.E.; Hernandez, T.L.; Kirwan, J.P.; Catalano, P.M.; Friedman, J.E. Cellular Mechanisms for Insulin Resistance in Normal Pregnancy and Gestational Diabetes. Diabetes Care 2007, 30 (Suppl. 2), S112–S119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes van Balen, V.A.; van Gansewinkel, T.a.G.; de Haas, S.; van Kuijk, S.M.J.; van Drongelen, J.; Ghossein-Doha, C.; Spaanderman, M.E.A. Physiological Adaptation of Endothelial Function to Pregnancy: Systematic Review and Meta-Analysis. Ultrasound Obstet. Gynecol. 2017, 50, 697–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carty, D.M.; Delles, C.; Dominiczak, A.F. Preeclampsia and Future Maternal Health. J. Hypertens. 2010, 28, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- Sibai, B.; Dekker, G.; Kupferminc, M. Pre-Eclampsia. Lancet 2005, 365, 785–799. [Google Scholar] [CrossRef]
- Rolnik, D.L.; Wright, D.; Poon, L.C.Y.; Syngelaki, A.; O’Gorman, N.; de Paco Matallana, C.; Akolekar, R.; Cicero, S.; Janga, D.; Singh, M.; et al. ASPRE Trial: Performance of Screening for Preterm Pre-Eclampsia. Ultrasound Obstet. Gynecol. 2017, 50, 492–495. [Google Scholar] [CrossRef]
- Kumar, A.; Cannon, C.P. Acute Coronary Syndromes: Diagnosis and Management, Part I. Mayo Clin. Proc. 2009, 84, 917–938. [Google Scholar] [CrossRef] [Green Version]
- Rafieian-Kopaei, M.; Setorki, M.; Doudi, M.; Baradaran, A.; Nasri, H. Atherosclerosis: Process, Indicators, Risk Factors and New Hopes. Int. J. Prev. Med. 2014, 5, 927–946. [Google Scholar]
- Weissgerber, T.L.; Milic, N.M.; Milin-Lazovic, J.S.; Garovic, V.D. Impaired Flow-Mediated Dilation Before, During, and After Preeclampsia: A Systematic Review and Meta-Analysis. Hypertension 2016, 67, 415–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crispi, F.; Figueras, F.; Cruz-Lemini, M.; Bartrons, J.; Bijnens, B.; Gratacos, E. Cardiovascular Programming in Children Born Small for Gestational Age and Relationship with Prenatal Signs of Severity. Am. J. Obstet. Gynecol. 2012, 207, 121.e1–121.e9. [Google Scholar] [CrossRef] [PubMed]
- Stergiotou, I.; Crispi, F.; Valenzuela-Alcaraz, B.; Cruz-Lemini, M.; Bijnens, B.; Gratacos, E. Aortic and Carotid Intima-Media Thickness in Term Small-for-Gestational-Age Newborns and Relationship with Prenatal Signs of Severity. Ultrasound Obstet. Gynecol. 2014, 43, 625–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanardo, V.; Fanelli, T.; Weiner, G.; Fanos, V.; Zaninotto, M.; Visentin, S.; Cavallin, F.; Trevisanuto, D.; Cosmi, E. Intrauterine Growth Restriction Is Associated with Persistent Aortic Wall Thickening and Glomerular Proteinuria during Infancy. Kidney Int. 2011, 80, 119–123. [Google Scholar] [CrossRef]
- McDonald, S.D.; Ray, J.; Teo, K.; Jung, H.; Salehian, O.; Yusuf, S.; Lonn, E. Measures of Cardiovascular Risk and Subclinical Atherosclerosis in a Cohort of Women with a Remote History of Preeclampsia. Atherosclerosis 2013, 229, 234–239. [Google Scholar] [CrossRef]
- Grand’Maison, S.; Pilote, L.; Okano, M.; Landry, T.; Dayan, N. Markers of Vascular Dysfunction After Hypertensive Disorders of Pregnancy: A Systematic Review and Meta-Analysis. Hypertension 2016, 68, 1447–1458. [Google Scholar] [CrossRef] [Green Version]
- Kirollos, S.; Skilton, M.; Patel, S.; Arnott, C. A Systematic Review of Vascular Structure and Function in Pre-Eclampsia: Non-Invasive Assessment and Mechanistic Links. Front. Cardiovasc. Med. 2019, 6, 166. [Google Scholar] [CrossRef]
- Nardozza, L.M.M.; Caetano, A.C.R.; Zamarian, A.C.P.; Mazzola, J.B.; Silva, C.P.; Marçal, V.M.G.; Lobo, T.F.; Peixoto, A.B.; Araujo Júnior, E. Fetal Growth Restriction: Current Knowledge. Arch. Gynecol. Obstet. 2017, 295, 1061–1077. [Google Scholar] [CrossRef]
- Stergiotou, I.; Bijnens, B.; Cruz-Lemini, M.; Figueras, F.; Gratacos, E.; Crispi, F. Maternal Subclinical Vascular Changes in Fetal Growth Restriction with and without Pre-Eclampsia. Ultrasound Obstet. Gynecol. 2015, 46, 706–712. [Google Scholar] [CrossRef]
- Gomez-Roig, M.D.; Mazarico, E.; Valladares, E.; Guirado, L.; Fernandez-Arias, M.; Vela, A. Aortic Intima-Media Thickness and Aortic Diameter in Small for Gestational Age and Growth Restricted Fetuses. PLoS ONE 2015, 10, e0126842. [Google Scholar] [CrossRef] [Green Version]
- Evanoff, N.G.; Dengel, D.R.; Narasimhan, S. Assessing Vascular Characteristics of the Fetal Descending Aorta: A Feasibility Study. J. Clin. Ultrasound 2020, 48, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Visentin, S.; Londero, A.P.; Calanducci, M.; Grisan, E.; Bongiorno, M.C.; Marin, L.; Cosmi, E. Fetal Abdominal Aorta: Doppler and Structural Evaluation of Endothelial Function in Intrauterine Growth Restriction and Controls. Ultraschall Med. 2019, 40, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Galjaard, S.; Pasman, S.A.; Ameye, L.; Timmerman, D.; Devlieger, R. Intima-Media Thickness Measurements in the Fetus and Mother during Pregnancy: A Feasibility Study. Ultrasound Med. Biol. 2014, 40, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Lemini, M.; Crispi, F.; Valenzuela-Alcaraz, B.; Figueras, F.; Sitges, M.; Bijnens, B.; Gratacós, E. Fetal Cardiovascular Remodeling Persists at 6 Months in Infants with Intrauterine Growth Restriction. Ultrasound Obstet. Gynecol. 2016, 48, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Visentin, S.; Grumolato, F.; Nardelli, G.B.; Di Camillo, B.; Grisan, E.; Cosmi, E. Early Origins of Adult Disease: Low Birth Weight and Vascular Remodeling. Atherosclerosis 2014, 237, 391–399. [Google Scholar] [CrossRef]
- Robbins, C.L.; Hutchings, Y.; Dietz, P.M.; Kuklina, E.V.; Callaghan, W.M. History of Preterm Birth and Subsequent Cardiovascular Disease: A Systematic Review. Am. J. Obstet. Gynecol. 2014, 210, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Skilton, M.R.; Celermajer, D.S.; Cosmi, E.; Crispi, F.; Gidding, S.S.; Raitakari, O.T.; Urbina, E.M. Natural History of Atherosclerosis and Abdominal Aortic Intima-Media Thickness: Rationale, Evidence, and Best Practice for Detection of Atherosclerosis in the Young. J. Clin. Med. 2019, 8, 1201. [Google Scholar] [CrossRef] [Green Version]
- Visentin, S.; Londero, A.P.; Bellamio, B.; Giunta, G.; Cosma, C.; Faggian, D.; Plebani, M.; Cosmi, E. Fetal Endothelial Remodeling in Late-Onset Gestational Hypertension. Am. J. Hypertens. 2016, 29, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Atabek, M.E.; Çağan, H.H.; Selver Eklioğlu, B.; Oran, B. Absence of Increase in Carotid Artery Intima-Media Thickness in Infants of Diabetic Mothers. J. Clin. Res. Pediatr. Endocrinol. 2011, 3, 144–148. [Google Scholar] [CrossRef]
- Epure, A.M.; Rios-Leyvraz, M.; Anker, D.; Bernardo, S.D.; da Costa, B.R.; Chiolero, A.; Sekarski, N. Risk Factors during First 1000 Days of Life for Carotid Intima-Media Thickness in Infants, Children, and Adolescents: A Systematic Review with Meta-Analyses. PLOS Med. 2020, 17, e1003414. [Google Scholar] [CrossRef]
- Iwashima, S.; Ishikawa, T.; Akira, O.; Itou, H. Association of Abdominal Aortic Wall Thickness in the Newborn with Maternal Factors. Am. J. Perinatol. 2012, 29, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Önal, Z.E.; Soydan, L.; Öztürk, H.E.; Sağ, Ç.; Gürbüz, T.; Nuhoğlu, Ç.; Şimşek, M.M. Carotid Intima Media Thickness in Obese Children: Is There an Association with Hyperlipidemia? J. Pediatric Endocrinol. Metab. 2016, 29, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Bjarnegård, N.; Morsing, E.; Cinthio, M.; Länne, T.; Brodszki, J. Cardiovascular Function in Adulthood Following Intrauterine Growth Restriction with Abnormal Fetal Blood Flow. Ultrasound Obstet. Gynecol. 2013, 41, 177–184. [Google Scholar] [CrossRef]
- Skilton, M.R.; Ayer, J.G.; Harmer, J.A.; Webb, K.; Leeder, S.R.; Marks, G.B.; Celermajer, D.S. Impaired Fetal Growth and Arterial Wall Thickening: A Randomized Trial of ω-3 Supplementation. Pediatrics 2012, 129, e698–e703. [Google Scholar] [CrossRef] [PubMed]
- Kagota, S.; Maruyama, K.; McGuire, J.J. Characterization and Functions of Protease-Activated Receptor 2 in Obesity, Diabetes, and Metabolic Syndrome: A Systematic Review. Biomed. Res. Int. 2016, 2016, 3130496. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boroń, D.; Kornacki, J.; Wender-Ozegowska, E. The Assessment of Maternal and Fetal Intima-Media Thickness in Perinatology. J. Clin. Med. 2022, 11, 1168. https://doi.org/10.3390/jcm11051168
Boroń D, Kornacki J, Wender-Ozegowska E. The Assessment of Maternal and Fetal Intima-Media Thickness in Perinatology. Journal of Clinical Medicine. 2022; 11(5):1168. https://doi.org/10.3390/jcm11051168
Chicago/Turabian StyleBoroń, Daniel, Jakub Kornacki, and Ewa Wender-Ozegowska. 2022. "The Assessment of Maternal and Fetal Intima-Media Thickness in Perinatology" Journal of Clinical Medicine 11, no. 5: 1168. https://doi.org/10.3390/jcm11051168
APA StyleBoroń, D., Kornacki, J., & Wender-Ozegowska, E. (2022). The Assessment of Maternal and Fetal Intima-Media Thickness in Perinatology. Journal of Clinical Medicine, 11(5), 1168. https://doi.org/10.3390/jcm11051168






